Archaea
 From Wikipedia - Reading time: 60 min
From Wikipedia - Reading time: 60 min
| Archaea Temporal range: Paleoarchean–Present
| |||
|---|---|---|---|

| |||
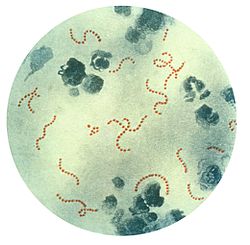
| Composite image to illustrate the morphological diversity of Archaea. Left column, from top to bottom: Methanosarcina barkeri; Ignicoccus hospitalis with two smaller Nanoarchaeum equitans; an Archaeal Richmond Mine acidophilic nanoorganism (ARMAN); Haloquadratum walsbyi. Right column, from top to bottom: Methanohalophilus mahii; an artist's rendering of Pyrococcus furiosus; a model of Promethearchaeum syntrophicum; Halobacterium species NRC-1. | |||
| Scientific classification | |||
| Domain: | Archaea Woese et al. 2024[1] | ||
| Type genus | |||
| Methanobacterium Kluyver and van Niel 1936 (Approved Lists 1980)[3]
| |||
| Kingdoms[1][3] | |||
And see text
| |||
| Synonyms | |||
| |||
Archaea (/ɑːrˈkiːə/ ⓘ ar-KEE-ə) is a domain of organisms. Traditionally, Archaea included only its prokaryotic members, but has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even though the domain Archaea cladistically includes eukaryotes, the term "archaea" (sg.: archaeon /ɑːrˈkiːɒn/ ar-KEE-on, from the Greek "ἀρχαῖον", which means ancient) in English still generally refers specifically to prokaryotic members of Archaea. Archaea were initially classified as bacteria, receiving the name archaebacteria (/ˌɑːrkibækˈtɪəriə/, in the Archaebacteria kingdom), but this term has fallen out of use.[4] Archaeal cells have unique properties separating them from Bacteria and Eukaryota, including: cell membranes made of ether-linked lipids; metabolisms such as methanogenesis; and a unique motility structure known as an archaellum.[5]Archaea are further divided into multiple recognized phyla. Classification is difficult because most have not been isolated in a laboratory and have been detected only by their gene sequences in environmental samples. It is unknown if they can produce endospores.
Archaea are often similar to bacteria in size and shape, although a few have very different shapes, such as the flat, square cells of Haloquadratum walsbyi.[6] Despite this, archaea possess genes and several metabolic pathways that are more closely related to those of eukaryotes, notably for the enzymes involved in transcription and translation. Other aspects of archaeal biochemistry are unique, such as their reliance on ether lipids in their cell membranes,[7] including archaeols. Archaea use more diverse energy sources than eukaryotes, ranging from organic compounds such as sugars, to ammonia, metal ions or even hydrogen gas. The salt-tolerant Haloarchaea use sunlight as an energy source, and other species of archaea fix carbon (autotrophy), but unlike cyanobacteria, no known species of archaea does both. Archaea reproduce asexually by binary fission, fragmentation, or budding; unlike bacteria, no known species of Archaea form endospores. The first observed archaea were extremophiles, living in extreme environments such as hot springs and salt lakes with no other organisms. Improved molecular detection tools led to the discovery of archaea in almost every habitat, including soil,[8] oceans, and marshlands. Archaea are particularly numerous in the oceans, and the archaea in plankton may be one of the most abundant groups of organisms on the planet.
Archaea are a major part of Earth's life. They are part of the microbiota of all organisms. In the human microbiome, they are important in the gut, mouth, and on the skin.[9] Their morphological, metabolic, and geographical diversity permits them to play multiple ecological roles: carbon fixation; nitrogen cycling; organic compound turnover; and maintaining microbial symbiotic and syntrophic communities, for example.[8][10] No archaea are known to be pathogens or parasites; many are mutualists or commensals, such as the methanogens (methane-producers) that inhabit the gastrointestinal tract in humans and ruminants, where their vast numbers facilitate digestion. Methanogens are used in biogas production and sewage treatment, while biotechnology exploits enzymes from extremophile archaea that can endure high temperatures and organic solvents.
