Angelicin
 From Wikipedia - Reading time: 13 min
From Wikipedia - Reading time: 13 min

| |
| Names | |
|---|---|
| Pronunciation | ˈeɪn.dʒəlaɪ.sɪn |
| Preferred IUPAC name
2H-Furo[2,3-h][1]benzopyran-2-one | |
| Other names
Isopsoralen, 2H-furo[2,3-h]chromen-2-one, furo[2,3-h]chromen-2-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.164.795 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H6O3 | |
| Molar mass | 186.166 g·mol−1 |
| Appearance | pale yellow crystals [1] |
| Melting point | 134°C |
| Boiling point | 362.6°C |
| 10 mM in DMSO | |
| log P | 1.97[2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
photosensitizer, vesicant, carcinogen |
| Flash point | 173.1°C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Angelicin is the parent compound in a family of naturally occurring organic compounds known as the angular furanocoumarins. Structurally, it can be considered as benzapyra-2-one fused with a furan moiety in the 7,8-position. Angelicin is commonly found in certain Apiaceae and Fabaceae plant species such as Bituminaria bituminosa. It has a skin permeability coefficient (LogKp) of -2.46.[2] The maximum absorption is observed at 300 nm.[3] The 1HNMR spectrum is available;[1] the infrared and mass spectra of angelicin can be found in this database. The sublimation of angelicin occurs at 120 °C and the pressure of 0.13 Pa.[4] Angelicin is a coumarin.
History and etymology
[edit]Humans have used plants rich in angelicin for centuries. The earliest known record dates back to 3000 BC when ancient Egyptians applied the oil and sap of local Apiaceae species exposing their skin to sunlight to cure vitiligo. In meantime, tribes in India used Psoralea corylifolia which contained psoralen, the isomer of angelicin. Humans also attempted to harvest the plants as an alternative food source. However, most of them turned out to be unpalatable and toxic such as Angelica archangelica due to the ability to irritate skin and damage internal organs.[5]

The name "angelicin" stems from the aforementioned plant, Angelica. This Latin name originated in medieval Europe where this plant was also used as a universal treatment to many types of disease not mentioning the bubonic plague. At this time, people believed that the plant could prevent the soul from being taken over by sorcery, curse and evil spirit (add reference). Angelica would have appeared in a dream with an angel explaining its applications, hence the name. Ironically, it was later discovered that the plant's oil is toxic when utilized in large quantities particularly when the plant was fresh.[6]
The species of plants where angelicin is found was introduced in Britain in the 19th century. Currently, it can be found in Canada and some parts of the United States and Europe. Because of the toxicity of certain plant parts and the ability of plant to proliferate, it is included in the list of invasive species.[7]
The leaves of Angelica archangelica, which are rich in angelicin, are used to extract the compound.[8] There were multiple studies on the toxicity of angelicin one of which showed that the compound elicits chromosomal damage in hamster cells exposed to 320-380 nm UV light.[9] The chromosomal aberrations were shown to be also induced in humans.
Nowadays, it is debated whether Angelica should be considered toxic. However, it is certain that the toxicity is dependent on the dose of angelicin administered and is solely the matter of experts when it comes to its application.
Biological synthesis
[edit]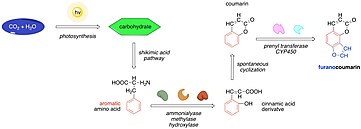
The biosynthesis of angelicin can be described as a variation in the biological synthesis of furanocoumarins. It begins from the capture of organic carbon by photosynthesis and the formation of carbohydrates. Subsequently, the carbohydrates become the substrates of the shikimic acid pathway where they are converted to phenylalanine and tyrosine. Enzymes such as ammonialyases, methylases and hydroxylases then transform these amino acids to cinnamic acid derivatives which undergo o-hydroxylation yielding coumarins. The coumarins can undergo further reactions such as prenylation and oxidation to give multiple furanocoumarins one of which is angelicin.[10]

Here, the biosynthesis of angelicin is described in more detail starting at L-phenylalanine as a precursor. The phenylalanine undergoes a non-oxidative deamination by phenylalanine ammonia-lyase (PAL) to trans-cinnamic acid. Afterwards, the trans-cinnamic acid is hydroxylated at the para position by trans-cinnamate 4-monooxygenase (C4H) which utilizes NADPH, H+ and O2. The product, p-coumaric acid, is then converted to umbelliferone, the important intermediate of biosynthesis pathway.[11]

4-Coumaric acid 2-hydroxylase (C2’H) hydroxylates the p-coumaric acid at the ortho position. Notably, this reaction uses alpha-ketoglutarate which is reduced to succinate both of which are involved in the Krebs cycle. The newly formed trans-dihydrocinnamic acid undergoes a photochemical isomerization to a cis isomer which spontaneously lactonizes to yield umbeliferone.[12]

