Anorexia nervosa
 From Wikipedia - Reading time: 62 min
From Wikipedia - Reading time: 62 min
| Anorexia nervosa | |
|---|---|
| Other names | Anorexia, AN |
 | |
| "Miss A—" depicted in 1866 and in 1870 after treatment. Her condition was one of the earliest case studies of anorexia, published in medical research papers of William Gull. | |
| Specialty | Psychiatry, clinical psychology |
| Symptoms | Fear of gaining weight, strong desire to be thin, food restrictions,[1] body image disturbance |
| Complications | Osteoporosis, infertility, heart damage, suicide,[1] whole-body swelling (edema), heart failure and/or lung failure, gastrointestinal problems, extensive muscle weakness, delirium, death[2] |
| Usual onset | Adolescence to early adulthood[1] |
| Causes | Unknown[3] |
| Risk factors | Family history, high-level athletics, bullying, social media, modelling, substance use disorder, being a dancer or gymnast[3][4][5] |
| Differential diagnosis | Body dysmorphic disorder, bulimia nervosa, hyperthyroidism, inflammatory bowel disease, dysphagia, cancer[6][7] |
| Treatment | Cognitive behavioral therapy, hospitalisation to restore weight[1][8] |
| Prognosis | 5% risk of death over 10 years[4][9] |
| Frequency | 2.9 million (2015)[10] |
| Deaths | 600 (2015)[11] |
Anorexia nervosa (AN), often referred to simply as anorexia,[12] is an eating disorder characterized by food restriction, body image disturbance, fear of gaining weight, and an overpowering desire to be thin.[1]
Individuals with anorexia nervosa have a fear of being overweight or being seen as such, despite the fact that they are typically underweight.[1][3] The DSM-5 describes this perceptual symptom as "disturbance in the way in which one's body weight or shape is experienced".[8] In research and clinical settings, this symptom is called "body image disturbance"[13] or body dysmorphia. Individuals with anorexia nervosa also often deny that they have a problem with low weight[4] due to their altered perception of appearance. They may weigh themselves frequently, eat small amounts, and only eat certain foods.[1] Some patients with anorexia nervosa binge eat and purge to influence their weight or shape.[1] Purging can manifest as induced vomiting, excessive exercise, and/or laxative abuse. Medical complications may include osteoporosis, infertility, and heart damage,[1] along with the cessation of menstrual periods.[4] In cases where the patients with anorexia nervosa continually refuse significant dietary intake and weight restoration interventions, a psychiatrist can declare the patient to lack capacity to make decisions. Then, these patients' medical proxies decide that the patient needs to be fed by restraint via nasogastric tube.[14]
Anorexia often develops during adolescence or young adulthood.[1] One psychologist found multiple origins of anorexia nervosa in a typical female patient, but primarily sexual abuse and problematic familial relations, especially those of overprotecting parents showing excessive possessiveness over their children.[15] The exacerbation of the mental illness is thought to follow a major life-change or stress-inducing events.[4] Ultimately however, causes of anorexia are varied and differ from individual to individual.[3] There is emerging evidence that there is a genetic component, with identical twins more often affected than fraternal twins.[3] Cultural factors play a very significant role, with societies that value thinness having higher rates of the disease.[4] Anorexia also commonly occurs in athletes who play sports where a low bodyweight is thought to be advantageous for aesthetics or performance, such as dance, gymnastics, running, and figure skating.[4][5][16]
Treatment of anorexia involves restoring the patient back to a healthy weight, treating their underlying psychological problems, and addressing underlying maladaptive behaviors.[1] A daily low dose of olanzapine has been shown to increase appetite and assist with weight gain in anorexia nervosa patients.[17] Psychiatrists may prescribe their anorexia nervosa patients medications to better manage their anxiety or depression.[1] Different therapy methods may be useful, such as cognitive behavioral therapy or an approach where parents assume responsibility for feeding their child, known as Maudsley family therapy.[1][18] Sometimes people require admission to a hospital to restore weight.[8] Evidence for benefit from nasogastric tube feeding is unclear.[19] Some people with anorexia will have a single episode and recover while others may have recurring episodes over years.[8] The largest risk of relapse occurs within the first year post-discharge from eating disorder therapy treatment. Within the first 2 years post-discharge from eating disorder treatment, approximately 31% of anorexia nervosa patients relapse.[20] Many complications, both physical and psychological, improve or resolve with nutritional rehabilitation and adequate weight gain.[8]
It is estimated to occur in 0.3% to 4.3% of women and 0.2% to 1% of men in Western countries at some point in their life.[21] About 0.4% of young women are affected in a given year and it is estimated to occur ten times more commonly among women than men.[4][21] It is unclear whether the increased incidence of anorexia observed in the 20th and 21st centuries is due to an actual increase in its frequency or simply due to improved diagnostic capabilities.[3] In 2013, it directly resulted in about 600 deaths globally, up from 400 deaths in 1990.[22] Eating disorders also increase a person's risk of death from a wide range of other causes, including suicide.[1][21] About 5% of people with anorexia die from complications over a ten-year period[4][9] with medical complications and suicide being the primary and secondary causes of death respectively.[23]
Signs and symptoms
[edit]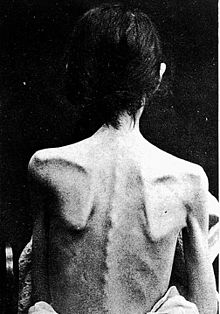
Anorexia nervosa is an eating disorder characterized by attempts to lose weight by way of starvation. A person with anorexia nervosa may exhibit a number of signs and symptoms, the type and severity of which may vary and be present but not readily apparent.[24] Though anorexia is typically recognized by the physical manifestations of the illness, it is a mental disorder that can be present at any weight.
Anorexia nervosa, and the associated malnutrition that results from self-imposed starvation, can cause complications in every major organ system in the body.[25] Hypokalemia, a drop in the level of potassium in the blood, is a sign of anorexia nervosa.[26][27] A significant drop in potassium can cause abnormal heart rhythms, constipation, fatigue, muscle damage, and paralysis.[28]
Signs and symptoms may be classified in various categories including: physical, cognitive, affective, behavioral and perceptual:
Physical symptoms
[edit]- A low body mass index for one's age and height (except in cases of "atypical anorexia")[29]
- Rapid, continuous weight loss[30]
- Dry hair and skin, hair thinning, as well as hair loss[31]
- Feeling cold all the time (hypothermia)[32]
- Raynaud Phenomenon[33]
- Hypotension or orthostatic hypotension
- Bradycardia or tachycardia
- Chronic fatigue[34]
- Insomnia
- Having severe muscle tension, aches and pains
- Irregular or absent menstrual[35] periods
- Infertility
- Gastrointestinal disease[36]
- Halitosis (from vomiting or starvation-induced ketosis)
- Abdominal distension
- Russell's Sign; can be a tell-tale sign of self-induced vomiting with scratches on the back of the hand
- Tooth erosion[37]
- Lanugo: soft, fine hair growing over the face and body[38]
- Orange discoloration of the skin, particularly the feet (Carotenosis)
Cognitive symptoms
[edit]- An obsession with counting calories and monitoring contents of food
- Preoccupation with food, recipes, or cooking; may cook elaborate dinners for others, but not eat the food themselves or consume a very small portion
- Admiration of thinner people
- Thoughts of being fat or not thin enough[39]
- An altered mental representation of one's body
- Impaired theory of mind, exacerbated by lower BMI and depression[40]
- Memory impairment
- Difficulty in abstract thinking and problem solving
- Rigid and inflexible thinking
- Poor self-esteem
- Hypercriticism and perfectionism
Affective symptoms
[edit]- Depression
- Ashamed of oneself or one's body
- Anxiety disorders
- Rapid mood swings
- Emotional dysregulation
- Alexithymia
Behavioral symptoms
[edit]- Compulsive weighing
- Regular body checking
- Food restriction, both in terms of caloric content and type (for example, macronutrient groups)
- Food rituals, such as cutting food into tiny pieces and measuring it, refusing to eat around others, and hiding or discarding of food
- Purging, which may be achieved through self-induced vomiting, laxatives, diet pills, emetics, diuretics, or exercise. The goals of purging are various, including the prevention of weight gain, discomfort with the physical sensation of being full or bloated, and feelings of guilt or impurity.[41]
- Excessive exercise[42] or compulsive movement,[43] such as pacing
- Self harming or self-loathing
- Social withdrawal and solitude, stemming from the avoidance of friends, family, and events where food may be present
- Excessive water consumption to create a false impression of satiety
- Excessive caffeine consumption
Perceptual symptoms
[edit]- Perception that one is not sick (anosognosia) or not sick "enough,"[44] which may prevent some from seeking recovery
- Perception of self as heavier or fatter than in reality, i.e., body image disturbance[13]
- Altered body schema, i.e., a distorted and unconscious perception of one's body size and shape that influences how the individual experiences their body during physical activities. For example, a patient with anorexia nervosa may genuinely fear that they cannot fit through a narrow passageway. However, due to their malnourished state, their body is significantly smaller than someone with a normal BMI who would actually struggle to fit through the same space. In spite of having a small frame, the patient's altered body schema leads them to perceive their body as larger than it is.[citation needed]
Interoception
[edit]Interoception involves the conscious and unconscious sense of the internal state of the body, and it has an important role in homeostasis and regulation of emotions.[45] Aside from noticeable physiological dysfunction, interoceptive deficits also prompt individuals with anorexia to concentrate on distorted perceptions of multiple elements of their body image.[46] This exists in both people with anorexia and in healthy individuals due to impairment in interoceptive sensitivity and interoceptive awareness.[46]
Aside from weight gain and outer appearance, people with anorexia also report abnormal bodily functions such as indistinct feelings of fullness.[47] This provides an example of miscommunication between internal signals of the body and the brain. Due to impaired interoceptive sensitivity, powerful cues of fullness may be detected prematurely in highly sensitive individuals, which can result in decreased calorie consumption and generate anxiety surrounding food intake in anorexia patients.[48] People with anorexia also report difficulty identifying and describing their emotional feelings and the inability to distinguish emotions from bodily sensations in general, called alexithymia.[47]
Interoceptive awareness and emotion are deeply intertwined, and could mutually impact each other in abnormalities.[48] Anorexia patients also exhibit emotional regulation difficulties that ignite emotionally-cued eating behaviors, such as restricting food or excessive exercising.[48] Impaired interoceptive sensitivity and interoceptive awareness can lead anorexia patients to adapt distorted interpretations of weight gain that are cued by physical sensations related to digestion (e.g., fullness).[48] Combined, these interoceptive and emotional elements could together trigger maladaptive and negatively reinforced behavioral responses that assist in the maintenance of anorexia.[48] In addition to metacognition, people with anorexia also have difficulty with social cognition including interpreting others' emotions, and demonstrating empathy.[49] Abnormal interoceptive awareness and interoceptive sensitivity shown through all of these examples have been observed so frequently in anorexia that they have become key characteristics of the illness.[47]
Comorbidity
[edit]Other psychological issues may factor into anorexia nervosa. Some pre-existing disorders can increase a person's likelihood to develop an eating disorder. Additionally, Anorexia Nervosa can contribute to the development of certain conditions.[50] The presence of psychiatric comorbidity has been shown to affect the severity and type of anorexia nervosa symptoms in both adolescents and adults.[51]
Post traumatic stress disorder remains highly prevalent among patients with anorexia nervosa, with more comorbid PTSD being associated with more severe eating disorder symptoms.[52] Obsessive-compulsive disorder (OCD) and obsessive-compulsive personality disorder (OCPD) are highly comorbid with AN.[53][54] OCD is linked with more severe symptomatology and worse prognosis.[55] The causality between personality disorders and eating disorders has yet to be fully established.[56] Other comorbid conditions include depression,[57] alcoholism,[58] substance abuse,[59] borderline and other personality disorders,[60][61] anxiety disorders,[62] attention deficit hyperactivity disorder,[63] and body dysmorphic disorder (BDD).[64] Depression and anxiety are the most common comorbidities,[65] and depression is associated with a worse outcome.[65]
Autism spectrum disorders occur more commonly among people with eating disorders than in the general population,[66] with about 30% of children and adults with AN likely having autism.[67] Zucker et al. (2007) proposed that conditions on the autism spectrum make up the cognitive endophenotype underlying anorexia nervosa and appealed for increased interdisciplinary collaboration.[68]
Causes
[edit]There is evidence for biological, psychological, developmental, and sociocultural risk factors, but the exact cause of eating disorders is unknown.[69]
Genetic
[edit]
Anorexia nervosa is highly heritable.[69] Twin studies have shown a heritability rate of 28–58%.[70] First-degree relatives of those with anorexia have roughly 12 times the risk of developing anorexia.[71] Association studies have been performed, studying 128 different polymorphisms related to 43 genes including genes involved in regulation of eating behavior, motivation and reward mechanics, personality traits and emotion. Consistent associations have been identified for polymorphisms associated with agouti-related peptide, brain derived neurotrophic factor, catechol-o-methyl transferase, SK3 and opioid receptor delta-1.[72] Epigenetic modifications, such as DNA methylation, may contribute to the development or maintenance of anorexia nervosa, though clinical research in this area is in its infancy.[73][74]
A 2019 study found a genetic relationship with mental disorders, such as schizophrenia, obsessive–compulsive disorder, anxiety disorder and depression; and metabolic functioning with a negative correlation with fat mass, type 2 diabetes and leptin.[75]
Environmental
[edit]Obstetric complications: prenatal and perinatal complications may factor into the development of anorexia nervosa, such as preterm birth,[76] maternal anemia, diabetes mellitus, preeclampsia, placental infarction, and neonatal heart abnormalities.[77] Neonatal complications may also have an influence on harm avoidance, one of the personality traits associated with the development of AN.[78]
Neuroendocrine dysregulation: altered signaling of peptides that facilitate communication between the gut, brain and adipose tissue, such as ghrelin, leptin, neuropeptide Y and orexin, may contribute to the pathogenesis of anorexia nervosa by disrupting regulation of hunger and satiety.[79][80]
Gastrointestinal diseases: people with gastrointestinal disorders may be more at risk of developing disorders of eating practices than the general population, principally restrictive eating disturbances.[81] An association of anorexia nervosa with celiac disease has been found.[37] Individuals with good dietary management may develop anxiety, food aversion and eating disorders because of concerns around cross contamination of their foods.[81] Some authors suggest that medical professionals should evaluate the presence of unrecognized celiac disease in all people with an eating disorder, especially if they present any gastrointestinal symptoms, (such as decreased appetite, abdominal pain, bloating, distension, vomiting, diarrhea or constipation), weight loss, or growth failure. With routinely asking celiac patients about weight or body shape concerns, dieting or vomiting for weight control, to evaluate the possible presence of an eating disorders,[37] especially in women.[82]
Anorexia nervosa is more likely to occur in a person's pubertal years. Some explanatory hypotheses for the rising prevalence of eating disorders in adolescence are "increase of adipose tissue in girls, hormonal changes of puberty, societal expectations of increased independence and autonomy that are particularly difficult for anorexic adolescents to meet; [and] increased influence of the peer group and its values."[83]
Anorexia as adaptation
[edit]Studies have hypothesized that disordered eating patterns may also arise secondary to starvation. The results of the Minnesota Starvation Experiment, for example, showed that normal controls will exhibit many of the same behavioral patterns associated with AN when subjected to starvation. Similarly, scientific experiments conducted using mice have suggested that other mammals exhibit these same behaviors, especially compulsive movement, when caloric restriction is induced,[84] likely mediated by various changes in the neuroendocrine system.[85][86][87] This has given further rise to the hypothesis that anorexia nervosa and other restrictive eating disorders may be an evolutionarily advantageous adaptive response to a perceived famine in the environment.[88][89] Recent research has further expanded this perspective, showing how caloric restriction may be adaptive in volatile or uncertain environment - thus potentially explaining the association between an increased risk to develop anorexia nervosa and adverse childhood experiences.[90]
Psychological
[edit]Early theories of the cause of anorexia linked it to childhood sexual abuse or dysfunctional families;[91] evidence is conflicting, and well-designed research is needed.[69] The fear of food is known as sitiophobia[92] or cibophobia,[93] and is part of the differential diagnosis.[94][95] Other psychological causes of anorexia include low self-esteem, feeling like there is lack of control, depression, anxiety, and loneliness.[96] People with anorexia are, in general, highly perfectionistic[97] and most have obsessive compulsive personality traits[98] which may facilitate sticking to a restricted diet.[99] It has been suggested that patients with anorexia are rigid in their thought patterns, and place a high level of importance upon being thin.[100][101]