Discovery and classification
[edit]
Early concept
[edit]
For much of the 20th century, prokaryotes were regarded as a single group of organisms and classified based on their biochemistry, morphology and metabolism. Microbiologists tried to classify microorganisms based on the structures of their cell walls, their shapes, and the substances they consume.[11] In 1965, Emile Zuckerkandl and Linus Pauling[12] instead proposed using the sequences of the genes in different prokaryotes to work out how they are related to each other. This phylogenetic approach is the main method used today.[13]
Archaea were first classified separately from bacteria in 1977 by Carl Woese and George E. Fox, based on their ribosomal RNA (rRNA) genes.[14] (At that time only the methanogens were known). They called these groups the Urkingdoms of Archaebacteria and Eubacteria, though other researchers treated them as kingdoms or subkingdoms. Woese and Fox gave the first evidence for Archaebacteria as a separate "line of descent": 1. lack of peptidoglycan in their cell walls, 2. two unusual coenzymes, 3. results of 16S ribosomal RNA gene sequencing. To emphasize this difference, Woese, Otto Kandler and Mark Wheelis later proposed reclassifying organisms into three then thought to be natural domains known as the three-domain system: the Eukarya, the Bacteria and the Archaea,[15] in what is now known as the Woesian Revolution.[16]
The word archaea comes from the Ancient Greek ἀρχαῖα, meaning "ancient things",[17] as the first representatives of the domain Archaea were methanogens and it was assumed that their metabolism reflected Earth's primitive atmosphere and the organisms' antiquity, but as new habitats were studied, more organisms were discovered. Extreme halophilic[18] and hyperthermophilic microbes[19] were also included in Archaea. For a long time, archaea were seen as extremophiles that exist only in extreme habitats such as hot springs and salt lakes, but by the end of the 20th century, archaea had been identified in non-extreme environments as well. Today, they are known to be a large and diverse group of organisms abundantly distributed throughout nature.[20] This new appreciation of the importance and ubiquity of archaea came from using polymerase chain reaction (PCR) to detect prokaryotes from environmental samples (such as water or soil) by multiplying their ribosomal genes. This allows the detection and identification of organisms that have not been cultured in the laboratory.[21][22]
Classification
[edit]
The classification of archaea, and of prokaryotes in general, is a rapidly moving and contentious field. Current classification systems aim to organize archaea into groups of organisms that share structural features and common ancestors.[23] These classifications rely heavily on the use of the sequence of ribosomal RNA genes to reveal relationships among organisms (molecular phylogenetics).[24] Most of the culturable and well-investigated species of archaea are members of two main kingdoms, the Methanobacteriati and the Thermoproteati (formerly TACK). Other groups have been tentatively created, such as the peculiar species Nanoarchaeum equitans — discovered in 2003 and assigned its own phylum, the Nanoarchaeota (reassigned to Nanobdellota in 2023[25]).[26] A new phylum "Candidatus Korarchaeota" (now Thermoproteota)[3] has also been proposed, containing a small group of unusual thermophilic species sharing features of both the main phyla.[27][28] Other detected species of archaea are only distantly related to any of these groups, such as the Archaeal Richmond Mine acidophilic nanoorganisms (ARMAN, comprising Micrarchaeota and Parvarchaeota), which were discovered in 2006[29] and are some of the smallest organisms known.[30]
A superphylum – "TACK" (now kingdom Thermoproteati)– which includes the Thaumarchaeota (now Nitrososphaerota), "Augarchaeota", Crenarchaeota (now Thermoproteota), and "Candidatus Korarchaeota" (now Thermoproteota)[3] was proposed in 2011 to be related to the origin of eukaryotes.[31] In 2017, the newly discovered and newly named "Asgard" (now kingdom Promethearchaeati) superphylum was proposed to be more closely related to the original eukaryote and a sister group to Thermoproteati / "TACK".[32]