Subsequently, umbelliferone 6-prenyltransferase (PT) couples umbelliferone with prenyl diphosphate to give osthenol and pyrophosphate. Osthenol is oxidized to (+)-columbianetin by (+)-columbianetin synthase (CS), a putative plant cytochrome P450, although the details of this reaction are not clear. The biosynthesis is terminated with the oxidation of (+)-columbianetin yielding angelicin by angelicin synthase (AS) which is also considered as the enzyme of cytochrome P450 family.[13]
It is noteworthy that the biosynthesis of angelicin diverges at the umbelliferone as it is also converted to psoralen, the isomer of angelicin. In fact, psoralen, from which the family of linear furanocoumarins descends, is by far much more abundant in plants than angelicin. As a result, most herbivorous insects are resistant to psoralen. Now, it is increasingly recognized that plants devised the pathway leading to angelicin as an alternative defense mechanism. For example, angelicin enhances the toxicity of psoralen by acting as an inhibitor of the detoxifying cytochrome P450 in insects.[14] Moreover, the comparison of the protein sequences of psoralen synthase and angelicin synthase shows a 70% identity overall and 40% identity in the substrate recognition sites.[13] This implies that the biosynthesis of angelicin is a relatively recently evolved trait.
Chemical synthesis
[edit]


Iodination of commercially available umbelliferone (7-hydroxycoumarin) yields 7-hydroxy-8-iodocoumarin. Acetoxy group can be introduced into hydroxyl of 7-hydroxy-8-iodocoumarin, which is used to create vaginol or vaginidiol with an isopropyl Grignard reagent and commercially available epoxy aldehydes. Subsequent acid-catalysed fragmentation of vaginol with dichloromethane in trifluoroacetic acid yields angelicin.[15]
The compound can be isolated from natural sources, albeit this affords a low yield due to the prevalence of other furanocoumarins. The popular technique is air drying the aerial parts and ground roots of plant followed by n-hexane extraction and column chromatography over silica gel.[1][16]
Medical use
[edit]Angelicin derivatives are used to treat psoriasis and cancer. One way of treating these diseases is by photochemotherapy (PUVA) which combines UV irradiation with photosensitizing chemical.[17][18] In most cases the 4,5’-dimethylangelicin is applied owing to its firm binding and specificity to DNA. Also, it was shown that it is actively inhibits the synthesis of nucleic acids in tumor cells thereby decreasing their growth.[19]
In PUVA, angelicin is less popular than psoralen, although both furanocoumarins are photosensitizing and used in couple with long-wave UV irradiation. Angelicin and psoralen are used in other skin disorders such as vitiligo and mycosis. DNA photobinding is the most studied aspect of the photobiology and photochemistry of angelicin. According to the mechanism, long-range UV light triggers angelicin to bind to the pyrimidine bases of DNA in the same manner as psoralen.[20] In this way, the inhibition of DNA replication via the formation of photoadducts can occur. This might be the basis for the desired therapeutic effect as in the case of psoralen derivatives.[17]
However, extreme care should be taken while using PUVA due to the side effects it may bring. Therefore, this type of treatment is sometimes used as a last resort and often corticosteroids are used instead.[18] One of the main adverse effects of PUVA is phototoxicity which can be tackled by heteroanalogues of angelicin. For example, recently researchers have shown that if furan ring is replaced by 1-substituted pyrazole or thiophene ring, the new angelicin heteroanalogues show virtually no phototoxicity.[21]
Interaction with biomolecules
[edit]
It was shown that angelicin exhibits a multifaceted effect on various biomolecules which stem from the compound's structure and photoreactivity. For example, the planar structure allows angelicin to intercalate between the DNA bases. When exposed to ultraviolet light, it undergoes a C4-photocycloaddition reaction with thymine and cytosine forming a monoadduct. The double bonds of angelicin involved in this reaction are the 3,4 and 4’,5’.[22] However, the rest of the angelicin's aromatic system cannot react with the pyrimidine of complementary strand owing to the unfavorable alignment of reactive double bonds.[23] Lipids are also susceptible to the photoinduced reactions with angelicin which can be either aerobic or anaerobic. The aerobic reactions cause lipid peroxidation [24] whereas the anaerobic pathway leads to the conjugation of angelicin with unsaturated fatty acid chains such as linolenic acid in a manner similar to the formation of pyrimidine adducts.[25]

Proteins were demonstrated to interact with angelicin in a non-covalent fashion. For instance, there is a measurable affinity of angelicin towards human serum albumin (19.10 × 104 mol−1L−1) which has one non-covalent binding site per angelicin molecule. The ultraviolet light (365 nm) facilitates its covalent binding to proteins which is enhanced in the presence of oxygen. At this wavelength, angelicin can also modify certain amino acids.[26][27][28]
Toxicity
[edit]According to the MSDS of Sigma-Aldrich,[29] the LD50 of angelicin is 322 mg/kg which shows acute toxicity if orally administered to rats. The possible consequences are alteration in circadian rhythm and righting reflex, ataxia and analgesia.