Although the prevalence rates vary greatly, between 37% and 100%,[102] there appears to be an association between traumatic events and eating disorder diagnosis.[103] Approximately 72% of individuals with anorexia report experiencing a traumatic event prior to the onset of eating disorder symptoms, with binge-purge subtype reporting the highest rates.[102][103] There are many traumatic events that have been identified as possible risk factors for the development of anorexia, the first of which was childhood sexual abuse.[104]
As mentioned previously, the prevalence of PTSD among anorexia nervosa patients ranges from 4% to 24%.[52] A complicated symptom profile develops when trauma and anorexia meld; the bodily experience of the individual is changed and intrusive thoughts and sensations may be experienced.[104] Traumatic events can lead to intrusive and obsessive thoughts, and the symptom of anorexia that has been most closely linked to a PTSD diagnosis is increased obsessive thoughts pertaining to food.[104] Similarly, impulsivity is linked to the purge and binge-purge subtypes of anorexia, trauma, and PTSD.[103] Emotional trauma (e.g., invalidation, chaotic family environment in childhood) may lead to difficulty with emotions, particularly the identification of and how physical sensations contribute to the emotional response.[104]
When trauma is perpetrated on an individual, it can lead to feelings of not being safe within their own body.[104] Both physical and sexual abuse can lead to an individual seeing their body as belonging to an "other" and not to the "self".[104] Individuals who feel as though they have no control over their bodies due to trauma may use food as a means of control because the choice to eat is an unmatched expression of control.[104] By controlling the intake of food, individuals can decide when and how much they eat. Individuals, particularly children experiencing abuse, may feel a loss of control over their life, circumstances, and their own bodies. Particularly sexual abuse, but also physical abuse, can make individuals feel that the body is not a safe place and an object over which another has control. Starvation, in the case of anorexia, may also lead to reduction in the body as a sexual object, making starvation a solution. Restriction may also be a means by which the pain an individual is experiencing can be communicated.[104]
Sociological
[edit]Anorexia nervosa has been increasingly diagnosed since 1950;[105] the increase has been linked to vulnerability and internalization of body ideals.[83] People in professions where there is a particular social pressure to be thin (such as models and dancers) were more likely to develop anorexia,[106] and those with anorexia have much higher contact with cultural sources that promote weight loss.[107] This trend can also be observed for people who partake in certain sports, such as jockeys and wrestlers.[108] There is a higher incidence and prevalence of anorexia nervosa in sports with an emphasis on aesthetics, where low body fat is advantageous, and sports in which one has to make weight for competition.[109] Family group dynamics can play a role in the perpetuation of anorexia including negative expressed emotion in overprotective families where blame is frequently experienced among its members.[110][111][112] In the face of constant pressure to be thin, often perpetuated by teasing and bullying, feelings of low self-esteem and self-worth can arise, including the perception that one is not "deserving" of food.[96]
Media effects
[edit]Persistent exposure to media that present thin ideal may constitute a risk factor for body dissatisfaction and anorexia nervosa. Cultures that equate thinness with beauty often have higher rates of anorexia nervosa.[113] The cultural ideal for body shape for men versus women continues to favor slender women and athletic, V-shaped muscular men. Media sources such as magazines, television shows, and social media can contribute to body dissatisfaction and disordered eating across the globe, by emphasizing Western ideals of slimness.[114] A 2002 review found that, of the magazines most popular among people aged 18 to 24 years, those read by men, unlike those read by women, were more likely to feature ads and articles on shape than on diet.[115] Body dissatisfaction and internalization of body ideals are risk factors for anorexia nervosa that threaten the health of both male and female populations.[116]
Another online aspect contributing to higher rates of eating disorders such as anorexia nervosa are websites and communities on social media that stress the importance of attainment of body ideals extol. These communities promote anorexia nervosa through the use of religious metaphors, lifestyle descriptions, "thinspiration" or "fitspiration" (inspirational photo galleries and quotes that aim to serve as motivators for attainment of body ideals).[117][118] Pro-anorexia websites reinforce internalization of body ideals and the importance of their attainment.[118]
Cultural
[edit]Cultural attitudes towards body image, beauty, and health also significantly impact the incidence of anorexia nervosa. There is a stark contrast between Western societies that idolize slimness and certain Eastern traditions that worship gods depicted with larger bodies,[119] and these varying cultural norms have varying influences on eating behaviors, self-perception, and anorexia in their respective cultures. For example, despite the fact that "fat phobia", or a fear of fat, is a key diagnostic criteria of anorexia by the DSM-5, anorexic patients in Asia rarely display this trait, as deep-rooted cultural values in Asian cultures praise larger bodies.[120] Fat phobia appears to be intricately linked to Western culture, encompassing how various cultural perceptions impact anorexia in various ways. It calls on the need for greater, diverse cultural consideration when looking at the diagnosis and experience of anorexia. For instance, in a cross-sectional study done on British South Asian adolescent English adolescent anorexia patients, it was found that both patients' symptom profiles differed. South Asians were less likely to exhibit fat-phobia as a symptom versus their English counterparts, instead exhibiting loss of appetite. However, both kinds of patients had distorted body images, implying the possibility of disordered eating and highlighting the need for cultural sensitivity when diagnosing anorexia.[121]
Notably, although these cultural distinctions persist, modernization and globalization slowly homogenize these attitudes.[119] Anorexia is increasingly tied to the pressures of a global culture that celebrates Western ideals of thinness. The spread of Western media, fashion, and lifestyle ideals across the globe has begun to shift perceptions and standards of beauty in diverse cultures, contributing to a rise in the incidence of anorexia in places they were once rare in.[122] Anorexia, once primarily associated with Western culture, seems more than ever to be linked to the cultures of modernity and globalization.[citation needed]
Mechanisms
[edit]Evidence from physiological, pharmacological and neuroimaging studies suggest serotonin (also called 5-HT) may play a role in anorexia. While acutely ill, metabolic changes may produce a number of biological findings in people with anorexia that are not necessarily causative of the anorexic behavior. For example, abnormal hormonal responses to challenges with serotonergic agents have been observed during acute illness, but not recovery. Nevertheless, increased cerebrospinal fluid concentrations of 5-hydroxyindoleacetic acid (a metabolite of serotonin), and changes in anorectic behavior in response to acute tryptophan depletion (tryptophan is a metabolic precursor to serotonin) support a role in anorexia. The activity of the 5-HT2A receptors has been reported to be lower in patients with anorexia in a number of cortical regions, evidenced by lower binding potential of this receptor as measured by PET or SPECT, independent of the state of illness. While these findings may be confounded by comorbid psychiatric disorders, taken as a whole they indicate serotonin in anorexia.[123][124] These alterations in serotonin have been linked to traits characteristic of anorexia such as obsessiveness, anxiety, and appetite dysregulation.[87]
Neuroimaging studies investigating the functional connectivity between brain regions have observed a number of alterations in networks related to cognitive control, introspection, and sensory function. Alterations in networks related to the dorsal anterior cingulate cortex may be related to excessive cognitive control of eating related behaviors. Similarly, altered somatosensory integration and introspection may relate to abnormal body image.[125] A review of functional neuroimaging studies reported reduced activations in "bottom up" limbic region and increased activations in "top down" cortical regions which may play a role in restrictive eating.[126]
Compared to controls, people who have recovered from anorexia show reduced activation in the reward system in response to food, and reduced correlation between self reported liking of a sugary drink and activity in the striatum and anterior cingulate cortex. Increased binding potential of 11C radiolabelled raclopride in the striatum, interpreted as reflecting decreased endogenous dopamine due to competitive displacement, has also been observed.[127]
Structural neuroimaging studies have found global reductions in both gray matter and white matter, as well as increased cerebrospinal fluid volumes. Regional decreases in the left hypothalamus, left inferior parietal lobe, right lentiform nucleus and right caudate have also been reported[128] in acutely ill patients. However, these alterations seem to be associated with acute malnutrition and largely reversible with weight restoration, at least in nonchronic cases in younger people.[129] In contrast, some studies have reported increased orbitofrontal cortex volume in currently ill and in recovered patients, although findings are inconsistent. Reduced white matter integrity in the fornix has also been reported.[130]
Diagnosis
[edit]A diagnostic assessment includes the person's current circumstances, biographical history, current symptoms, and family history. The assessment also includes a mental state examination, which is an assessment of the person's current mood and thought content, focusing on views on weight and patterns of eating.
DSM-5
[edit]Anorexia nervosa is classified under the Feeding and Eating Disorders in the latest revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM 5). There is no specific BMI cut-off that defines low weight required for the diagnosis of anorexia nervosa.[131][4]
The diagnostic criteria for anorexia nervosa (all of which needing to be met for diagnosis) are:[8][132]
- Restriction of energy intake relative to requirements leading to a low body weight. (Criterion A)
- Intense fear of gaining weight or persistent behaviors that interfere with gaining weight. (Criterion B)
- Disturbance in the way a person's weight or body shape is experienced or a lack of recognition about the risks of the low body weight. (Criterion C)
Relative to the previous version of the DSM (DSM-IV-TR), the 2013 revision (DSM5) reflects changes in the criteria for anorexia nervosa. Most notably, the amenorrhea (absent period) criterion was removed.[8][133] Amenorrhea was removed for several reasons: it does not apply to males, it is not applicable for females before or after the age of menstruation or taking birth control pills, and some women who meet the other criteria for AN still report some menstrual activity.[8]
Subtypes
[edit]There are two subtypes of AN:[25][134]
- Restrictive Type: In the most recent months leading up to the evaluation, the patient has not engaged in binging and purging via laxative or diuretic abuse, enemas, or self-induced vomiting. The weight loss accomplished in this patient is mainly through the use of one or more of the following methods: fasting, dieting, and excessive exercise.[135]
- Binge-eating / Purging Type: In the last few months, the patient has recurrently engaged in binge-purge cycles.[135]
Levels of severity
[edit]The use of the body mass index in the diagnosis of eating disorders has been controversial, largely owing to its oversimplification of health and failure to take into account complicating factors such as body composition or the initial bodyweight of the patient prior to the onset of AN.[136] As such, the DSM-5 does not have a strict BMI cutoff for the diagnosis of anorexia nervosa,[137] but it nevertheless uses BMI to establish levels of severity, which it states as follows:[138]
- Mild: BMI of greater than 17
- Moderate: BMI of 16–16.99
- Severe: BMI of 15–15.99
- Extreme: BMI of less than 15
Investigations
[edit]Medical tests to check for signs of physical deterioration in anorexia nervosa may be performed by a general physician or psychiatrist.
Physical examination:
- Blinded weight: The patient will strip and put on a surgical gown alone. The patient will step backwards onto the scale as the healthcare provider blocks the reading from the patient's line of vision.[citation needed]
- Orthostatic vitals: The patient lies completely flat for five minutes, and then, the medical provider measures the patient's blood pressure and heart rate. The patient stands up and stays stationary for two minutes. Then, the blood pressure and heart rate are assessed again, making note of any patient symptoms upon standing like dizziness. According to the College of Family Physicians of Canada, a change in orthostatic heart rate greater than 20 beats/minute or a change in orthostatic blood pressure greater than 10mmHg can warrant admission for an adolescent.[139]
- Examination of hands and arms for brittle nails, Russell's sign, swollen joints, lanugo, and self harm.[140]
- Auscultation of the chest for rubs, gallops, thrills, murmurs, and apex beat.[140]
- Examination of the face for puffiness, dental decay, swollen parotid glands, and conjunctival hemorrhage.[140]
Blood tests:
- Complete blood count (CBC): a test of the white blood cells, red blood cells and platelets used to assess the presence of various disorders such as leukocytosis, leukopenia, thrombocytosis and anemia which may result from malnutrition.[141]
- Chem-20: Chem-20, also known as SMA-20, is a group of twenty separate chemical tests performed on blood serum. Tests include protein and electrolytes such as potassium, chlorine and sodium, and tests specific to liver and kidney function.[142]
- Glucose tolerance test: Oral glucose tolerance test (OGTT) used to assess the body's ability to metabolize glucose. Can be useful in detecting various disorders such as diabetes, an insulinoma, Cushing's Syndrome, hypoglycemia and polycystic ovary syndrome.[143]
- Lipid profile: includes cholesterol (including total cholesterol, HDL and LDL) and triglycerides.[144]
- Serum cholinesterase test: a test of liver enzymes (acetylcholinesterase and pseudocholinesterase) useful as a test of liver function and to assess the effects of malnutrition.[145]
- Liver function tests: A series of tests used to assess liver function some of the tests are also used in the assessment of malnutrition, protein deficiency, kidney function, bleeding disorders, and Crohn's Disease.[146]
- Luteinizing hormone (LH) response to gonadotropin-releasing hormone (GnRH): Tests the pituitary glands' response to GnRh, a hormone produced in the hypothalamus. Hypogonadism is often seen in anorexia nervosa cases.[26]
- Creatine kinase (CK) test: measures the circulating blood levels of creatine kinase an enzyme found in the heart (CK-MB), brain (CK-BB) and skeletal muscle (CK-MM).[147]
- Blood urea nitrogen (BUN) test: urea nitrogen is the byproduct of protein metabolism first formed in the liver then removed from the body by the kidneys. The BUN test is primarily used to test kidney function. A low BUN level may indicate the effects of malnutrition.[148]
- BUN-to-creatinine ratio: A BUN to creatinine ratio is used to predict various conditions. A high BUN/creatinine ratio can occur in severe hydration, acute kidney failure, congestive heart failure, and intestinal bleeding. A low BUN/creatinine ratio can indicate a low protein diet, celiac disease, rhabdomyolysis, or cirrhosis of the liver.[149][150]
- Thyroid function tests: tests used to assess thyroid functioning by checking levels of thyroid-stimulating hormone (TSH), thyroxine (T4), and triiodothyronine (T3).[151]
Additional medical screenings:
- Urinalysis: a variety of tests performed on the urine used in the diagnosis of medical disorders, to test for substance abuse, and as an indicator of overall health[152]
- Electrocardiogram (EKG or ECG): measures electrical activity of the heart. It can be used to detect various disorders such as hyperkalemia.[153]
- Electroencephalogram (EEG): measures the electrical activity of the brain. It can be used to detect abnormalities such as those associated with pituitary tumors.[154]
Differential diagnoses
[edit]A variety of medical and psychological conditions have been misdiagnosed as anorexia nervosa; in some cases the correct diagnosis was not made for more than ten years.[citation needed]
The distinction between binge purging anorexia, bulimia nervosa and Other Specified Feeding or Eating Disorders (OSFED) is often difficult for non-specialist clinicians. A main factor differentiating binge-purge anorexia from bulimia is the gap in physical weight. Patients with bulimia nervosa are ordinarily at a healthy weight, or slightly overweight. Patients with binge-purge anorexia are commonly underweight.[155] Moreover, patients with the binge-purging subtype may be significantly underweight and typically do not binge-eat large amounts of food.[155] In contrast, those with bulimia nervosa tend to binge large amounts of food.[155] It is not unusual for patients with an eating disorder to "move through" various diagnoses as their behavior and beliefs change over time.[68]
Treatment
[edit]Treatment for people with anorexia nervosa should be individualized and tailored to each person's medical, psychological, and nutritional circumstances. Treating this condition with an interdisciplinary team is suggested so that the different health care professional specialties can help addresses the different challenges that can be associated with recovery.[156] Treatment for anorexia typically involves a combination of medical, psychological interventions such as therapy, and nutritional interventions (diet) interventions. Hospitalization may also be needed in some cases,[157] and the person requires a comprehensive medical assessment to help direct the treatment options. There is no conclusive evidence that any particular treatment approach for anorexia nervosa works better than others.[12][158] In some clinical settings a specific body image intervention is performed to reduce body dissatisfaction and body image disturbance. Although restoring the person's weight is the primary task at hand, optimal treatment also includes and monitors behavioral change in the individual as well.[19]
In general, treatment for anorexia nervosa aims to address three main areas:[159]
- Restoring the person to a healthy weight;
- Treating the psychological disorders related to the illness;
- Reducing or eliminating behaviors or thoughts that originally led to the disordered eating.[159]
Psychological support
[edit]Psychological support, often in the form of cognitive-behavioral therapy (CBT), family-bases treatment, or psychotherapy aims to change distorted thoughts and behaviors around food, body image, and self-worth, with family-based therapy also being a key approach for younger patients.