In 2013, the superphylum "DPANN" (now kingdom Nanobdellati) was proposed to group "Nanoarchaeota", "Nanohaloarchaeota", Archaeal Richmond Mine acidophilic nanoorganisms (ARMAN, comprising "Micrarchaeota" and "Parvarchaeota"), and other similar archaea. This archaeal superphylum encompasses at least 10 different lineages and includes organisms with extremely small cell and genome sizes and limited metabolic capabilities. Therefore, Nanobdellati/"DPANN" may include members obligately dependent on symbiotic interactions, and may even include novel parasites. However, other phylogenetic analyses found that Nanobdellati/"DPANN" does not form a monophyletic group, and that the apparent grouping is caused by long branch attraction (LBA), suggesting that all these lineages belong to Methanobacteriati.[33][34]
Phylogeny
[edit]According to Tom A. Williams et al. 2017,[35] Castelle & Banfield (2018)[36] and GTDB release 10-RS226 (16th April 2025).[37][38][39]
| Tom A. Williams et al. 2017[35] and Castelle & Banfield 2018[36] | 10-RS226 (16th April 2025)[37][38][39] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Concept of species
[edit]The classification of archaea into species is also controversial. Ernst Mayr's species definition — a reproductively isolated group of interbreeding organisms — does not apply, as archaea reproduce only asexually.[41]
Archaea show high levels of horizontal gene transfer between lineages. Some researchers suggest that individuals can be grouped into species-like populations given highly similar genomes and infrequent gene transfer to/from cells with less-related genomes, as in the genus Ferroplasma.[42] On the other hand, studies in Halorubrum found significant genetic transfer to/from less-related populations, limiting the criterion's applicability.[43] Some researchers question whether such species designations have practical meaning.[44]
Current knowledge on genetic diversity in archaeans is fragmentary, so the total number of species cannot be estimated with any accuracy.[24] Estimates of the number of phyla range from 18 to 23, of which only 8 have representatives that have been cultured and studied directly. Many of these hypothesized groups are known from a single rRNA sequence, so the level of diversity remains obscure.[45] This situation is also seen in the Bacteria; many uncultured microbes present similar issues with characterization.[46]
Prokaryotic phyla
[edit]Valid phyla
[edit]The following phyla have been validly published according to the Prokaryotic Code; belonging to the four kingdoms of archaea:[47][48][3]
Candidate phyla
[edit]The following phyla have been proposed, but have not been validly published according to the Prokaryotic Code; phyla that do not belong to any kingdom are shown in bold:[49][3]
- "Ca. Aenigmatarchaeota"
- "Ca. Altarchaeota"
- "Ca. Augarchaeota"
- "Ca. Geoarchaeota"
- "Ca. Hadarchaeota"
- "Ca. Hadesarchaeota"
- "Ca. Huberarchaeota"
- "Ca. Hydrothermarchaeota"
- "Ca. Iainarchaeota"
- "Ca. Micrarchaeota"
- "Ca. Nanohalarchaeota"
- "Ca. Nezhaarchaeota"
- "Ca. Parvarchaeota"
- "Ca. Poseidoniota"
- "Ca. Undinarchaeota"
Origin and evolution
[edit]The age of the Earth is about 4.54 billion years.[50][51][52] Scientific evidence suggests that life began on Earth at least 3.5 billion years ago.[53][54] The earliest evidence for life on Earth is graphite found to be biogenic in 3.7-billion-year-old metasedimentary rocks discovered in Western Greenland[55] and microbial mat fossils found in 3.48-billion-year-old sandstone discovered in Western Australia.[56][57] In 2015, possible remains of biotic matter were found in 4.1-billion-year-old rocks in Western Australia.[58][59]
Although probable prokaryotic cell fossils date to almost 3.5 billion years ago, most prokaryotes do not have distinctive morphologies, and fossil shapes cannot be used to identify them as archaea.[60] Instead, chemical fossils of unique lipids are more informative because such compounds do not occur in other organisms.[61] Some publications suggest that archaeal or eukaryotic lipid remains are present in shales dating from 2.7 billion years ago,[62] though such data have since been questioned.[63] These lipids have also been detected in even older rocks from west Greenland. The oldest such traces come from the Isua district, which includes Earth's oldest known sediments, formed 3.8 billion years ago.[64] The archaeal lineage may be the most ancient that exists on Earth.[65]