Angelicin demonstrates phototoxic and photomutagenic effects when in contact with skin. It enhances the sensitivity of skin to UV light [30] leading to severe skin damage such as erythema and blisters.[31][32] Upon irradiation with UV light of longer wavelength, angelicin forms DNA monoadducts which can cause skin cancer.[32] In contrast, the isomer of angelicin, psoralen, was reported to be five to ten times more active than angelicin and cross-link DNA . This impedes DNA replication more prominently due to the inability for the two strands of DNA helix to separate.[33] Both psoralen and angelicin can be used in cancer therapeutics to suppress DNA replication in tumor cells and induce apoptosis – as mentioned in medical use – but they should be handled with care as they can cause photodermatitis in healthy cells as a side effect.[30][33]
In mammalian cell cultures, angelicin showed mutagenic and cytotoxic effects while playing a role of strong inhibitor of drug metabolism.[34] The inhibition is due to the fact that angelicin decreases the activity and expression of CYP1A1 which is regulated by aryl hydrocarbon receptors (AhR). There are three hypotheses proposed to explain the phenomenon:[34]
- Angelicin attenuates the catalytic activity performed by CYP1A1 regardless the presence of UV light.
- Angelicin triggers the gene expression of CYP1A1 by activation of AhR when no UV light is available.
- Angelicin leads to CYP1A1 gene expression without the involvement of AhR.
The phototoxic properties of angelicin were deployed by its use as a natural pesticide and disinfectant. Note that it is difficult to readily determine whether only angelicin poses the highest risk of phototoxicity and photomutagenicity as in plants angelicin always occurs in a mixture with angelicin derivatives, psoralen and other furanocoumarins. Moreover, the furanocoumarin composition of most plant species is not definitely known as well as the toxic properties of some furanocoumarins.[32]
References
[edit]- ^ a b c Dehghan, Hossein; Sarrafi, Yaghoub; Salehi, Peyman; Ebrahimi, Samad Nejad (2017-04-01). "α-Glucosidase inhibitory and antioxidant activity of furanocoumarins from Heracleum persicum". Medicinal Chemistry Research. 26 (4): 849–855. doi:10.1007/s00044-017-1796-y. ISSN 1054-2523. S2CID 31293666.
- ^ a b "BioByte". www.biobyte.com. Retrieved 2018-03-15.
- ^ Bordin, F.; Dall'Acqua, F.; Guiotto, A. (December 1991). "Angelicins, angular analogs of psoralens: chemistry, photochemical, photobiological and phototherapeutic properties". Pharmacology & Therapeutics. 52 (3): 331–363. doi:10.1016/0163-7258(91)90031-G. ISSN 0163-7258. PMID 1820581.
- ^ Böhme, Horst; Severin, Theodor (1957). "Optische Untersuchungen an Cumarinen Mitteilung: Die Ultraviolettabsorption einiger Cumarine pflanzlicher Herkunft". Archiv der Pharmazie. 290 (10): 486–494. doi:10.1002/ardp.19572901010. ISSN 1521-4184. PMID 13471015. S2CID 84020911.
- ^ Lenković, Maja; Cabrijan, Leo; Gruber, Franjo; Saftić, Marina; Stanić Zgombić, Zrinka; Stasić, Adalbert; Peharda, Vesna (October 2008). "Phytophotodermatitis in Rijeka region, Croatia". Collegium Antropologicum. 32 (Suppl 2): 203–205. ISSN 0350-6134. PMID 19138025.
- ^ "Angelica Herb Uses, Health Benefits and Side Effects". The Herbal Resource. Retrieved 2018-03-16.
- ^ "Giant Hogweed: a new contribution to understanding this plant in the UK". Dr M Goes Wild. 2014-01-09. Retrieved 2018-03-16.
- ^ Steck, Warren; Bailey, B. K. (1969). "Leaf coumarins of Angelicaarchangelica". Canadian Journal of Chemistry. 47 (13): 2425–2430. doi:10.1139/v69-396.
