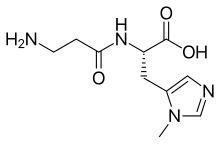
Anserine
 From Wikipedia - Reading time: 5 min
From Wikipedia - Reading time: 5 min
| |
| Names | |
|---|---|
| Systematic IUPAC name
(Z)-N-(3-Amino-1-hydroxypropylidene)-3-methyl-L-histidine | |
| Other names
beta-Alanyl-3-methyl-L-histidine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.679 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H16N4O3 | |
| Molar mass | 240.25904 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Anserine (β-alanyl-3-methylhistidine) is a dipeptide containing β-alanine and 3-methylhistidine.[1] Anserine is a derivative of carnosine, which has been methylated.[2]
Both anserine and carnosine chelate copper.[3] Due to its methylation, anserine is more stable in serum and resistant to degradation than carnosine.[4] Anserine can be found in the skeletal muscle and brain of mammals and birds.[2]
The pKa of the imidazole ring of histidine, when contained in anserine, is 7.04.[5][6]
Neuroprotective effects
[edit]An animal model study of Alzheimer's disease using mice found that treatment with anserine reduced memory loss.[7] Anserine reduced glial inflammatory activity (particularly of astrocyte).[6] The study also found that anserine-treated mice had greater pericyte surface area.[6] The greater area of pericytes was commensurate with improved memory (pericytes wrap around brain capillary to control blood flow and gate cells from neurotoxin, blocking inflammation).[6] The anserine-treated mice overall performed better on a spatial memory test (Morris Water Maze).[6]
A human study on 84 elderly subjects showed that subjects who took anserine and carnosine supplements for one year showed increased blood flow in the prefrontal cortex on MRI.[7]
A study demonstrated that the free N-terminal of histidine on anserine and carnosine protect against zinc-caused neurotoxicity and regulate the Arc pathway in which Arc protein is used to produce dendrite protein for connecting nerve cells.[8]
Both Anserine and Carnosine are chelating agents for copper and other transition metals.[9] Chelation of transition metals is one method used by antioxidants to protect their targets from oxidative stress as it prevents them from undergoing Fenton reactions with peroxides. In the olfactory bulbs, the concentration of both of these molecules was found to be in the millimolar range, whereas the concentration of copper was approximately 50μM. these found concentrations indicate the chelation of copper by Anserine and Carnosine.[3]
See also
[edit]References
[edit]- ^ Garrett CM, Grisham RH (2012). Biochemistry (5th ed.). Cengage Learning. p. 46. ISBN 978-1-133-10629-6.
- ^ a b Blancquaert L, Baba SP, Kwiatkowski S, Stautemas J, Stegen S, Barbaresi S, Chung W, Boakye AA, Hoetker JD, Bhatnagar A, Delanghe J (2016-09-01). "Carnosine and anserine homeostasis in skeletal muscle and heart is controlled by β-alanine transamination". The Journal of Physiology. 594 (17): 4849–4863. doi:10.1113/JP272050. ISSN 1469-7793. PMC 5009790. PMID 27062388.
- ^ a b Kohen R, Yamamoto Y, Cundy KC, Ames BN (1988). "Antioxidant Activity of Carnosine, Homocarnosine, and Anserine Present in Muscle and Brain". Proceedings of the National Academy of Sciences of the United States of America. 85 (9): 3175–3179. Bibcode:1988PNAS...85.3175K. doi:10.1073/pnas.85.9.3175. ISSN 0027-8424. JSTOR 31967. PMC 280166. PMID 3362866.
- ^ Everaert I, Baron G, Barbaresi S, Gilardoni E, Coppa C, Carini M, Vistoli G, Bex T, Stautemas J, Blancquaert L, Derave W (January 2019). "Development and validation of a sensitive LC-MS/MS assay for the quantification of anserine in human plasma and urine and its application to pharmacokinetic study". Amino Acids. 51 (1): 103–114. doi:10.1007/s00726-018-2663-y. hdl:2434/599742. ISSN 1438-2199. PMID 30302566. S2CID 52945820.
- ^ Wu G (March 2020). "Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health". Amino Acids. 52 (3): 329–360. doi:10.1007/s00726-020-02823-6. ISSN 1438-2199. PMC 7088015. PMID 32072297.
- ^ a b c d e Kaneko J, Enya A, Enomoto K, Ding Q, Hisatsune T (October 2017). "Anserine (beta-alanyl-3-methyl-L-histidine) improves neurovascular-unit dysfunction and spatial memory in aged AβPPswe/PSEN1dE9 Alzheimer's-model mice". Scientific Reports. 7 (1): 12571. Bibcode:2017NatSR...712571K. doi:10.1038/s41598-017-12785-7. PMC 5626714. PMID 28974740.
- ^ a b Ding Q, Tanigawa K, Kaneko J, Totsuka M, Katakura Y, Imabayashi E, et al. (June 2018). "Anserine/Carnosine Supplementation Preserves Blood Flow in the Prefrontal Brain of Elderly People Carrying APOE e4". Aging and Disease. 9 (3): 334–345. doi:10.14336/ad.2017.0809. PMC 5988590. PMID 29896423.
- ^ Ding Q, Tanigawa K, Kaneko J, Totsuka M, Katakura Y, Imabayashi E, et al. (June 2018). "Anserine/Carnosine Supplementation Preserves Blood Flow in the Prefrontal Brain of Elderly People Carrying APOE e4". Aging and Disease. 9 (3): 334–345. doi:10.14336/ad.2017.0809. PMC 5988590. PMID 29896423.
- ^ Kohen R, Misgav R, Ginsburg I (1991). "The SOD like activity of copper:carnosine, copper:anserine and copper:homocarnosine complexes". Free Radical Research Communications. 12-13 Pt 1: 179–185. doi:10.3109/10715769109145784. ISSN 8755-0199. PMID 1649087.
 KSF
KSF