Family-based therapy
[edit]Family-based treatment (FBT) may be more successful than individual therapy for adolescents with AN.[9][160] Various forms of family-based treatment have been proven to work in the treatment of adolescent AN including conjoint family therapy (CFT), in which the parents and child are seen together by the same therapist, and separated family therapy (SFT) in which the parents and child attend therapy separately with different therapists.[9] Proponents of family therapy for adolescents with AN assert that it is important to include parents in the adolescent's treatment.[9] The evidence supporting family based therapy for adults is weak and despite the evidence that it is effective and the primary choice for treatment in adolescents, there is no evidence it is helpful for adults.[12][161][162] A four- to five-year follow up study of the Maudsley family therapy, an evidence-based manualized model, showed full recovery at rates up to 90%.[163] The Maudsley model of family therapy is problem focused, and the treatment targets re-establishing regular eating, weight restoration, and the reduction of illness behaviors like purging.[164] The Maudsley model is split into three phases, with phase one focusing on the parents implementing weight restoration in the child; phase two transitioning control over food back to the individual at an age-appropriate level; and phase three focusing on other issues related to typical adolescent development (e.g., social and other psychological developments), and helps parents learn how to interact with their child.[164] Although this model is recommended by the National Institute of Mental Health (NIMH),[165] critics claim that it has the potential to create power struggles in an intimate relationship and may disrupt equal partnerships.[163]
Cognitive behavioral therapy
[edit]Cognitive behavioral therapy (CBT) is useful in adolescents and adults with anorexia nervosa.[166] One of the most known psychotherapy in the field is CBT-E, an enhanced cognitive-behavior therapy specifically focus to eating disorder psychopathology. Acceptance and commitment therapy is a third-wave cognitive-behavioral therapy which has shown promise in the treatment of AN.[167] Cognitive remediation therapy (CRT) is also used in treating anorexia nervosa.[168] Schema-Focused Therapy (a form of CBT) was developed by Dr. Jeffrey Young and is effective in helping patients identify origins and triggers for disordered eating.[169]
Psychotherapy
[edit]Psychotherapy for individuals with AN is challenging as they may value being thin and may seek to maintain control and resist change.[170] Initially, developing a desire to change is fundamental.[171] There is no strong evidence to suggest one type of psychotherapy over another for treating anorexia nervosa in adults or adolescents.[156]
Diet
[edit]Diet is the most essential factor to work on in people with anorexia nervosa, and must be tailored to each person's needs. Food variety is important when establishing meal plans as well as foods that are higher in energy density, especially in carbohydrates and dietary fat, which are easier for the undernourished body to break down.[172] Evidence of a role for zinc supplementation during refeeding is unclear.[19] Dieticians work with the medical team to add dietary supplements like iron, every other day, or calcium.
Historically, practitioners have slowly increased calories at a measured pace from a starting point of around 1,200 kcal/day.[42][173] However, as understanding of the process of weight restoration has improved, an approach that favors a higher starting point and a more rapid rate of increase has become increasingly common. In either approach, the end goal is typically in the range of 3,000 to 3,500 kcal/day.[173]
Extreme hunger
[edit]People who have undergone significant caloric deficits often report experiencing hyperphagia, or extreme hunger. With adequate refeeding and the full restoration of both fat mass and fat-free mass, hunger eventually becomes normalized. However, the restoration of fat-free mass typically takes longer than that of body fat, leading to "fat overshoot" or "overshoot weight," wherein the patient's body fat levels are greater than pre-starvation levels.[174] The timeline of the complete normalization of hunger varies considerably from individual to individual, from a few months to multiple years.[175]
Refeeding syndrome
[edit]Treatment professionals tend to be conservative with refeeding in anorexic patients due to the risk of refeeding syndrome (RFS), which occurs when a malnourished person is refed too quickly for their body to be able to adapt. Two of the most common indicators that RFS is occurring are low phosophate levels and low potassium levels.[176] RFS is most likely to happen in severely or extremely underweight anorexics, as well as when medical comorbidities, such as infection or cardiac failure, are present. In these circumstances, it is recommended to start refeeding more slowly but to build up rapidly as long as RFS does not occur. Recommendations on energy requirements in the most medically compromised patients vary, from 5–10 kcal/kg/day to 1900 kcal/day.[177][178] This risk-averse approach can lead to underfeeding, which results in poorer outcomes for short- and long-term recovery.[173]
Medication
[edit]Pharmaceuticals have limited benefit for anorexia itself.[179][131] There is a lack of good information from which to make recommendations concerning the effectiveness of antidepressants in treating anorexia.[180] Administration of olanzapine has been shown to result in a modest but statistically significant increase in body weight of anorexia nervosa patients.[181]
Admission to hospital
[edit]Patients with AN may be deemed to have a lack of insight regarding the necessity of treatment, and thus may be involuntarily treated without their consent.[157]: 1038 AN has a high mortality and patients admitted in a severely ill state to medical units are at particularly high risk.[182] Diagnosis can be challenging, risk assessment may not be performed accurately, consent and the need for compulsion may not be assessed appropriately, refeeding syndrome may be missed or poorly treated and the behavioural and family problems in AN may be missed or poorly managed.[183] Guidelines published by the Royal College of Psychiatrists recommend that medical and psychiatric experts work together in managing severely ill people with AN.[184]
Experience of treatment
[edit]Patients involved in treatment sometimes felt that treatment focused on biological aspects of body weight and eating behaviour change rather than their perceptions or emotional state.[185]: 8 Patients felt that a therapists trust in them shown by being treated as a complete person with their own capacities was significant.[185]: 9 Some patients defined recovery from AN in terms of reclaiming a lost identity.[185]: 10 Additionally, access to timely treatment can be hindered by systemic challenges within the medical system. Some individuals have reported experiencing delays in treatment, particularly when transitioning from adolescence to adulthood.[186]
Healthcare workers involved in the treatment of anorexia reported frustration and anger to setbacks in treatment and noncompliance and were afraid of patients dying. Some healthcare workers felt that they did not understand the treatment and that medical doctors were making decisions.[187]: 11 They may feel powerless to improve a patient's situation and deskilled as a result.[187]: 12 Healthcare workers involved in monitoring patients consumption of food felt watched themselves.[187]: 12 Healthcare workers often feel a degree of moral dissonance of not being in control of outcomes which they may protect against by focusing on individual tasks, avoiding identifying with patients (for example by making their eating behavior very different and not sharing personal information with patients), and blaming patients for their distress.[187]: 13,14 Healthcare workers would inflexibly follow process to avoid responsibility.[187]: 13 Healthcare workers attempted to reach balance by gradually giving patients back control avoiding feeling sole responsibility for outcomes, being mindful of their emotional state, and trying to view eating disorders as external from patients.[187]: 13
Prognosis
[edit]
AN has the highest mortality rate of any psychological disorder.[9] The mortality rate is 11 to 12 times greater than in the general population, and the suicide risk is 56 times higher.[26] Half of women with AN achieve a full recovery, while an additional 20–30% may partially recover.[9][26] Not all people with anorexia recover completely: about 20% develop anorexia nervosa as a chronic disorder.[158] If anorexia nervosa is not treated, serious complications such as heart conditions[24] and kidney failure can arise and eventually lead to death.[188] The average number of years from onset to remission of AN is seven for women and three for men. After ten to fifteen years, 70% of people no longer meet the diagnostic criteria, but many still continue to have eating-related problems.[189] People who have autism recover more slowly, probably due to autism's effects on thinking patterns, such as reduced cognitive flexibility.[190]
Alexithymia (inability to identify and describe one's own emotions) influences treatment outcome.[179] Recovery is also viewed on a spectrum rather than black and white. According to the Morgan-Russell criteria, individuals can have a good, intermediate, or poor outcome. Even when a person is classified as having a "good" outcome, weight only has to be within 15% of average, and normal menstruation must be present in females. The good outcome also excludes psychological health. Recovery for people with anorexia nervosa is undeniably positive, but recovery does not mean a return to normal.[191]
Complications
[edit]Anorexia nervosa can have serious implications if its duration and severity are significant and if onset occurs before the completion of growth, pubertal maturation, or the attainment of peak bone mass.[192][medical citation needed] Complications specific to adolescents and children with anorexia nervosa can include growth retardation, as height gain may slow and can stop completely with severe weight loss or chronic malnutrition. In such cases, provided that growth potential is preserved, height increase can resume and reach full potential after normal intake is resumed.[193] Height potential is normally preserved if the duration and severity of illness are not significant or if the illness is accompanied by delayed bone age (especially prior to a bone age of approximately 15 years), as hypogonadism may partially counteract the effects of undernutrition on height by allowing for a longer duration of growth compared to controls.[medical citation needed] Appropriate early treatment can preserve height potential, and may even help to increase it in some post-anorexic subjects, due to factors such as long-term reduced estrogen-producing adipose tissue levels compared to premorbid levels.[medical citation needed] In some cases, especially where onset is before puberty, complications such as stunted growth and pubertal delay are usually reversible.[194]
Anorexia nervosa causes alterations in the female reproductive system; significant weight loss, as well as psychological stress and intense exercise, typically results in a cessation of menstruation in women who are past puberty. In patients with anorexia nervosa, there is a reduction of the secretion of gonadotropin releasing hormone in the central nervous system which prevents ovulation.[195] Anorexia nervosa can also result in pubertal delay or arrest. Both height gain and pubertal development are dependent on the release of growth hormone and gonadotropins (LH and FSH) from the pituitary gland. Suppression of gonadotropins in people with anorexia nervosa has been documented.[196] Typically, growth hormone (GH) levels are high, but levels of IGF-1, the downstream hormone that should be released in response to GH are low; this indicates a state of "resistance" to GH due to chronic starvation.[197] IGF-1 is necessary for bone formation, and decreased levels in anorexia nervosa contribute to a loss of bone density and potentially contribute to osteopenia or osteoporosis.[197] Anorexia nervosa can also result in reduction of peak bone mass. Buildup of bone is greatest during adolescence, and if onset of anorexia nervosa occurs during this time and stalls puberty, low bone mass may be permanent.[198]
Hepatic steatosis, or fatty infiltration of the liver, can also occur, and is an indicator of malnutrition in children.[199] Neurological disorders that may occur as complications include seizures and tremors. Wernicke encephalopathy, which results from vitamin B1 deficiency, has been reported in patients who are extremely malnourished; symptoms include confusion, problems with the muscles responsible for eye movements and abnormalities in walking gait.[citation needed]
The most common gastrointestinal complications of anorexia nervosa are delayed stomach emptying and constipation, but also include elevated liver function tests, diarrhea, acute pancreatitis, gastroesophageal reflux disease (GERD), difficulty swallowing, and, rarely, superior mesenteric artery syndrome.[200] Acid exposure from GERD and self-induced vomiting can cause dental problems, such as tooth enamel erosion and gum disease.[201][202] Delayed stomach emptying, or gastroparesis, often develops following food restriction and weight loss; the most common symptom is bloating with gas and abdominal distension, and often occurs after eating. Other symptoms of gastroparesis include early satiety, fullness, nausea, and vomiting. The symptoms may inhibit efforts at eating and recovery, but can be managed by limiting high-fiber foods, using liquid nutritional supplements, or using metoclopramide to increase emptying of food from the stomach.[200] Gastroparesis generally resolves when weight is regained.[citation needed]
Cardiac complications
[edit]Anorexia nervosa increases the risk of sudden cardiac death, though the precise cause is unknown. Cardiac complications include structural and functional changes to the heart.[203] Some of these cardiovascular changes are mild and are reversible with treatment, while others may be life-threatening. Cardiac complications can include arrhythmias, abnormally slow heart beat, low blood pressure, decreased size of the heart muscle, reduced heart volume, mitral valve prolapse, myocardial fibrosis, and pericardial effusion.[203]
Abnormalities in conduction and repolarization of the heart that can result from anorexia nervosa include QT prolongation, increased QT dispersion, conduction delays, and junctional escape rhythms.[203] Electrolyte abnormalities, particularly hypokalemia and hypomagnesemia, can cause anomalies in the electrical activity of the heart, and result in life-threatening arrhythmias. Hypokalemia most commonly results in patients with anorexia when restricting is accompanied by purging (induced vomiting or laxative use). Hypotension (low blood pressure) is common, and symptoms include fatigue and weakness. Orthostatic hypotension, a marked decrease in blood pressure when standing from a supine position, may also occur. Symptoms include lightheadedness upon standing, weakness, and cognitive impairment, and may result in fainting or near-fainting.[203] Orthostasis in anorexia nervosa indicates worsening cardiac function and may indicate a need for hospitalization.[203] Hypotension and orthostasis generally resolve upon recovery to a normal weight. The weight loss in anorexia nervosa also causes atrophy of cardiac muscle. This leads to decreased ability to pump blood, a reduction in the ability to sustain exercise, a diminished ability to increase blood pressure in response to exercise, and a subjective feeling of fatigue.[204]