Woese argued that the bacteria, archaea, and eukaryotes represent separate lines of descent that diverged early on from an ancestral colony of organisms.[66][67] One possibility[67][68] is that this occurred before the evolution of cells, when the lack of a typical cell membrane allowed unrestricted lateral gene transfer, and that the common ancestors of the three domains arose by fixation of specific subsets of genes.[67][68] It is possible that the last common ancestor of bacteria and archaea was a thermophile, which raises the possibility that lower temperatures are "extreme environments" for archaea, and organisms that live in cooler environments appeared only later.[69] Since archaea and bacteria are no more related to each other than they are to eukaryotes, the term prokaryote may suggest a false similarity between them.[70] However, structural and functional similarities between lineages often occur because of shared ancestral traits or evolutionary convergence. These similarities are known as a grade, and prokaryotes are best thought of as a grade of life, characterized by such features as an absence of membrane-bound organelles.
Comparison with other domains
[edit]The following table compares some major characteristics of the three domains, to illustrate their similarities and differences.[71]
| Property | Archaea | Bacteria | Eukaryota |
|---|---|---|---|
| Cell membrane | Ether-linked lipids | Ester-linked lipids | Ester-linked lipids |
| Cell wall | Glycoprotein, or S-layer; rarely pseudopeptidoglycan | Peptidoglycan, S-layer, or no cell wall | Various structures |
| Gene structure | Circular chromosomes, similar translation and transcription to Eukaryota | Circular chromosomes, unique translation and transcription | Multiple, linear chromosomes, but translation and transcription similar to Archaea |
| Internal cell structure | No membrane-bound organelles (?[72]) or nucleus | No membrane-bound organelles or nucleus | Membrane-bound organelles and nucleus |
| Metabolism[73] | Various, including diazotrophy, with methanogenesis unique to Archaea | Various, including photosynthesis, aerobic and anaerobic respiration, fermentation, diazotrophy, and autotrophy | Photosynthesis, cellular respiration, and fermentation; no diazotrophy |
| Reproduction | Asexual reproduction, horizontal gene transfer | Asexual reproduction, horizontal gene transfer | Sexual and asexual reproduction |
| Protein synthesis initiation | Methionine | Formylmethionine | Methionine |
| RNA polymerase | One | One | Many |
| EF-2/EF-G | Sensitive to diphtheria toxin | Resistant to diphtheria toxin | Sensitive to diphtheria toxin |
Archaea were split off as a third domain because of the large differences in their ribosomal RNA structure. The particular molecule 16S rRNA is key to the production of proteins in all organisms. Because this function is so central to life, organisms with mutations in their 16S rRNA are unlikely to survive, leading to great (but not absolute) stability in the structure of this polynucleotide over generations. 16S rRNA is large enough to show organism-specific variations, but still small enough to be compared quickly. In 1977, Carl Woese, a microbiologist studying the genetic sequences of organisms, developed a new comparison method that involved splitting the RNA into fragments that could be sorted and compared with other fragments from other organisms.[14] The more similar the patterns between species, the more closely they are related.[74]
Woese used his new rRNA comparison method to categorize and contrast different organisms. He compared a variety of species and happened upon a group of methanogens with rRNA vastly different from any known prokaryotes or eukaryotes.[14] These methanogens were much more similar to each other than to other organisms, leading Woese to propose the new domain of Archaea.[14] His experiments showed that the archaea were genetically more similar to eukaryotes than prokaryotes, even though they were more similar to prokaryotes in structure.[75] This led to the conclusion that Archaea and Eukarya shared a common ancestor more recent than Eukarya and Bacteria.[75] The development of the nucleus occurred after the split between Bacteria and this common ancestor.[75][15]