- ^ Ashwood-Smith, M.J.; Grant, E.L.; Heddle, J.A.; Friedman, G.B. (1977-06-01). "Chromosome damage in chinese hamster cells sensitized to near-ultraviolet light by psoralen and angelicin". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 43 (3): 377–385. doi:10.1016/0027-5107(77)90059-8. ISSN 0027-5107. PMID 561302.
- ^ a b Bonner, James; Varner, J. E. (2016-07-29). Plant Biochemistry. Elsevier. ISBN 9781483267807.
- ^ a b Jacob, Claus; Kirsch, Gilbert; Slusarenko, Alan; Winyard, Paul G.; Burkholz, Torsten (2014-11-25). Recent Advances in Redox Active Plant and Microbial Products: From Basic Chemistry to Widespread Applications in Medicine and Agriculture. Springer. ISBN 9789401789530.
- ^ a b Arnold, J.W.E. (1976). The Biology of Plant Phenolics. Vol. 4. Biochemical education.
- ^ a b c Larbat, Romain; Hehn, Alain; Hans, Joachim; Schneider, Sarah; Jugdé, Hélène; Schneider, Bernd; Matern, Ulrich; Bourgaud, Frédéric (2009-02-20). "Isolation and functional characterization of CYP71AJ4 encoding for the first P450 monooxygenase of angular furanocoumarin biosynthesis" (PDF). The Journal of Biological Chemistry. 284 (8): 4776–4785. doi:10.1074/jbc.M807351200. ISSN 0021-9258. PMID 19098286. S2CID 33058404.
- ^ Stanjek, Volker; Boland, Wilhelm (1998-09-09). "Biosynthesis of Angular Furanocoumarins: Mechanism and Stereochemistry of the Oxidative Dealkylation of Columbianetin to Angelicin in Heracleum mantegazzianum (Apiaceae)". Helvetica Chimica Acta. 81 (9): 1596–1607. doi:10.1002/(SICI)1522-2675(19980909)81:9<1596::AID-HLCA1596>3.0.CO;2-F.
- ^ a b c d Zou, Yefen; Lobera, Mercedes; Snider, Barry B. (2005-03-04). "Synthesis of 2,3-dihydro-3-hydroxy-2-hydroxylalkylbenzofurans from epoxy aldehydes. One-step syntheses of brosimacutin G, vaginidiol, vaginol, smyrindiol, xanthoarnol, and Avicenol A. Biomimetic syntheses of angelicin and psoralen". The Journal of Organic Chemistry. 70 (5): 1761–1770. doi:10.1021/jo047974k. ISSN 0022-3263. PMID 15730299.
- ^ Shulˈts, E. E.; Ganbaatar, Zh; Petrova, T. N.; Shakirov, M. M.; Bagryanskaya, I. Yu; Taraskin, V. V.; Radnaeva, L. D.; Otgonsuren, D.; Pokrovskii, A. G. (2012-05-01). "Plant coumarins. IX.* Phenolic compounds of Ferulopsis hystrix growing in Mongolia. Cytotoxic activity of 8,9-dihydrofurocoumarins". Chemistry of Natural Compounds. 48 (2): 211–217. doi:10.1007/s10600-012-0207-3. ISSN 0009-3130. S2CID 46726721.
- ^ a b Young, A. R. (June 1990). "Photocarcinogenicity of psoralens used in PUVA treatment: present status in mouse and man". Journal of Photochemistry and Photobiology B: Biology. 6 (1–2): 237–247. doi:10.1016/1011-1344(90)85093-C. ISSN 1011-1344. PMID 2121937.
- ^ a b Matz, Hagit (January 2010). "Phototherapy for psoriasis: what to choose and how to use: facts and controversies". Clinics in Dermatology. 28 (1): 73–80. doi:10.1016/j.clindermatol.2009.04.003. ISSN 1879-1131. PMID 20082955.
- ^ Bordin, F.; Carlassare, F.; Baccichetti, F.; Guiotto, A.; Rodighiero, P.; Vedaldi, D.; Dall‘Acqua, F. (1979-06-01). "4,5'-Dimethylangelicin: A New Dna-Photobinding Monofunctional Agent*". Photochemistry and Photobiology. 29 (6): 1063–1070. doi:10.1111/j.1751-1097.1979.tb07821.x. ISSN 1751-1097. PMID 388472. S2CID 40307307.
- ^ Dall'Acqua, F.; Terbojevich, M.; Marciani, S.; Vedaldi, D.; Recher, M. (1978-04-01). "Investigation on the dark interaction between furocoumarins and DNA". Chemico-Biological Interactions. 21 (1): 103–115. doi:10.1016/0009-2797(78)90071-6. ISSN 0009-2797. PMID 566637.