Some individuals may also have a decrease in cardiac contractility. Cardiac complications can be life-threatening, but the heart muscle generally improves with weight gain, and the heart normalizes in size over weeks to months, with recovery.[204] Atrophy of the heart muscle is a marker of the severity of the disease, and while it is reversible with treatment and refeeding, it is possible that it may cause permanent, microscopic changes to the heart muscle that increase the risk of sudden cardiac death.[203] Individuals with anorexia nervosa may experience chest pain or palpitations; these can be a result of mitral valve prolapse. Mitral valve prolapse occurs because the size of the heart muscle decreases while the tissue of the mitral valve remains the same size. Studies have shown rates of mitral valve prolapse of around 20 percent in those with anorexia nervosa, while the rate in the general population is estimated at 2–4 percent.[205] It has been suggested that there is an association between mitral valve prolapse and sudden cardiac death, but it has not been proven to be causative, either in patients with anorexia nervosa or in the general population.[203]
Relapse
[edit]Rates of relapse after treatment range 30–72% over a period of 2–26 months, with a rate of approximately 50% in 12 months after weight restoration.[206] Relapse occurs in approximately a third of people in hospital, and is greatest in the first six to eighteen months after release from an institution.[207] BMI or measures of body fat and leptin levels at discharge were the strongest predictors of relapse, as well as signs of eating psychopathology at discharge.[206] Duration of illness, age, severity, the proportion of AN binge-purge subtype, and presence of comorbidities are also contributing factors.[citation needed]
Epidemiology
[edit]This section needs expansion with: possible reasons for the higher prevalence in women. You can help by adding to it. (December 2024) |
Anorexia is estimated to occur in 0.9% to 4.3% of women and 0.2% to 0.3% of men in Western countries at some point in their life.[21] About 0.4% of young females are affected in a given year and it is estimated to occur three to ten times less commonly in males.[4][21][207][208] The cause of this disparity is not well-established but is thought to be linked to both biological and socio-cultural factors.[209] Rates in most of the developing world are unclear.[4] Often it begins during the teen years or young adulthood.[1] Medical students are a high risk group, with an overall estimated prevalence of 10.4% globally.[210]
The lifetime rate of atypical anorexia nervosa, a form of ED-NOS in which the person loses a significant amount of weight and is at risk for serious medical complications despite having a higher body-mass index, is much higher, at 5–12%.[211] Additionally, a UCSF study showed severity of illness is independent of current BMI, and "patients with large, rapid, or long-duration of weight loss were more severely ill regardless of their current weight."[212]
While anorexia became more commonly diagnosed during the 20th century it is unclear if this was due to an increase in its frequency or simply better diagnosis.[3] Most studies show that since at least 1970 the incidence of AN in adult women is fairly constant, while there is some indication that the incidence may have been increasing for girls aged between 14 and 20.[21]
Underrepresentation
[edit]In non-Westernized countries, including those in Africa (excluding South Africa), eating disorders are less frequently reported and studied compared to Western countries,[213] with available data mostly limited to case reports and isolated studies rather than prevalence investigations. Theories to explain these lower rates of eating disorders, lower reporting, and lower research rates in these countries include the attention to effects of westernisation and culture change on the prevalence of anorexia.[214][clarification needed]
Athletes are often overlooked as anorexic.[215] Research emphasizes the importance to take athletes' diet, weight and symptoms into account when diagnosing anorexia, instead of just looking at weight and BMI. For athletes, ritualized activities such as weigh-ins place emphasis on gaining and losing large amounts of weight, which may promote the development of eating disorders among them.[216]
Males
[edit]While anorexia nervosa is more commonly found in women, it can also affect men, with a lifetime prevalence of 0.3% in men.[217] However, a lack of awareness of eating disorders in males may lead to underdiagnosis and underreporting. This can include a lack of knowledge about what kinds of behaviors males with eating disorders might display, as they differ slightly from those found in females, with a 2009 survey showing that females are more inclined to report fasting, body checking, and body avoidance, whereas males are more prone to report overeating.[218] An additional difference is in the use of supplements to affect bodyweight, with women being more prone to using diet pills and men being more prone to using anabolic steroids.[69]
Moreover, men who exhibit symptoms of anorexia may not meet the BMI criteria outlined in the DSM-IV due to having more muscle mass and therefore a higher bodyweight.[215] Consequently, a subclinical diagnosis, such as Eating Disorder Not Otherwise Specified (ED-NOS) in the DSM-IV or Other Specified Feeding or Eating Disorder (OSFED) in the DSM-5, is often made instead.[219]
Men with anorexia may also experience body dysmorphia, reporting their bodies to be twice as large than in actuality, and body dissatisfaction, especially with regard to muscularity and body composition. As in the case of women, men are more prone to develop an eating disorder if their occupation or sport emphasizes having a slim physique or lighter weight, like modeling, dancing, horse racing, wrestling, and gymnastics. Hormonal changes may also be observed in males with anorexia nervosa, with marked changes in their serum testosterone, luteinizing hormone, and follicle stimulating hormone. Such extreme endocrine disturbances can potentially result in infertility.[220]
Anorexic men are sometimes colloquially referred to as manorexic[221] or as having bigorexia.
Elderly
[edit]An increasing trend of anorexia among the elderly, termed "Anorexia of Aging,"[222] is observed, characterized by behaviors similar to those seen in typical anorexia nervosa but often accompanied by excessive laxative use.[222] Most geriatric anorexia patients limit their food intake to dairy or grains, whereas an adolescent anorexic has a more general limitation.[222]
This eating disorder that affects older adults has two types – early onset and late onset.[222] Early onset refers to a recurrence of anorexia in late life in an individual who experienced the disease during their youth.[222] Late onset describes instances where the eating disorder begins for the first time late in life.[222]
The stimulus for anorexia in elderly patients is typically a loss of control over their lives, which can be brought on by many events, including moving into an assisted living facility.[223] This is also a time when most older individuals experience a rise in conflict with family members, such as limitations on driving or limitations on personal freedom, which increases the likelihood of an issue with anorexia.[223] There can be physical issues in the elderly that leads to anorexia of aging, including a decline in chewing ability, a decline in taste and smell, and a decrease in appetite.[224] Psychological reasons for the elderly to develop anorexia can include depression and bereavement, and even an indirect attempt at suicide.[224] There are also common comorbid psychiatric conditions with aging anorexics, including major depression, anxiety disorder, obsessive compulsive disorder, bipolar disorder, schizophrenia, and dementia.[225]
The signs and symptoms that go along with anorexia of aging are similar to what is observed in adolescent anorexia, including sudden weight loss, unexplained hair loss or dental problems, and a desire to eat alone.[223]
There are also several medical conditions that can result from anorexia in the elderly. An increased risk of illness and death can be a result of anorexia.[224] There is also a decline in muscle and bone mass as a result of a reduction in protein intake during anorexia.[224] Another result of anorexia in the aging population is irreparable damage to kidneys, heart or colon and an imbalance of electrolytes.[226]
Many assessments are available to diagnose anorexia in the aging community. These assessments include the Simplified Nutritional Assessment Questionnaire (SNAQ)[227] and Functional Assessment of Anorexia/Cachexia Therapy (FAACT).[228][222] Specific to the geriatric populace, the interRAI system[229] identifies detrimental conditions in assisted living facilities and nursing homes.[222] Even a simple screening for nutritional insufficiencies such as low levels of important vitamins, can help to identify someone who has anorexia of aging.[222]
Anorexia in the elderly should be identified by the retirement communities but is often overlooked,[223] especially in patients with dementia.[226] Some studies report that malnutrition is prevalent in nursing homes, with up to 58% of residents suffering from it, which can lead to the difficulty of identifying anorexia.[226] One of the challenges with assisted living facilities is that they often serve bland, monotonous food, which lessens residents' desire to eat.[226]
The treatment for anorexia of aging is undifferentiated as anorexia for any other age group. Some of the treatment options include outpatient and inpatient facilities, antidepressant medication and behavioral therapy such as meal observation and discussing eating habits.[225]
History
[edit]
The history of anorexia nervosa begins with descriptions of religious fasting dating from the Hellenistic era[230] and continuing into the medieval period. The medieval practice of self-starvation by women, including some young women, in the name of religious piety and purity also concerns anorexia nervosa; it is sometimes referred to as anorexia mirabilis.[231][232] The earliest medical descriptions of anorexic illnesses are generally credited to English physician Richard Morton in 1689.[230]
Etymologically, anorexia is a term of Greek origin: an- (ἀν-, prefix denoting negation) and orexis (ὄρεξις, "appetite"), translating literally to "a loss of appetite". In and of itself, this term does not have a harmful connotation, e.g., exercise-induced anorexia simply means that hunger is naturally suppressed during and after sufficiently intense exercise sessions.[233] It is the adjective nervosa that indicates the functional and non-organic nature of the disorder, but this adjective is also often omitted when the context is clear. Despite the literal translation of anorexia, the feeling of hunger in anorexia nervosa is frequently present and the pathological control of this instinct is a source of satisfaction for the patients.[234]
The term "anorexia nervosa" was coined in 1873 by Sir William Gull, one of Queen Victoria's personal physicians.[235] Gull published a seminal paper providing a number of detailed case descriptions of patients with anorexia nervosa.[236] In the same year, French physician Ernest-Charles Lasègue similarly published details of a number of cases in a paper entitled De l'Anorexie hystérique.[237]
In the late 19th century anorexia nervosa became widely accepted by the medical profession as a recognized condition. Awareness of the condition was largely limited to the medical profession until the latter part of the 20th century, when German-American psychoanalyst Hilde Bruch published The Golden Cage: the Enigma of Anorexia Nervosa in 1978. Despite major advances in neuroscience,[238] Bruch's theories tend to dominate popular thinking. A further important event was the death of the popular singer and drummer Karen Carpenter in 1983, which prompted widespread ongoing media coverage of eating disorders.[239]
See also
[edit]- Body image
- Eating recovery
- Evolutionary psychiatry
- Idée fixe
- Inedia
- List of people with anorexia nervosa
- National Association of Anorexia Nervosa and Associated Disorders
- Muscle dysmorphia
- Orthorexia nervosa
- Pro-ana
References
[edit]- ^ a b c d e f g h i j k l m n o "What are Eating Disorders?". NIMH. Archived from the original on 23 May 2015. Retrieved 24 May 2015.
- ^ "Anorexia Nervosa". My.clevelandclinic.org. Retrieved 9 June 2022.
- ^ a b c d e f g Attia E (2010). "Anorexia nervosa: current status and future directions". Annual Review of Medicine. 61 (1): 425–435. doi:10.1146/annurev.med.050208.200745. PMID 19719398.
- ^ a b c d e f g h i j k l Diagnostic and statistical manual of mental disorders : DSM-5 (5th ed.). Washington: American Psychiatric Publishing. 2013. pp. 338–345. ISBN 978-0-89042-555-8.
- ^ a b Arcelus J, Witcomb GL, Mitchell A (March 2014). "Prevalence of eating disorders amongst dancers: a systemic review and meta-analysis". European Eating Disorders Review. 22 (2): 92–101. doi:10.1002/erv.2271. PMID 24277724.
- ^ Parker R, Sharma A (2008). General Medicine. Elsevier Health Sciences. p. 56. ISBN 978-0-7234-3461-0.
- ^ First MB (19 November 2013). DSM-5 Handbook of Differential Diagnosis. American Psychiatric Pub. ISBN 978-1-58562-462-1 – via Google Books.
- ^ a b c d e f g h "Feeding and eating disorders" (PDF). American Psychiatric Publishing. 2013. Archived from the original (PDF) on 1 May 2015. Retrieved 9 April 2015.
- ^ a b c d e f g Espie J, Eisler I (2015). "Focus on anorexia nervosa: modern psychological treatment and guidelines for the adolescent patient". Adolescent Health, Medicine and Therapeutics. 6: 9–16. doi:10.2147/AHMT.S70300. PMC 4316908. PMID 25678834.
- ^ Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators) (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. ISSN 0140-6736. PMC 5055577. PMID 27733282.
- ^ Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. (GBD 2015 Mortality and Causes of Death Collaborators) (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ^ a b c Treasure J, Zipfel S, Micali N, Wade T, Stice E, Claudino A, et al. (November 2015). "Anorexia nervosa". Nature Reviews. Disease Primers. 1: 15074. doi:10.1038/nrdp.2015.74. PMID 27189821. S2CID 21580134.
- ^ a b Artoni P, Chierici ML, Arnone F, Cigarini C, De Bernardis E, Galeazzi GM, et al. (March 2021). "Body perception treatment, a possible way to treat body image disturbance in eating disorders: a case-control efficacy study". Eating and Weight Disorders. 26 (2): 499–514. doi:10.1007/s40519-020-00875-x. PMID 32124409. S2CID 211728899.
- ^ "Force-Feeding of Anorexic Patients and the Right to Die" (PDF). Archived from the original (PDF) on 23 November 2020. Retrieved 2 October 2020.
- ^ Matt Lacoste S (1 September 2017). "Looking for the origins of anorexia nervosa in adolescence - A new treatment approach". Aggression and Violent Behavior. 36: 76–80. doi:10.1016/j.avb.2017.07.006. ISSN 1359-1789.
- ^ Sudi K, Öttl K, Payerl D, Baumgartl P, Tauschmann K, Müller W (2004). "Anorexia athletica". Nutrition. 20 (7–8): 657–661. doi:10.1016/j.nut.2004.04.019. PMID 15212748.
- ^ Walsh T (2020). Eating Disorders: What Everyone Needs to Know. Oxford University Press. pp. 105–113. ISBN 978-0190926595.
- ^ Hay P (July 2013). "A systematic review of evidence for psychological treatments in eating disorders: 2005–2012". The International Journal of Eating Disorders. 46 (5): 462–469. doi:10.1002/eat.22103. PMID 23658093.
- ^ a b c "Eating Disorders: Core Interventions in the Treatment and Management of Anorexia Nervosa, Bulimia Nervosa and Related Eating Disorders". 2004. p. 103. PMID 23346610.
- ^ Berends T (2018). "Relapse in anorexia nervosa: a systematic review and meta-analysis". Current Opinion in Psychiatry. 31 (6): 445–455. doi:10.1097/YCO.0000000000000453. hdl:1874/389359. PMID 30113325.
- ^ a b c d e f Smink FR, van Hoeken D, Hoek HW (August 2012). "Epidemiology of eating disorders: incidence, prevalence and mortality rates". Current Psychiatry Reports. 14 (4): 406–414. doi:10.1007/s11920-012-0282-y. PMC 3409365. PMID 22644309.
- ^ Murray CJ, Barber RM, Foreman KJ, Ozgoren AA, Abd-Allah F, Abera SF, et al. (GBD 2013 Mortality and Causes of Death Collaborators) (January 2015). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013". Lancet. 385 (9963): 117–171. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442.