One property unique to archaea is the abundant use of ether-linked lipids in their cell membranes. Ether linkages are more chemically stable than the ester linkages found in bacteria and eukarya, which may be a contributing factor to the ability of many archaea to survive in extreme environments that place heavy stress on cell membranes, such as extreme heat and salinity. Comparative analysis of archaeal genomes has also identified several molecular conserved signature indels and signature proteins uniquely present in either all archaea or different main groups within archaea.[76][77][78] Another unique feature of archaea, found in no other organisms, is methanogenesis (the metabolic production of methane). Methanogenic archaea play a pivotal role in ecosystems with organisms that derive energy from oxidation of methane, many of which are bacteria, as they are often a major source of methane in such environments and can play a role as primary producers. Methanogens also play a critical role in the carbon cycle, breaking down organic carbon into methane, which is also a major greenhouse gas.[79]
This difference in the biochemical structure of Bacteria and Archaea has been explained by researchers through evolutionary processes.[vague] It is theorized that both domains originated at deep sea alkaline hydrothermal vents. At least twice, microbes evolved lipid biosynthesis and cell wall biochemistry. It has been suggested that the last universal common ancestor was a non-free-living organism.[80] It may have had a permeable membrane composed of bacterial simple chain amphiphiles (fatty acids), including archaeal simple chain amphiphiles (isoprenoids). These stabilize fatty acid membranes in seawater; this property may have driven the divergence of bacterial and archaeal membranes, "with the later biosynthesis of phospholipids giving rise to the unique G1P and G3P headgroups of archaea and bacteria respectively. If so, the properties conferred by membrane isoprenoids place the lipid divide as early as the origin of life".[81]
Relationship to bacteria
[edit]
The relationships among the three domains are of central importance for understanding the origin of life. Most of the metabolic pathways, which are the object of the majority of an organism's genes, are common between Archaea and Bacteria, while most genes involved in gene expression are common between Archaea and Eukarya.[83] Within prokaryotes, archaeal cell structure is most similar to that of Gram-positive bacteria, largely because both have a single lipid bilayer[84] and usually contain a thick sacculus (exoskeleton) of varying chemical composition.[85] In some phylogenetic trees based upon different gene / protein sequences of prokaryotic homologs, the archaeal homologs are more closely related to those of gram-positive bacteria.[84] Archaea and gram-positive bacteria also share conserved indels in a number of important proteins, such as Hsp70 and glutamine synthetase I;[84][86] but the phylogeny of these genes was interpreted to reveal inter-domain gene transfer,[87][88] and might not reflect the organismal relationship(s).[89]
It has been proposed that the archaea evolved from Gram-positive bacteria in response to antibiotic selection pressure.[84][86][90] This is suggested by the observation that archaea are resistant to a wide variety of antibiotics that are produced primarily by Gram-positive bacteria,[84][86] and that these antibiotics act primarily on the genes that distinguish archaea from bacteria. The proposal is that the selective pressure towards resistance generated by the gram-positive antibiotics was eventually sufficient to cause extensive changes in many of the antibiotics' target genes, and that these strains represented the common ancestors of present-day Archaea.[90] The evolution of Archaea in response to antibiotic selection, or any other competitive selective pressure, could also explain their adaptation to extreme environments (such as high temperature or acidity) as the result of a search for unoccupied niches to escape from antibiotic-producing organisms;[90][91] Cavalier-Smith has made a similar suggestion, the Neomura hypothesis.[92] This proposal is also supported by other work investigating protein structural relationships[93] and studies that suggest that gram-positive bacteria may constitute the earliest branching lineages within the prokaryotes.[94]