- ^ Mosti, L.; Lo Presti, E.; Menozzi, G.; Marzano, C.; Baccichetti, F.; Falcone, G.; Filippelli, W.; Piucci, B. (August 1998). "Synthesis of angelicin heteroanalogues: preliminary photobiological and pharmacological studies". Farmaco (Societa Chimica Italiana: 1989). 53 (8–9): 602–610. doi:10.1016/S0014-827X(98)00076-7. hdl:11577/2470046. ISSN 0014-827X. PMID 10081825.
- ^ a b Caffieri, S.; Lucchini, V.; Rodighiero, P.; Miolo, G.; Dall'Acqua, F. (November 1988). "3,4 and 4',5'-photocycloadducts between 4'-methylangelicin and thymine from DNA". Photochemistry and Photobiology. 48 (5): 573–577. doi:10.1111/j.1751-1097.1988.tb02866.x. ISSN 0031-8655. PMID 3241830. S2CID 32844266.
- ^ Dall'Acqua, F.; Marciani, S.; Ciavatta, L.; Rodighiero, G. (1971). "Formation of inter-strand cross-linkings in the photoreactions between furanocoumarins and DNA". Zeitschrift für Naturforschung B. 26 (6): 561–569. doi:10.1515/znb-1971-0613. PMID 4397973.
- ^ Dall'Acqua, F.; Martelli, P. (February 1991). "Photosensitizing action of furocoumarins on membrane components and consequent intracellular events". Journal of Photochemistry and Photobiology B: Biology. 8 (3): 235–254. doi:10.1016/1011-1344(91)80082-S. ISSN 1011-1344. PMID 1904925.
- ^ a b Caffieri, S.; Daga, A.; Vedaldi, D.; Dall'Acqua, F. (1988-12-01). "Photoaddition of angelicin to linolenic acid methyl ester". Journal of Photochemistry and Photobiology B: Biology. 2 (4): 515–521. doi:10.1016/1011-1344(88)85080-2. ISSN 1011-1344. PMID 3150003.
- ^ Veronese, FM; Bevilacqua, R; Schiavon, O; Rodighiero, G (1979). "Drug-protein interaction: plasma protein binding of furocoumarins". Il Farmaco; Edizione Scientifica. 34 (8): 716–25. ISSN 0430-0920. PMID 467637.
- ^ Veronese, F. M.; Schiavon, O.; Bevilacqua, R.; Bordin, F.; Rodighiero, G. (1982-07-01). "Photoinactivation of Enzymes by Linear and Angular Furocoumarins". Photochemistry and Photobiology. 36 (1): 25–30. doi:10.1111/j.1751-1097.1982.tb04335.x. ISSN 1751-1097. PMID 6287507. S2CID 42986954.
- ^ Veronese, F. M.; Schiavon, O.; Bevilacqua, R.; Bordin, F.; Rodighiero, G. (1981-09-01). "The Effect of Psoralens and Angelicins on Proteins in the Presence of Uv-a Irradiation". Photochemistry and Photobiology. 34 (3): 351–354. doi:10.1111/j.1751-1097.1981.tb09369.x. ISSN 1751-1097. PMID 7280051.
- ^ "Material Safety Data Sheet" (PDF). Sigma-Aldrich.
- ^ a b E. Gorgus, C. Lohr, N. Raquet, S. Guth, and D. Schrenk. Limettin and furocoumarins in beverages containing citrus juices or extracts. Food and Chemical Toxicology, 48(1):93–98, 2010.
- ^ B. V. Davidov A. Ya. Potapenko, V. L. Sukhorukov. A comparison between skin-photosensitizing activities of 8-methoxypsoralen and angelicin. Experientia 40, pages 264–265, 1982.
- ^ a b c Christiane Lohr, Nicole Raquet, and Dieter Schrenk. Application of the concept of relative photomutagenic potencies to selected furocoumarins in V79 cells. Toxicology in Vitro, 24(2):558–566, 2010.
- ^ a b Alley, Amanda (August 1987). "Parsnips and furocoumarins". Food and Chemical Toxicology. 25 (8): 634–635. doi:10.1016/0278-6915(87)90033-0.
- ^ a b Baumgart, Annette; Schmidt, Melanie; Schmitz, Hans-Joachim; Schrenk, Dieter (15 February 2005). "Natural furocoumarins as inducers and inhibitors of cytochrome P450 1A1 in rat hepatocytes". Biochemical Pharmacology. 69 (4): 657–667. doi:10.1016/j.bcp.2004.11.017. PMID 15670584.
 KSF
KSF