- ^ Smith AR, Zuromski KL, Dodd DR (1 August 2018). "Eating disorders and suicidality: what we know, what we don't know, and suggestions for future research". Current Opinion in Psychology. Suicide. 22: 63–67. doi:10.1016/j.copsyc.2017.08.023. ISSN 2352-250X. PMID 28846874.
- ^ a b Surgenor LJ, Maguire S (2013). "Assessment of anorexia nervosa: an overview of universal issues and contextual challenges". Journal of Eating Disorders. 1 (1): 29. doi:10.1186/2050-2974-1-29. PMC 4081667. PMID 24999408.
- ^ a b Strumia R (September 2009). "Skin signs in anorexia nervosa". Dermato-Endocrinology. 1 (5): 268–270. doi:10.4161/derm.1.5.10193. PMC 2836432. PMID 20808514.
- ^ a b c d Miller KK (September 2013). "Endocrine effects of anorexia nervosa". Endocrinology and Metabolism Clinics of North America. 42 (3): 515–528. doi:10.1016/j.ecl.2013.05.007. PMC 3769686. PMID 24011884.
- ^ Walsh JM, Wheat ME, Freund K (August 2000). "Detection, evaluation, and treatment of eating disorders the role of the primary care physician". Journal of General Internal Medicine. 15 (8): 577–590. doi:10.1046/j.1525-1497.2000.02439.x. PMC 1495575. PMID 10940151.
- ^ Stargrove MB, Treasure J, McKee DL (2008). Herb, Nutrient, and Drug Interactions: Clinical Implications and Therapeutic Strategies. Elsevier Health Sciences. ISBN 978-0-323-02964-3. Retrieved 9 April 2015.
- ^ American Psychiatric Association (22 May 2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). American Psychiatric Association. p. 339. doi:10.1176/appi.books.9780890425596. ISBN 978-0-89042-555-8. Retrieved 29 February 2024.
- ^ "Anorexia Nervosa". National Association of Anorexia Nervosa and Associated Disorders. Archived from the original on 13 April 2014. Retrieved 15 April 2014.
- ^ Mehler PS, Brown C (31 March 2015). "Anorexia nervosa – medical complications". Journal of Eating Disorders. 3 (1): 11. doi:10.1186/s40337-015-0040-8. ISSN 2050-2974. PMC 4381361. PMID 25834735.
- ^ Smith LL (6 August 2021). "The Central Role of Hypothermia and Hyperactivity in Anorexia Nervosa: A Hypothesis". Frontiers in Behavioral Neuroscience. 15. doi:10.3389/fnbeh.2021.700645. ISSN 1662-5153. PMC 8377352. PMID 34421554.
- ^ Sirufo MM, Ginaldi L, De Martinis M (10 May 2021). "Peripheral Vascular Abnormalities in Anorexia Nervosa: A Psycho-Neuro-Immune-Metabolic Connection". International Journal of Molecular Sciences. 22 (9): 5043. doi:10.3390/ijms22095043. ISSN 1422-0067. PMC 8126077. PMID 34068698.
- ^ Nolen-Hoeksema S (2013). Abnormal Psychology. New York: McGraw Hill. pp. 339–41. ISBN 978-0-07-803538-8.
- ^ Poyastro Pinheiro A, Thornton LM, Plotonicov KH, Tozzi F, Klump KL, Berrettini WH, et al. (July 2007). "Patterns of menstrual disturbance in eating disorders". The International Journal of Eating Disorders. 40 (5): 424–434. doi:10.1002/eat.20388. ISSN 0276-3478. PMID 17497704.
- ^ Hedrick T (August 2022). "The Overlap Between Eating Disorders and Gastrointestinal Disorders" (PDF). Nutrition issues in Gastroenterology. University of Virginia. Archived from the original (PDF) on 8 February 2024.
- ^ a b c Bern EM, O'Brien RF (August 2013). "Is it an eating disorder, gastrointestinal disorder, or both?". Current Opinion in Pediatrics (Review). 25 (4): 463–470. doi:10.1097/MOP.0b013e328362d1ad. PMID 23838835. S2CID 5417088.
Several case reports brought attention to the association of anorexia nervosa and celiac disease.(...) Some patients present with the eating disorder prior to diagnosis of celiac disease and others developed anorexia nervosa after the diagnosis of celiac disease. Healthcare professionals should screen for celiac disease with eating disorder symptoms especially with gastrointestinal symptoms, weight loss, or growth failure.(...) Celiac disease patients may present with gastrointestinal symptoms such as diarrhea, steatorrhea, weight loss, vomiting, abdominal pain, anorexia, constipation, bloating, and distension due to malabsorption. Extraintestinal presentations include anemia, osteoporosis, dermatitis herpetiformis, short stature, delayed puberty, fatigue, aphthous stomatitis, elevated transaminases, neurologic problems, or dental enamel hypoplasia.(...) it has become clear that symptomatic and diagnosed celiac disease is the tip of the iceberg; the remaining 90% or more of children are asymptomatic and undiagnosed.
- ^ Walsh JM, Wheat ME, Freund K (August 2000). "Detection, evaluation, and treatment of eating disorders the role of the primary care physician". Journal of General Internal Medicine. 15 (8): 577–590. doi:10.1046/j.1525-1497.2000.02439.x. PMC 1495575. PMID 10940151.
- ^ Walsh BT (May 2013). "The Enigmatic Persistence of Anorexia Nervosa". American Journal of Psychiatry. 170 (5): 477–484. doi:10.1176/appi.ajp.2012.12081074. ISSN 0002-953X. PMC 4095887. PMID 23429750.
- ^ Bora E, Köse S (August 2016). "Meta-analysis of theory of mind in anorexia nervosa and bulimia nervosa: A specific İmpairment of cognitive perspective taking in anorexia nervosa?". The International Journal of Eating Disorders. 49 (8): 739–740. doi:10.1002/eat.22572. hdl:11343/291969. PMID 27425037.
- ^ "Anorexia nervosa". National Eating Disorders Collaboration (NEDC). 23 August 2017. Retrieved 19 September 2022.
- ^ a b Marzola E, Nasser JA, Hashim SA, Shih PA, Kaye WH (November 2013). "Nutritional rehabilitation in anorexia nervosa: review of the literature and implications for treatment". BMC Psychiatry. 13 (1): 290. doi:10.1186/1471-244X-13-290. PMC 3829207. PMID 24200367.
- ^ Robinson PH (2006). Community treatment of eating disorders. Chichester: John Wiley & Sons. p. 66. ISBN 978-0-470-01676-3.
- ^ Gaudiani J (2 October 2018). Sick Enough: A Guide to the Medical Complications of Eating Disorders (1st ed.). Routledge. ISBN 978-0815382454.
- ^ Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, et al. (June 2018). "Interoception and Mental Health: A Roadmap". Biological Psychiatry. Cognitive Neuroscience and Neuroimaging. 3 (6): 501–513. doi:10.1016/j.bpsc.2017.12.004. PMC 6054486. PMID 29884281.
- ^ a b Badoud D, Tsakiris M (June 2017). "From the body's viscera to the body's image: Is there a link between interoception and body image concerns?". Neuroscience and Biobehavioral Reviews. 77: 237–246. doi:10.1016/j.neubiorev.2017.03.017. PMID 28377099. S2CID 768206.
- ^ a b c Khalsa SS, Lapidus RC (2016). "Can Interoception Improve the Pragmatic Search for Biomarkers in Psychiatry?". Frontiers in Psychiatry. 7: 121. doi:10.3389/fpsyt.2016.00121. PMC 4958623. PMID 27504098.
- ^ a b c d e Boswell JF, Anderson LM, Anderson DA (June 2015). "Integration of Interoceptive Exposure in Eating Disorder Treatment". Clinical Psychology: Science and Practice. 22 (2): 194–210. doi:10.1111/cpsp.12103.
- ^ Kasperek-Zimowska BJ, Zimowski JG, Biernacka K, Kucharska-Pietura K, Rybakowski F (2014). "Impaired social cognition processes in Asperger syndrome and anorexia nervosa. In search for endophenotypes of social cognition". Psychiatria Polska. 50 (3): 533–542. doi:10.12740/PP/OnlineFirst/33485. PMID 27556112.
- ^ Strober M, Freeman R, Lampert C, Diamond J (November 2007). "The association of anxiety disorders and obsessive compulsive personality disorder with anorexia nervosa: evidence from a family study with discussion of nosological and neurodevelopmental implications". The International Journal of Eating Disorders. 40 (S3): S46 – S51. doi:10.1002/eat.20429. PMID 17610248.
- ^ Brand-Gothelf A, Leor S, Apter A, Fennig S (October 2014). "The impact of comorbid depressive and anxiety disorders on severity of anorexia nervosa in adolescent girls". The Journal of Nervous and Mental Disease. 202 (10): 759–762. doi:10.1097/NMD.0000000000000194. PMID 25265267. S2CID 6023688.
- ^ a b Rijkers C (2019). "Eating disorders and posttraumatic stress disorder" (PDF). Current Opinion in Psychiatry. 32 (6): 510–517. doi:10.1097/YCO.0000000000000545. PMID 31313708.
- ^ Godier LR, Park RJ (2014). "Compulsivity in anorexia nervosa: a transdiagnostic concept". Frontiers in Psychology. 5: 778. doi:10.3389/fpsyg.2014.00778. PMC 4101893. PMID 25101036.
- ^ Halmi KA, Tozzi F, Thornton LM, Crow S, Fichter MM, Kaplan AS, et al. (December 2005). "The relation among perfectionism, obsessive-compulsive personality disorder and obsessive-compulsive disorder in individuals with eating disorders". The International Journal of Eating Disorders. 38 (4): 371–374. doi:10.1002/eat.20190. PMID 16231356.
- ^ Crane AM, Roberts ME, Treasure J (November 2007). "Are obsessive-compulsive personality traits associated with a poor outcome in anorexia nervosa? A systematic review of randomized controlled trials and naturalistic outcome studies". The International Journal of Eating Disorders. 40 (7): 581–588. doi:10.1002/eat.20419. PMID 17607713.
- ^ Gárriz M, Andrés-Perpiñá S, Plana MT, Flamarique I, Romero S, Julià L, et al. (March 2021). "Personality disorder traits, obsessive ideation and perfectionism 20 years after adolescent-onset anorexia nervosa: a recovered study". Eating and Weight Disorders. 26 (2): 667–677. doi:10.1007/s40519-020-00906-7. PMID 32350776. S2CID 216649851.
However, prospective studies are still scarce and the results from current literature regarding causal connections between AN and personality are unavailable.
- ^ Casper RC (1998). "Depression and eating disorders". Depression and Anxiety. 8 (Suppl 1): 96–104. doi:10.1002/(SICI)1520-6394(1998)8:1+<96::AID-DA15>3.0.CO;2-4. PMID 9809221. S2CID 36772859.
- ^ Zernig G, Saria A, Kurz M, O'Malley S (24 March 2000). Handbook of Alcoholism. CRC Press. p. 293. ISBN 978-1-4200-3696-1.
- ^ Devoe DJ, Dimitropoulos G, Anderson A, Bahji A, Flanagan J, Soumbasis A, et al. (11 December 2021). "The prevalence of substance use disorders and substance use in anorexia nervosa: a systematic review and meta-analysis". Journal of Eating Disorders. 9 (1): 161. doi:10.1186/s40337-021-00516-3. ISSN 2050-2974. PMC 8666057. PMID 34895358.
- ^ Sansone RA, Levitt JL (21 August 2013). Personality Disorders and Eating Disorders: Exploring the Frontier. Routledge. p. 28. ISBN 978-1-135-44280-4.
- ^ Halmi KA (November 2013). "Perplexities of treatment resistance in eating disorders". BMC Psychiatry. 13: 292. doi:10.1186/1471-244X-13-292. PMC 3829659. PMID 24199597.
- ^ Swinbourne JM, Touyz SW (July 2007). "The co-morbidity of eating disorders and anxiety disorders: a review". European Eating Disorders Review. 15 (4): 253–274. doi:10.1002/erv.784. PMID 17676696.
- ^ Cortese S, Bernardina BD, Mouren MC (September 2007). "Attention-deficit/hyperactivity disorder (ADHD) and binge eating". Nutrition Reviews. 65 (9): 404–411. doi:10.1111/j.1753-4887.2007.tb00318.x. PMID 17958207.
- ^ Wilhelm S, Phillips KA, Steketee G (18 December 2012). Cognitive-Behavioral Therapy for Body Dysmorphic Disorder: A Treatment Manual. Guilford Press. p. 270. ISBN 978-1-4625-0790-0.
- ^ a b Berkman ND, Bulik CM, Brownley KA, Lohr KN, Sedway JA, Rooks A, et al. (April 2006). "Management of eating disorders" (PDF). Evidence Report/Technology Assessment (135): 1–166. PMC 4780981. PMID 17628126. Archived (PDF) from the original on 22 December 2014.
- ^ Huke V, Turk J, Saeidi S, Kent A, Morgan JF (September 2013). "Autism spectrum disorders in eating disorder populations: a systematic review". European Eating Disorders Review. 21 (5): 345–351. doi:10.1002/erv.2244. PMID 23900859.
- ^ Inal-Kaleli I, Dogan N, Kose S, Bora E (12 November 2024). "Investigating the Presence of Autistic Traits and Prevalence of Autism Spectrum Disorder Symptoms in Anorexia Nervosa: A Systematic Review and Meta-Analysis". The International Journal of Eating Disorders. 58 (1): 66–90. doi:10.1002/eat.24307. ISSN 1098-108X. PMC 11784827. PMID 39530423.
- ^ a b Zucker NL, Losh M, Bulik CM, LaBar KS, Piven J, Pelphrey KA (November 2007). "Anorexia nervosa and autism spectrum disorders: guided investigation of social cognitive endophenotypes" (PDF). Psychological Bulletin. 133 (6): 976–1006. doi:10.1037/0033-2909.133.6.976. PMID 17967091. Archived (PDF) from the original on 20 April 2010.
- ^ a b c d Rikani AA, Choudhry Z, Choudhry AM, Ikram H, Asghar MW, Kajal D, et al. (October 2013). "A critique of the literature on etiology of eating disorders". Annals of Neurosciences. 20 (4): 157–161. doi:10.5214/ans.0972.7531.200409. PMC 4117136. PMID 25206042.
- ^ Thornton LM, Mazzeo SE, Bulik CM (2011). "The heritability of eating disorders: methods and current findings". Behavioral Neurobiology of Eating Disorders. Current Topics in Behavioral Neurosciences. Vol. 6. pp. 141–56. doi:10.1007/7854_2010_91. ISBN 978-3-642-15130-9. PMC 3599773. PMID 21243474.
- ^ Hildebrandt T, Downey A (4 July 2013). "The Neurobiology of Eating Disorders". In Charney D, Sklar P, Buxbaum J, Nestler E (eds.). Neurobiology of Mental Illness (4th ed.). Oxford University Press. ISBN 978-0-19-993495-9.