Relation to eukaryotes
[edit]
The evolutionary relationship between archaea and eukaryotes remains unclear. Aside from the similarities in cell structure and function that are discussed below, many genetic trees group the two.[96]
Complicating factors include claims that the relationship between eukaryotes and the archaeal phylum Thermoproteota is closer than the relationship between the Methanobacteriati and the phylum Thermoproteota[97] and the presence of archaea-like genes in certain bacteria, such as Thermotoga maritima, from horizontal gene transfer.[98] The standard hypothesis states that the ancestor of the eukaryotes diverged early from the Archaea,[99][100] and that eukaryotes arose through symbiogenesis, the fusion of an archaean and a eubacterium, which formed the mitochondria; this hypothesis explains the genetic similarities between the groups.[95] The eocyte hypothesis instead posits that Eukaryota emerged relatively late from the Archaea.[101]
A lineage of archaea discovered in 2015, Lokiarchaeum (of the proposed new phylum "Lokiarchaeota"), named for a hydrothermal vent called Loki's Castle in the Arctic Ocean, was found to be the most closely related to eukaryotes known at that time. It has been called a transitional organism between prokaryotes and eukaryotes.[102][103]
Several sister phyla of "Lokiarchaeota" have since been found ("Thorarchaeota", "Odinarchaeota", "Heimdallarchaeota"), all together comprising a newly proposed supergroup "Asgard".[32][104][105]
Details of the relation of Promethearchaeati / "Asgard" members and eukaryotes are still under consideration,[106] although, in January 2020, scientists reported that Promethearchaeum syntrophicum, a type of Promethearchaeati / "Asgard" archaea, may be a possible link between simple prokaryotic and complex eukaryotic microorganisms about two billion years ago.[107][108][109]
Morphology
[edit]Individual archaea range from 0.1 micrometers (μm) to over 15 μm in diameter, and occur in various shapes, commonly as spheres, rods, spirals or plates.[110] Other morphologies in the Thermoproteota include irregularly shaped lobed cells in Sulfolobus, needle-like filaments that are less than half a micrometer in diameter in Thermofilum, and almost perfectly rectangular rods in Thermoproteus and Pyrobaculum.[111] Archaea in the genus Haloquadratum such as Haloquadratum walsbyi are flat, square specimens that live in hypersaline pools.[112] These unusual shapes are probably maintained by both their cell walls and a prokaryotic cytoskeleton. Proteins related to the cytoskeleton components of other organisms exist in archaea,[113] and filaments form within their cells,[114] but in contrast with other organisms, these cellular structures are poorly understood.[115] In Thermoplasma and Ferroplasma the lack of a cell wall means that the cells have irregular shapes, and can resemble amoebae.[116]
Some species form aggregates or filaments of cells up to 200 μm long.[110] These organisms can be prominent in biofilms.[117] Notably, aggregates of Thermococcus coalescens cells fuse together in culture, forming single giant cells.[118] Archaea in the genus Pyrodictium produce an elaborate multicell colony involving arrays of long, thin hollow tubes called cannulae that stick out from the cells' surfaces and connect them into a dense bush-like agglomeration.[119] The function of these cannulae is not settled, but they may allow communication or nutrient exchange with neighbors.[120] Multi-species colonies exist, such as the "string-of-pearls" community that was discovered in 2001 in a German swamp. Round whitish colonies of a novel Methanobacteriati species are spaced along thin filaments that can range up to 15 centimetres (5.9 in) long; these filaments are made of a particular bacteria species.[121]
Structure, composition development, and operation
[edit]Archaea and bacteria have generally similar cell structure, but cell composition and organization set the archaea apart. Like bacteria, archaea lack interior membranes and organelles.[70] Like bacteria, the cell membranes of archaea are usually bounded by a cell wall and they swim using one or more flagella.[122] Structurally, archaea are most similar to gram-positive bacteria. Most have a single plasma membrane and cell wall, and lack a periplasmic space; the exception to this general rule is Ignicoccus, which possess a particularly large periplasm that contains membrane-bound vesicles and is enclosed by an outer membrane.[123]