- ^ Rask-Andersen M, Olszewski PK, Levine AS, Schiöth HB (March 2010). "Molecular mechanisms underlying anorexia nervosa: focus on human gene association studies and systems controlling food intake". Brain Research Reviews. 62 (2): 147–164. doi:10.1016/j.brainresrev.2009.10.007. PMID 19931559. S2CID 37635456.
- ^ Pjetri E, Schmidt U, Kas MJ, Campbell IC (July 2012). "Epigenetics and eating disorders". Current Opinion in Clinical Nutrition and Metabolic Care. 15 (4): 330–335. doi:10.1097/MCO.0b013e3283546fd3. PMID 22617563. S2CID 27183934.
- ^ Hübel C, Marzi SJ, Breen G, Bulik CM (June 2019). "Epigenetics in eating disorders: a systematic review". Molecular Psychiatry. 24 (6): 901–915. doi:10.1038/s41380-018-0254-7. PMC 6544542. PMID 30353170.
- ^ Watson HJ, Yilmaz Z, Thornton LM, Hübel C, Coleman JR, Gaspar HA, et al. (August 2019). "Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa". Nature Genetics. 51 (8): 1207–1214. doi:10.1038/s41588-019-0439-2. PMC 6779477. PMID 31308545.
- ^ Larsen JT, Bulik CM, Thornton LM, Koch SV, Petersen L (April 2021). "Prenatal and perinatal factors and risk of eating disorders". Psychological Medicine. 51 (5): 870–880. doi:10.1017/S0033291719003945. PMID 31910913. S2CID 210086516.
- ^ Jones C, Pearce B, Barrera I, Mummert A (September 2017). "Fetal programming and eating disorder risk". Journal of Theoretical Biology. 428: 26–33. Bibcode:2017JThBi.428...26J. doi:10.1016/j.jtbi.2017.05.028. PMID 28571669.
- ^ Favaro A, Tenconi E, Santonastaso P (January 2006). "Perinatal factors and the risk of developing anorexia nervosa and bulimia nervosa". Archives of General Psychiatry. 63 (1): 82–88. doi:10.1001/archpsyc.63.1.82. PMID 16389201. S2CID 45181444.
- ^ Davis JF, Choi DL, Benoit SC (2011). "24. Orexigenic Hypothalamic Peptides Behavior and Feeding – 24.5 Orexin". In Preedy VR, Watson RR, Martin CR (eds.). Handbook of Behavior, Food and Nutrition. Springer. pp. 361–2. ISBN 978-0-387-92271-3.
- ^ Smitka K, Papezova H, Vondra K, Hill M, Hainer V, Nedvidkova J (2013). "The role of "mixed" orexigenic and anorexigenic signals and autoantibodies reacting with appetite-regulating neuropeptides and peptides of the adipose tissue-gut-brain axis: relevance to food intake and nutritional status in patients with anorexia nervosa and bulimia nervosa". International Journal of Endocrinology. 2013: 483145. doi:10.1155/2013/483145. PMC 3782835. PMID 24106499.
- ^ a b Satherley R, Howard R, Higgs S (January 2015). "Disordered eating practices in gastrointestinal disorders" (PDF). Appetite (Review). 84: 240–250. doi:10.1016/j.appet.2014.10.006. PMID 25312748. S2CID 25805182. Archived from the original (PDF) on 24 September 2019. Retrieved 4 July 2019.
- ^ Quick VM, Byrd-Bredbenner C, Neumark-Sztainer D (May 2013). "Chronic illness and disordered eating: a discussion of the literature". Advances in Nutrition (Review). 4 (3): 277–286. doi:10.3945/an.112.003608. PMC 3650496. PMID 23674793.
- ^ a b Herpertz-Dahlmann B, Bühren K, Remschmidt H (June 2013). "Growing up is hard: mental disorders in adolescence". Deutsches Ärzteblatt International. 110 (25): 432–9, quiz 440. doi:10.3238/arztebl.2013.0432. PMC 3705204. PMID 23840288.
- ^ Chen D, Steele AD, Lindquist S, Guarente L (9 December 2005). "Increase in activity during calorie restriction requires Sirt1". Science. 310 (5754): 1641. doi:10.1126/science.1118357. ISSN 1095-9203. PMID 16339438.
- ^ Zandian M, Ioakimidis I, Bergh C, Södersten P (September 2007). "Cause and treatment of anorexia nervosa". Physiology & Behavior. 92 (1–2): 283–290. doi:10.1016/j.physbeh.2007.05.052. PMID 17585973. S2CID 43620773.
- ^ Thambirajah MS (2007). Case Studies in Child and Adolescent Mental Health. Radcliffe Publishing. p. 145. ISBN 978-1-85775-698-2. OCLC 84150452.
- ^ a b Kaye W (April 2008). "Neurobiology of anorexia and bulimia nervosa". Physiology & Behavior. 94 (1): 121–135. doi:10.1016/j.physbeh.2007.11.037. PMC 2601682. PMID 18164737.
- ^ Guisinger S (October 2003). "Adapted to flee famine: adding an evolutionary perspective on anorexia nervosa". Psychological Review. 110 (4): 745–761. doi:10.1037/0033-295X.110.4.745. ISSN 0033-295X. PMID 14599241.
- ^ Guisinger S. "Adapted to Famine: An Evolutionary Approach to Understanding Eating Behaviors". Archived from the original on 22 June 2024.
- ^ Rossi E, Cassioli E, Dani C, Marchesoni G, Monteleone AM, Wonderlich SA, et al. (1 May 2024). "The maltreated eco-phenotype of eating disorders: A new diagnostic specifier? A systematic review of the evidence and comprehensive description". Neuroscience & Biobehavioral Reviews. 160: 105619. doi:10.1016/j.neubiorev.2024.105619. hdl:2158/1352632. ISSN 0149-7634. PMID 38462152.
- ^ Connors ME, Morse W (January 1993). "Sexual abuse and eating disorders: a review". The International Journal of Eating Disorders. 13 (1): 1–11. doi:10.1002/1098-108x(199301)13:1<1::aid-eat2260130102>3.0.co;2-p. PMID 8477269.
- ^ Worthen D (2001). P & G Pharmacy Handbook. Boca Raton, FL: Routledge. p. 65. ISBN 978-1-4822-9648-8.
- ^ Ensminger AH (1983). Foods and Nutrition Encyclopedia (1st ed.). Clovis, California: Pegus Press. p. 423. ISBN 978-0-941218-05-4.
- ^ Colman A (2015). A Dictionary of Psychology. OUP Oxford. p. 851. ISBN 978-0-19-105784-7.
- ^ Rogers AI, Vanderveldt HS, Deshpande AR (2012). "Weight Loss". In Hawkey CJ, Bosch J, Richter JE, Garcia-Tsao G, Chan FK (eds.). Textbook of Clinical Gastroenterology and Hepatology (2nd ed.). John Wiley & Sons. p. 69. ISBN 978-1-118-32142-3.
- ^ a b "Factors That May Contribute to Eating Disorders". National Eating Disorders Association. Archived from the original on 3 March 2016. Retrieved 1 March 2016.
- ^ Halmi KA, Sunday SR, Strober M, Kaplan A, Woodside DB, Fichter M, et al. (November 2000). "Perfectionism in anorexia nervosa: variation by clinical subtype, obsessionality, and pathological eating behavior". The American Journal of Psychiatry. 157 (11): 1799–1805. doi:10.1176/appi.ajp.157.11.1799. PMID 11058477.
- ^ Anderluh MB, Tchanturia K, Rabe-Hesketh S, Treasure J (February 2003). "Childhood obsessive-compulsive personality traits in adult women with eating disorders: defining a broader eating disorder phenotype". The American Journal of Psychiatry. 160 (2): 242–247. doi:10.1176/appi.ajp.160.2.242. PMID 12562569.
- ^ "Anorexia Nervosa". Mayo Clinic.
- ^ Flett GL, Newby J, Hewitt PL, Persaud C (September 2011). "Perfectionistic automatic thoughts, trait perfectionism, and bulimic automatic thoughts in young women". Journal of Rational-Emotive & Cognitive-Behavior Therapy. 29 (3): 192–206. doi:10.1007/s10942-011-0135-3. S2CID 144731404.
- ^ Garner DM, Bemis KM (June 1982). "A cognitive-behavioral approach to anorexia nervosa". Cognitive Therapy and Research. 6 (2): 123–50. doi:10.1007/BF01183887. S2CID 10469356.
- ^ a b Tagay S, Schlottbohm E, Reyes-Rodriguez ML, Repic N, Senf W (January 2014). "Eating disorders, trauma, PTSD, and psychosocial resources". Eating Disorders. 22 (1): 33–49. doi:10.1080/10640266.2014.857517. PMC 3966425. PMID 24365526.
- ^ a b c Reyes-Rodríguez ML, Von Holle A, Ulman TF, Thornton LM, Klump KL, Brandt H, et al. (July 2011). "Posttraumatic stress disorder in anorexia nervosa". Psychosomatic Medicine. 73 (6): 491–497. doi:10.1097/PSY.0b013e31822232bb. PMC 3132652. PMID 21715295.
- ^ a b c d e f g h Malecki J, Rhodes P, Ussher J (August 2018). "Childhood trauma and anorexia nervosa: from body image to embodiment". Health Care for Women International. 39 (8): 936–951. doi:10.1080/07399332.2018.1492268. PMID 30152723. S2CID 205580678.
- ^ "Eating disorders and culture". The Harvard Mental Health Letter. 20 (9): 7. March 2004. PMID 15044128.
- ^ Francisco R (2018), "Studies on Body Image, Eating, and Weight in Models, Dancers, and Aesthetic Athletes", in Cuzzolaro M, Fassino S (eds.), Body Image, Eating, and Weight, Springer International Publishing, pp. 401–411, doi:10.1007/978-3-319-90817-5_29, hdl:10400.14/33058, ISBN 978-3-319-90817-5, S2CID 150323306
- ^ Becker AE (2018). "Sociocultural influences on body image and eating disturbance". In Brownell KD, Walsh BT (eds.). Eating Disorders and Obesity: A Comprehensive Handbook (Third ed.). Guilford Publications. pp. 127–133. ISBN 978-1-4625-3609-2.
- ^ Anderson-Fye EP, Becker AE (2004). "Sociocultural Aspects of Eating Disorders". In Thompson KJ (ed.). Handbook of Eating Disorders and Obesity. Hoboken, NJ: John Wiley & Sons. pp. 565–89. ISBN 978-0-471-23073-1.
- ^ Baum A (2006). "Eating disorders in the male athlete" (PDF). Sports Medicine. 36 (1): 1–6. doi:10.2165/00007256-200636010-00001. PMID 16445307. S2CID 15296296. Archived (PDF) from the original on 4 June 2015.
- ^ Kyriacou O, Treasure J, Schmidt U (January 2008). "Expressed emotion in eating disorders assessed via self-report: an examination of factors associated with expressed emotion in carers of people with anorexia nervosa in comparison to control families". The International Journal of Eating Disorders. 41 (1): 37–46. doi:10.1002/eat.20469. PMID 17922532.
- ^ Yager J (April 2020). "Managing Patients With Severe and Enduring Anorexia Nervosa: When Is Enough, Enough?". The Journal of Nervous and Mental Disease. 208 (4): 277–282. doi:10.1097/NMD.0000000000001124. PMID 32221180. S2CID 209342890.
- ^ "Eating Disorders Anorexia Causes | Eating Disorders". Psychiatric Disorders and Mental Health Issues. 13 June 2014. Archived from the original on 7 March 2016. Retrieved 1 March 2016.
- ^ Simpson KJ (February 2002). "Anorexia nervosa and culture". Journal of Psychiatric and Mental Health Nursing. 9 (1): 65–71. doi:10.1046/j.1351-0126.2001.00443.x. PMID 11896858.
- ^ Spettigue W, Henderson KA (February 2004). "Eating disorders and the role of the media". The Canadian Child and Adolescent Psychiatry Review. 13 (1): 16–19. PMC 2533817. PMID 19030149.
- ^ Labre MP (April 2002). "Adolescent boys and the muscular male body ideal". The Journal of Adolescent Health. 30 (4): 233–242. doi:10.1016/S1054-139X(01)00413-X. PMID 11927235.
- ^ Izydorczyk B, Sitnik-Warchulska K (29 March 2018). "Sociocultural Appearance Standards and Risk Factors for Eating Disorders in Adolescents and Women of Various Ages". Frontiers in Psychology. 9: 429. doi:10.3389/fpsyg.2018.00429. PMC 5885084. PMID 29651268.
- ^ Drenten J, Gurrieri L (2018). Crossing the #BIKINIBRIDGE: Exploring the Role of Social Media in Propagating Body Image Trends (1st ed.). New York, NY: Routledge. ISBN 9781138052550. Retrieved 15 March 2024.
- ^ a b Norris ML, Boydell KM, Pinhas L, Katzman DK (September 2006). "Ana and the Internet: a review of pro-anorexia websites". The International Journal of Eating Disorders. 39 (6): 443–447. doi:10.1002/eat.20305. PMID 16721839. S2CID 29355957.
- ^ a b Lee S (January 1996). "Reconsidering the status of anorexia nervosa as a western culture-bound syndrome". Social Science & Medicine. 42 (1): 21–34. doi:10.1016/0277-9536(95)00074-7. PMID 8745105.
- ^ Demarque M, Guzman G, Morrison E, Ahovi J, Moro MR, Blanchet-Collet C (April 2015). "Anorexia nervosa in a girl of Chinese origin: Psychological, somatic and transcultural factors". Clinical Child Psychology and Psychiatry. 20 (2): 276–288. doi:10.1177/1359104513514067. ISSN 1359-1045. PMID 24363225. S2CID 13036347.
- ^ Tareen A, Hodes M, Rangel L (February 2005). "Non-fat-phobic anorexia nervosa in British South Asian adolescents". International Journal of Eating Disorders. 37 (2): 161–165. doi:10.1002/eat.20080. ISSN 0276-3478. PMID 15732077.
- ^ Kim YR, Nakai Y, Thomas JJ (January 2021). "Introduction to a special issue on eating disorders in Asia". International Journal of Eating Disorders. 54 (1): 3–6. doi:10.1002/eat.23444. ISSN 0276-3478. PMID 33340374. S2CID 229325699.
- ^ Kaye WH, Frank GK, Bailer UF, Henry SE, Meltzer CC, Price JC, et al. (May 2005). "Serotonin alterations in anorexia and bulimia nervosa: new insights from imaging studies". Physiology & Behavior. 85 (1): 73–81. doi:10.1016/j.physbeh.2005.04.013. PMID 15869768. S2CID 25676759.
- ^ Kaye WH, Bailer UF, Frank GK, Wagner A, Henry SE (September 2005). "Brain imaging of serotonin after recovery from anorexia and bulimia nervosa". Physiology & Behavior. 86 (1–2): 15–17. doi:10.1016/j.physbeh.2005.06.019. PMID 16102788. S2CID 17250708.
- ^ Gaudio S, Wiemerslage L, Brooks SJ, Schiöth HB (December 2016). "A systematic review of resting-state functional-MRI studies in anorexia nervosa: Evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration". Neuroscience and Biobehavioral Reviews. 71: 578–589. doi:10.1016/j.neubiorev.2016.09.032. PMID 27725172.
- ^ Fuglset TS, Landrø NI, Reas DL, Rø Ø (2016). "Functional brain alterations in anorexia nervosa: a scoping review". Journal of Eating Disorders. 4: 32. doi:10.1186/s40337-016-0118-y. PMC 5125031. PMID 27933159.
- ^ Kaye WH, Wagner A, Fudge JL, Paulus M (2011). "Neurocircuitry of Eating Disorders". In Adan RA, Kaye WH (eds.). Behavioral Neurobiology of Eating Disorders: 6. Current Topics in Behavioral Neurosciences. Springer Berlin Heidelberg. pp. 59–80. ISBN 978-3-642-15131-6.
- ^ Titova OE, Hjorth OC, Schiöth HB, Brooks SJ (April 2013). "Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: a meta-analysis of VBM studies". BMC Psychiatry. 13: 110. doi:10.1186/1471-244X-13-110. PMC 3664070. PMID 23570420.
- ^ King JA, Frank GK, Thompson PM, Ehrlich S (February 2018). "Structural Neuroimaging of Anorexia Nervosa: Future Directions in the Quest for Mechanisms Underlying Dynamic Alterations". Biological Psychiatry. 83 (3): 224–234. doi:10.1016/j.biopsych.2017.08.011. PMC 6053269. PMID 28967386.
- ^ Frank GK (August 2015). "Advances from neuroimaging studies in eating disorders". CNS Spectrums. 20 (4): 391–400. doi:10.1017/S1092852915000012. PMC 4989857. PMID 25902917.
- ^ a b Mitchell JE, Peterson CB (April 2020). "Anorexia Nervosa". The New England Journal of Medicine. 382 (14): 1343–1351. doi:10.1056/NEJMcp1803175. PMID 32242359. S2CID 214769639.
- ^ "DSM-5 Changes: Implications for Child Serious Emotional Disturbance" (PDF). Substance Abuse and Mental Health Services Administration. June 2016. p. 48 (Table 19, DSM-IV to DSM-5 Anorexia Nervosa Comparison). Retrieved 17 September 2021.
- ^ Estour B, Galusca B, Germain N (2014). "Constitutional thinness and anorexia nervosa: a possible misdiagnosis?". Frontiers in Endocrinology. 5: 175. doi:10.3389/fendo.2014.00175. PMC 4202249. PMID 25368605.
- ^ Peat C, Mitchell JE, Hoek HW, Wonderlich SA (November 2009). "Validity and utility of subtyping anorexia nervosa". The International Journal of Eating Disorders. 42 (7): 590–594. doi:10.1002/eat.20717. PMC 2844095. PMID 19598270.
- ^ a b "Other Mental Disorders", Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, American Psychiatric Publishing, Inc, 2013, doi:10.1176/appi.books.9780890425596.529303, ISBN 978-0-89042-559-6, retrieved 17 December 2024
- ^ Mattar L (8 March 2011). "Anorexia nervosa and nutritional assessment: contribution of body composition measurements". Nutrition Research Reviews. 24 (1): 39–45. doi:10.1017/S0954422410000284. PMID 21382223.
- ^ Himmerich H, Treasure J (1 April 2024). "Anorexia nervosa: diagnostic, therapeutic, and risk biomarkers in clinical practice". Trends in Molecular Medicine. Special issue: Eating disorders. 30 (4): 350–360. doi:10.1016/j.molmed.2024.01.002. ISSN 1471-4914. PMID 38331700.
- ^ Singleton JK (12 November 2014). Primary Care, Second Edition: An Interprofessional Perspective. Springer Publishing Company. ISBN 978-0-8261-7147-4. Retrieved 9 April 2015.
- ^ Khalifa I (February 2019). "Anorexia nervosa requiring admission in adolescents". L'Anorexie mentale nécessitant une hospitalisation chez les adolescents. 65 (2): 107–108. PMC 6515507. PMID 30765357.
- ^ a b c "Physical examination in Anorexia nervosa" (PDF).
- ^ "CBC blood test". MedlinePlus. U.S. National Library of Medicine. Archived from the original on 25 May 2013. Retrieved 31 May 2013.
- ^ "Comprehensive metabolic panel". MedlinePlus. U.S. National Library of Medicine. Archived from the original on 5 April 2015. Retrieved 4 February 2012.
- ^ Lee H, Oh JY, Sung YA, Chung H, Cho WY (October 2009). "The prevalence and risk factors for glucose intolerance in young Korean women with polycystic ovary syndrome". Endocrine. 36 (2): 326–332. doi:10.1007/s12020-009-9226-7. PMID 19688613. S2CID 207361456.
- ^ "Guide to Common Laboratory Tests for Eating Disorder Patients" (PDF). Maudsleyparents.org. Retrieved 29 June 2022.
- ^ Montagnese C, Scalfi L, Signorini A, De Filippo E, Pasanisi F, Contaldo F (December 2007). "Cholinesterase and other serum liver enzymes in underweight outpatients with eating disorders". The International Journal of Eating Disorders. 40 (8): 746–750. doi:10.1002/eat.20432. PMID 17610252.
- ^ Narayanan V, Gaudiani JL, Harris RH, Mehler PS (May 2010). "Liver function test abnormalities in anorexia nervosa—cause or effect". The International Journal of Eating Disorders. 43 (4): 378–381. doi:10.1002/eat.20690. PMID 19424979.
- ^ Walder A, Baumann P (December 2008). "Increased creatinine kinase and rhabdomyolysis in anorexia nervosa". The International Journal of Eating Disorders. 41 (8): 766–767. doi:10.1002/eat.20548. PMID 18521917.
- ^ "BUN – blood test". MedlinePlus. U.S. National Library of Medicine. 26 January 2012. Archived from the original on 9 April 2010. Retrieved 4 February 2012.
- ^ Sheridan AM, Bonventre JV (July 2000). "Cell biology and molecular mechanisms of injury in ischemic acute renal failure". Current Opinion in Nephrology and Hypertension. 9 (4): 427–434. doi:10.1097/00041552-200007000-00015. PMID 10926180.
- ^ Nelsen DA (December 2002). "Gluten-sensitive enteropathy (celiac disease): more common than you think". American Family Physician. 66 (12): 2259–2266. PMID 12507163.
- ^ Misra M, Klibanski A (2011). "The neuroendocrine basis of anorexia nervosa and its impact on bone metabolism". Neuroendocrinology. 93 (2): 65–73. doi:10.1159/000323771. PMC 3214929. PMID 21228564.
- ^ "Urinalysis". MedlinePlus. U.S. National Library of Medicine. 26 January 2012. Archived from the original on 4 April 2010. Retrieved 4 February 2012.
- ^ Pepin J, Shields C (February 2012). "Advances in diagnosis and management of hypokalemic and hyperkalemic emergencies". Emergency Medicine Practice. 14 (2): 1–17, quiz 17–8. PMID 22413702.
- ^ "Electroencephalogram". Medline Plus. 26 January 2012. Archived from the original on 27 January 2012. Retrieved 4 February 2012.
- ^ a b c Nolen-Hoeksema S (2014). "Eating disorders". Abnormal Psychology (Sixth ed.). New York: McGraw-Hill Education. p. 341. ISBN 978-0-07-803538-8.
- ^ a b Hay PJ, Claudino AM, Touyz S, Abd Elbaky G (27 July 2015). Cochrane Common Mental Disorders Group (ed.). "Individual psychological therapy in the outpatient treatment of adults with anorexia nervosa". Cochrane Database of Systematic Reviews. 2018 (2): CD003909. doi:10.1002/14651858.CD003909.pub2. PMC 6491116. PMID 26212713.
- ^ a b Atti AR, Mastellari T, Valente S, Speciani M, Panariello F, De Ronchi D (May 2021). "Compulsory treatments in eating disorders: a systematic review and meta-analysis". Eating and Weight Disorders. 26 (4): 1037–1048. doi:10.1007/s40519-020-01031-1. PMC 8062396. PMID 33099675.
- ^ a b Lock JD, Fitzpatrick KK (March 2009). "Anorexia nervosa". BMJ Clinical Evidence. 2009. PMC 2907776. PMID 19445758.
- ^ a b National Institute of Mental Health. "Eating disorders". Archived from the original on 23 March 2015. Retrieved 23 March 2015.
- ^ Russell GF, Szmukler GI, Dare C, Eisler I (December 1987). "An evaluation of family therapy in anorexia nervosa and bulimia nervosa". Archives of General Psychiatry. 44 (12): 1047–1056. doi:10.1001/archpsyc.1987.01800240021004. PMID 3318754.
- ^ Blessitt E, Voulgari S, Eisler I (November 2015). "Family therapy for adolescent anorexia nervosa". Current Opinion in Psychiatry. 28 (6): 455–460. doi:10.1097/yco.0000000000000193. PMID 26382158. S2CID 33438815.
- ^ Fisher CA, Skocic S, Rutherford KA, Hetrick SE (1 May 2019). "Family therapy approaches for anorexia nervosa". The Cochrane Database of Systematic Reviews. 2019 (5): CD004780. doi:10.1002/14651858.CD004780.pub4. ISSN 1469-493X. PMC 6497182. PMID 31041816.
- ^ a b le Grange D, Eisler I (January 2009). "Family interventions in adolescent anorexia nervosa". Child and Adolescent Psychiatric Clinics of North America. 18 (1): 159–173. doi:10.1016/j.chc.2008.07.004. PMID 19014864.
- ^ a b Treasure J, Parker S, Oyeleye O, Harrison A (May 2021). "The value of including families in the treatment of anorexia nervosa". European Eating Disorders Review. 29 (3): 393–401. doi:10.1002/erv.2816. PMC 8246805. PMID 33351987.
- ^ "Eating Disorders". National Institute of Mental Health (NIMH). 2011. Archived from the original on 1 October 2013. Retrieved 29 September 2013.
- ^ Whitfield G, Davidson A (2007). Cognitive Behavioural Therapy Explained. Radcliffe Publishing. ISBN 978-1-85775-603-6. Retrieved 9 April 2015.
- ^ Keltner NL, Steele D (6 August 2014). Psychiatric Nursing. Elsevier Health Sciences. ISBN 978-0-323-29352-5. Retrieved 9 April 2015.
- ^ Tchanturia K, Lounes N, Holttum S (November 2014). "Cognitive remediation in anorexia nervosa and related conditions: a systematic review". European Eating Disorders Review. 22 (6): 454–462. doi:10.1002/erv.2326. PMID 25277720.
- ^ Edwards DJ (2017). "An Interpretative Phenomenological Analysis of Schema Modes in a Single Case of Anorexia Nervosa: Part 1- Background, Method, and Child and Parent Modes". Indo-Pacific Journal of Phenomenology. 17: 1–13. doi:10.1080/20797222.2017.1326728. S2CID 148977898.
- ^ Nolen-Hoeksema S (2014). Abnormal Psychology (Sixth ed.). McGraw-Hill Education. p. 357. ISBN 978-1-259-06072-4.
- ^ Garner DM, Garfinkel PE (1 January 1997). Handbook of Treatment for Eating Disorders. Guilford Press. ISBN 978-1-57230-186-3.
- ^ Whitnet E, Rolfes SR (2011). Understanding Nutrition. United States: Wadsworth Cengage Learning. p. 255. ISBN 978-1-133-58752-1. Archived from the original on 24 November 2015.
- ^ a b c Garber AK, Sawyer SM, Golden NH, Guarda AS, Katzman DK, Kohn MR, et al. (March 2016). "A systematic review of approaches to refeeding in patients with anorexia nervosa". The International Journal of Eating Disorders. 49 (3): 293–310. doi:10.1002/eat.22482. PMC 6193754. PMID 26661289.
- ^ Dulloo AG, Jacquet J, Girardier L (March 1997). "Poststarvation hyperphagia and body fat overshooting in humans: a role for feedback signals from lean and fat tissues". The American Journal of Clinical Nutrition. 65 (3): 717–723. doi:10.1093/ajcn/65.3.717. ISSN 0002-9165. PMID 9062520.
- ^ Garfinkel PE, Garner DM (1997). Handbook of Treatment for Eating Disorders. Guilford Press. pp. 156–157.
During the weekends in particular, some of the men found it difficult to stop eating. Their daily intake commonly ranged between 8,000 and 10,000 calories. [...] After about 5 months of refeeding, the majority of the men reported some normalization of their eating patterns, but for some the extreme overconsumption persisted. [...] More than 8 months after renourishment began, most men had returned to normal eating patterns; however, a few were still eating abnormal amounts.
- ^ Maniscalco J (2007). "Malnutrition: Refeeding Syndrome". In Zaoutis LB, Chiang VW (eds.). Comprehensive Pediatric Hospital Medicine. Elsevier Inc. pp. 633–640 (637–638). doi:10.1016/B978-032303004-5.50103-4. ISBN 978-0-323-03004-5. Archived from the original on 21 January 2024.
Hypophosphatemia is considered the hallmark of refeeding syndrome, although other imbalances may occur as well, including hypokalemia and hypomagnesemia.
- ^ O'Connor G, Nicholls D (June 2013). "Refeeding hypophosphatemia in adolescents with anorexia nervosa: a systematic review". Nutrition in Clinical Practice. 28 (3): 358–364. doi:10.1177/0884533613476892. PMC 4108292. PMID 23459608.
- ^ "Nutrition support for adults: oral nutrition support, enteral tube feeding and parenteral nutrition". NICE. 4 August 2017. Retrieved 8 March 2021.
- ^ a b Pinna F, Sanna L, Carpiniello B (2015). "Alexithymia in eating disorders: therapeutic implications". Psychology Research and Behavior Management. 8: 1–15. doi:10.2147/PRBM.S52656. PMC 4278740. PMID 25565909.
- ^ Claudino AM, Hay P, Lima MS, Bacaltchuk J, Schmidt U, Treasure J (January 2006). "Antidepressants for anorexia nervosa". The Cochrane Database of Systematic Reviews (1): CD004365. doi:10.1002/14651858.CD004365.pub2. PMID 16437485.
- ^ Meftah AM, Deckler E, Citrome L, Kantrowitz JT (January 2020). "New discoveries for an old drug: a review of recent olanzapine research". Postgraduate Medicine. 132 (1): 80–90. doi:10.1080/00325481.2019.1701823. PMID 31813311. S2CID 208957067.
- ^ Arcelus J, Mitchell AJ, Wales J, Nielsen S (July 2011). "Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies". Archives of General Psychiatry. 68 (7): 724–731. doi:10.1001/archgenpsychiatry.2011.74. PMID 21727255.
- ^ Robinson P (2012). "Avoiding deaths in hospital from anorexia nervosa: the MARSIPAN project". The Psychiatrist. 36 (3): 109–13. doi:10.1192/pb.bp.111.036699.
- ^ MARSIPAN Working Group (2014). "CR189: Management of Really Sick Patients with Anorexia Nervosa" (Second ed.). Royal College of Psychiatrists. p. 6. Archived from the original on 21 April 2016.
- ^ a b c Conti JE, Joyce C, Hay P, Meade T (October 2020). ""Finding my own identity": a qualitative metasynthesis of adult anorexia nervosa treatment experiences". BMC Psychology. 8 (1): 110. doi:10.1186/s40359-020-00476-4. PMC 7583290. PMID 33092638.
- ^ doi: 10.1192/bjo.2018.78
- ^ a b c d e f Graham MR, Tierney S, Chisholm A, Fox JR (March 2020). "The lived experience of working with people with eating disorders: A meta-ethnography" (PDF). The International Journal of Eating Disorders. 53 (3): 422–441. doi:10.1002/eat.23215. PMID 31904870. S2CID 209894802.
- ^ Bouquegneau A, Dubois BE, Krzesinski JM, Delanaye P (August 2012). "Anorexia nervosa and the kidney". American Journal of Kidney Diseases. 60 (2): 299–307. doi:10.1053/j.ajkd.2012.03.019. PMID 22609034.
- ^ Nolen-Hoeksema S (2014). "Eating Disorders". Abnormal Psychology (Sixth ed.). New York: McGraw Hill Education. p. 342. ISBN 978-0-07-803538-8.
- ^ Saure E, Laasonen M, Lepistö-Paisley T, Mikkola K, Ålgars M, Raevuori A (July 2020). "Characteristics of autism spectrum disorders are associated with longer duration of anorexia nervosa: A systematic review and meta-analysis". The International Journal of Eating Disorders. 53 (7): 1056–1079. doi:10.1002/eat.23259. ISSN 1098-108X. PMID 32181530.
- ^ Leigh S (19 November 2019). "Many Patients with Anorexia Nervosa Get Better, But Complete Recovery Elusive to Most". University of California San Francisco. The Regents of The University of California. Retrieved 23 June 2021.
- ^ Donaldson AA, Gordon CM (September 2015). "Skeletal complications of eating disorders". Metabolism. 64 (9). Metabolism: Clinical and Experimental: 943–951. doi:10.1016/j.metabol.2015.06.007. PMC 4546560. PMID 26166318.
- ^ Downey AE, Richards A, Tanner AB (3 March 2023). "Linear growth in young people with restrictive eating disorders: "Inching" toward consensus". Frontiers in Psychiatry. 14: 1094222. doi:10.3389/fpsyt.2023.1094222. PMC 10020618. PMID 36937727.
- ^ "Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders" (PDF). National Collaborating Centre for Mental Health. 2004. Archived (PDF) from the original on 27 March 2014.
- ^ Vyver E, Steinegger C, Katzman DK (2008). "Eating disorders and menstrual dysfunction in adolescents". Annals of the New York Academy of Sciences. 1135 (1): 253–264. Bibcode:2008NYASA1135..253V. doi:10.1196/annals.1429.013. PMID 18574232. S2CID 42042720.
- ^ Devlin MJ, Walsh BT, Katz JL, Roose SP, Linkie DM, Wright L, et al. (April 1989). "Hypothalamic-pituitary-gonadal function in anorexia nervosa and bulimia". Psychiatry Research. 28 (1): 11–24. doi:10.1016/0165-1781(89)90193-5. PMID 2500676. S2CID 39940665.
- ^ a b Støving RK, Chen JW, Glintborg D, Brixen K, Flyvbjerg A, Hørder K, et al. (June 2007). "Bioactive insulin-like growth factor (IGF) I and IGF-binding protein-1 in anorexia nervosa". The Journal of Clinical Endocrinology and Metabolism. 92 (6): 2323–2329. doi:10.1210/jc.2006-1926. PMID 17389700.
- ^ Misra M, Klibanski A (June 2014). "Anorexia nervosa and bone". The Journal of Endocrinology. 221 (3): R163 – R176. doi:10.1530/JOE-14-0039. PMC 4047520. PMID 24898127.
- ^ Kleinman R (1 April 2008). Walker's Pediatric Gastrointestinal Disease. PMPH-USA. ISBN 978-1-55009-364-3. Retrieved 9 April 2015.
- ^ a b Norris ML, Harrison ME, Isserlin L, Robinson A, Feder S, Sampson M (March 2016). "Gastrointestinal complications associated with anorexia nervosa: A systematic review". The International Journal of Eating Disorders. 49 (3): 216–237. doi:10.1002/eat.22462. PMID 26407541.
- ^ Gibson D (12 December 2024). "The Effects of Purging on Oral Health". ACUTE Center for Eating Disorders & Severe Malnutrition. Retrieved 12 February 2025.
- ^ "FACT SHEET: Eating Disorders". College of Dental Hygienists of Ontario. Archived from the original on 13 February 2025. Retrieved 12 February 2025.
- ^ a b c d e f g Sachs KV, Harnke B, Mehler PS, Krantz MJ (March 2016). "Cardiovascular complications of anorexia nervosa: A systematic review". The International Journal of Eating Disorders. 49 (3): 238–248. doi:10.1002/eat.22481. PMID 26710932.
- ^ a b Goldberg SJ, Comerci GD, Feldman L (January 1988). "Cardiac output and regional myocardial contraction in anorexia nervosa". Journal of Adolescent Health Care. 9 (1): 15–21. doi:10.1016/0197-0070(88)90013-7. PMID 3335466.
- ^ Johnson GL, Humphries LL, Shirley PB, Mazzoleni A, Noonan JA (August 1986). "Mitral valve prolapse in patients with anorexia nervosa and bulimia". Archives of Internal Medicine. 146 (8): 1525–1529. doi:10.1001/archinte.1986.00360200083014. PMID 3460535.
- ^ a b Frostad S, Rozakou-Soumalia N, Dârvariu Ş, Foruzesh B, Azkia H, Larsen MP, et al. (May 2022). "BMI at Discharge from Treatment Predicts Relapse in Anorexia Nervosa: A Systematic Scoping Review". Journal of Personalized Medicine. 12 (5): 836. doi:10.3390/jpm12050836. PMC 9144864. PMID 35629258.
- ^ a b Hasan TF, Hasan H (2011). "Anorexia nervosa: a unified neurological perspective". International Journal of Medical Sciences. 8 (8): 679–703. doi:10.7150/ijms.8.679. PMC 3204438. PMID 22135615.
- ^ Striegel-Moore RH, Rosselli F, Perrin N, DeBar L, Wilson GT, May A, et al. (July 2009). "Gender difference in the prevalence of eating disorder symptoms". The International Journal of Eating Disorders. 42 (5): 471–474. doi:10.1002/eat.20625. PMC 2696560. PMID 19107833.
- ^ Tarchi L, Stanghellini G, Ricca V, Castellini G (13 January 2024). "The primacy of ocular perception: a narrative review on the role of gender identity in eating disorders". Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity. 29 (1): 8. doi:10.1007/s40519-023-01632-6. ISSN 1590-1262. PMC 10787908. PMID 38217553.
- ^ Jahrami H, Sater M, Abdulla A, Faris MA, AlAnsari A (June 2019). "Eating disorders risk among medical students: a global systematic review and meta-analysis". Eating and Weight Disorders. 24 (3): 397–410. doi:10.1007/s40519-018-0516-z. PMID 29785631. S2CID 29156560.
- ^ Zanetti T (2013). "Epidemiology of Eating Disorders". Eating Disorders and the Skin. pp. 9–15. doi:10.1007/978-3-642-29136-4_2. ISBN 978-3-642-29135-7.
- ^ Garber AK, Cheng J, Accurso EC, Adams SH, Buckelew SM, Kapphahn CJ, et al. (December 2019). "Weight Loss and Illness Severity in Adolescents With Atypical Anorexia Nervosa". Pediatrics. 144 (6). doi:10.1542/peds.2019-2339. PMC 6889949. PMID 31694978.
- ^ Makino M, Tsuboi K, Dennerstein L (September 2004). "Prevalence of eating disorders: a comparison of Western and non-Western countries". MedGenMed. 6 (3): 49. PMC 1435625. PMID 15520673.
- ^ Tsai G (December 2000). "Eating disorders in the Far East". Eating and Weight Disorders. 5 (4): 183–197. doi:10.1007/BF03354445. PMID 11216126. S2CID 41396054.
- ^ a b Bonci CM, Bonci LJ, Granger LR, Johnson CL, Malina RM, Milne LW, et al. (2008). "National athletic trainers' association position statement: preventing, detecting, and managing disordered eating in athletes". Journal of Athletic Training. 43 (1): 80–108. doi:10.4085/1062-6050-43.1.80. PMC 2231403. PMID 18335017.
- ^ Thiel A, Gottfried H, Hesse FW (October 1993). "Subclinical eating disorders in male athletes. A study of the low weight category in rowers and wrestlers". Acta Psychiatrica Scandinavica. 88 (4): 259–265. doi:10.1111/j.1600-0447.1993.tb03454.x. PMID 8256643. S2CID 10232724.
- ^ "Male Anorexia: Understanding Eating Disorders in Men and Boys". Psych Central. 20 May 2022. Retrieved 29 February 2024.
- ^ Striegel-Moore RH, Rosselli F, Perrin N, DeBar L, Wilson GT, May A, et al. (July 2009). "Gender difference in the prevalence of eating disorder symptoms". The International Journal of Eating Disorders. 42 (5): 471–474. doi:10.1002/eat.20625. PMC 2696560. PMID 19107833.
- ^ Thomas JJ, Vartanian LR, Brownell KD (May 2009). "The relationship between eating disorder not otherwise specified (EDNOS) and officially recognized eating disorders: meta-analysis and implications for DSM". Psychological Bulletin. 135 (3): 407–433. doi:10.1037/a0015326. PMC 2847852. PMID 19379023.
- ^ Wooldridge T (17 September 2012). "An Overview of Anorexia Nervosa in Males". Eating Disorders: The Journal of Treatment and Prevention. 20 (5): 368–378. doi:10.1080/10640266.2012.715515. PMID 22985234.
- ^ Crilly L (2 April 2012). Hope with Eating Disorders. Hay House, Inc. ISBN 978-1-84850-906-1. Retrieved 9 April 2015.
- ^ a b c d e f g h i Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, et al. (January 2016). "Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments". Nutrients. 8 (2): 69. doi:10.3390/nu8020069. PMC 4772033. PMID 26828516.
- ^ a b c d Ekern B (25 February 2016). "Common Types of Eating Disorders Observed in the Elderly Population". Eating Disorder Hope. Retrieved 12 June 2022.
- ^ a b c d Donini LM, Poggiogalle E, Piredda M, Pinto A, Barbagallo M, Cucinotta D, et al. (2 May 2013). "Anorexia and eating patterns in the elderly". PLOS ONE. 8 (5): e63539. Bibcode:2013PLoSO...863539D. doi:10.1371/journal.pone.0063539. PMC 3642105. PMID 23658838.
- ^ a b Lapid MI, Prom MC, Burton MC, McAlpine DE, Sutor B, Rummans TA (June 2010). "Eating disorders in the elderly". International Psychogeriatrics. 22 (4): 523–536. doi:10.1017/S1041610210000104. PMID 20170590. S2CID 38114971.
- ^ a b c d Morley JE, Silver AJ (1 January 1988). "Anorexia in the elderly". Neurobiology of Aging. 9 (1): 9–16. doi:10.1016/S0197-4580(88)80004-6. PMID 2898107. S2CID 3099767.
- ^ Lau S, Pek K, Chew J, Lim JP, Ismail NH, Ding YY, et al. (September 2020). "The Simplified Nutritional Appetite Questionnaire (SNAQ) as a Screening Tool for Risk of Malnutrition: Optimal Cutoff, Factor Structure, and Validation in Healthy Community-Dwelling Older Adults". Nutrients. 12 (9): 2885. doi:10.3390/nu12092885. PMC 7551805. PMID 32967354.
- ^ "Functional Assessment of Anorexia/Cachexia Treatment (FAACT)". FACIT.org.
- ^ Devriendt E, Wellens NI, Flamaing J, Declercq A, Moons P, Boonen S, et al. (September 2013). "The interRAI Acute Care instrument incorporated in an eHealth system for standardized and web-based geriatric assessment: strengths, weaknesses, opportunities and threats in the acute hospital setting". BMC Geriatrics. 13: 90. doi:10.1186/1471-2318-13-90. PMC 3766642. PMID 24007312.
- ^ a b Pearce JM (2004). "Richard Morton: origins of anorexia nervosa". European Neurology. 52 (4): 191–192. doi:10.1159/000082033. PMID 15539770. S2CID 30482453.
- ^ Espi Forcen F (April 2013). "Anorexia mirabilis: the practice of fasting by Saint Catherine of Siena in the late Middle Ages". The American Journal of Psychiatry. 170 (4): 370–371. doi:10.1176/appi.ajp.2012.12111457. PMID 23545792.
- ^ Harris JC (November 2014). "Anorexia nervosa and anorexia mirabilis: Miss K. R--and St Catherine Of Siena". JAMA Psychiatry. 71 (11): 1212–1213. doi:10.1001/jamapsychiatry.2013.2765. PMID 25372187.
- ^ King NA, Burley VJ, Blundell JE (October 1994). "Exercise-induced suppression of appetite: effects on food intake and implications for energy balance". European Journal of Clinical Nutrition. 48 (10). United States government: 715–724. PMID 7835326.
Subjective feelings of hunger were significantly suppressed during and after intense exercise sessions (P 0.01), but the suppression was short-lived.
- ^ Grant JE, Phillips KA (2004). "Is anorexia nervosa a subtype of body dysmorphic disorder? Probably not, but read on". Harvard Review of Psychiatry. 12 (2): 123–126. doi:10.1080/10673220490447236. PMC 1622894. PMID 15204807.
- ^ Gull WW (September 1997). "Anorexia nervosa (apepsia hysterica, anorexia hysterica). 1868". Obesity Research. 5 (5): 498–502. doi:10.1002/j.1550-8528.1997.tb00677.x. PMID 9385628.
- ^ Gull WW (1894). Acland TD (ed.). Medical Papers. p. 309.
- ^ Lasègue E (6 September 1873). "On Hysterical Anorexia". Medical Times and Gazette. See also "On hysterical anorexia (a). 1873". Obesity Research. 5 (5): 492–497. September 1997. doi:10.1002/j.1550-8528.1997.tb00676.x. PMID 9385627.
- ^ Arnold C (2012). Decoding Anorexia: How Breakthroughs in Science Offer Hope for Eating Disorders. Routledge Press. ISBN 978-0-415-89867-6.
- ^ Arnold C (29 March 2016). "Anorexia: you don't just grow out of it". The Guardian. Archived from the original on 29 March 2016. Retrieved 29 March 2016.
Further reading
[edit]- Bailey AP, Parker AG, Colautti LA, Hart LM, Liu P, Hetrick SE (2014). "Mapping the evidence for the prevention and treatment of eating disorders in young people". Journal of Eating Disorders. 2 (1): 5. doi:10.1186/2050-2974-2-5. PMC 4081733. PMID 24999427.
- Coelho GM, Gomes AI, Ribeiro BG, Soares E (2014). "Prevention of eating disorders in female athletes". Open Access Journal of Sports Medicine. 5: 105–113. doi:10.2147/OAJSM.S36528. PMC 4026548. PMID 24891817.
- Luca A, Luca M, Calandra C (February 2015). "Eating Disorders in Late-life". Aging and Disease. 6 (1): 48–55. doi:10.14336/AD.2014.0124. PMC 4306473. PMID 25657852.
 KSF
KSF