Cell wall and archaella
[edit]Most archaea (but not Thermoplasma and Ferroplasma) possess a cell wall.[116] In most archaea, the wall is assembled from surface-layer proteins, which form an S-layer.[124] An S-layer is a rigid array of protein molecules that cover the outside of the cell (like chain mail).[125] This layer provides both chemical and physical protection, and can prevent macromolecules from contacting the cell membrane.[126] Unlike bacteria, archaea lack peptidoglycan in their cell walls.[127] Methanobacteriales do have cell walls containing pseudopeptidoglycan, which resembles eubacterial peptidoglycan in morphology, function, and physical structure, but pseudopeptidoglycan is distinct in chemical structure; it lacks D-amino acids and N-acetylmuramic acid, substituting the latter with N-Acetyltalosaminuronic acid.[126]
Archaeal flagella are known as archaella, that operate like bacterial flagella – their long stalks are driven by rotatory motors at the base. These motors are powered by a proton gradient across the membrane, but archaella are notably different in composition and development.[122] The two types of flagella evolved from different ancestors. The bacterial flagellum shares a common ancestor with the type III secretion system,[128][129] while archaeal flagella appear to have evolved from bacterial type IV pili.[130] In contrast with the bacterial flagellum, which is hollow and assembled by subunits moving up the central pore to the tip of the flagella, archaeal flagella are synthesized by adding subunits at the base.[131]
Membranes
[edit]
Archaeal membranes are made of molecules that are distinctly different from those in all other life forms, showing that archaea are related only distantly to bacteria and eukaryotes.[132] In all organisms, cell membranes are made of molecules known as phospholipids. These molecules possess both a polar part that dissolves in water (the phosphate "head"), and a "greasy" non-polar part that does not (the lipid tail). These dissimilar parts are connected by a glycerol moiety. In water, phospholipids cluster, with the heads facing the water and the tails facing away from it. The major structure in cell membranes is a double layer of these phospholipids, which is called a lipid bilayer.[133]
The phospholipids of archaea are unusual in four ways:
- They have membranes composed of glycerol-ether lipids, whereas bacteria and eukaryotes have membranes composed mainly of glycerol-ester lipids.[134] The difference is the type of bond that joins the lipids to the glycerol moiety; the two types are shown in yellow in the figure at the right. In ester lipids, this is an ester bond, whereas in ether lipids this is an ether bond.[135]
- The stereochemistry of the archaeal glycerol moiety is the mirror image of that found in other organisms. The glycerol moiety can occur in two forms that are mirror images of one another, called enantiomers. Just as a right hand does not fit easily into a left-handed glove, enantiomers of one type generally cannot be used or made by enzymes adapted for the other. The archaeal phospholipids are built on a backbone of sn-glycerol-1-phosphate, which is an enantiomer of sn-glycerol-3-phosphate, the phospholipid backbone found in bacteria and eukaryotes. This suggests that archaea use entirely different enzymes for synthesizing phospholipids as compared to bacteria and eukaryotes. Such enzymes developed very early in life's history, indicating an early split from the other two domains.[132]
- Archaeal lipid tails differ from those of other organisms in that they are based upon long isoprenoid chains with multiple side-branches, sometimes with cyclopropane or cyclohexane rings.[136] By contrast, the fatty acids in the membranes of other organisms have straight chains without side branches or rings. Although isoprenoids play an important role in the biochemistry of many organisms, only the archaea use them to make phospholipids. These branched chains may help prevent archaeal membranes from leaking at high temperatures.[137]
- In some archaea, the lipid bilayer is replaced by a monolayer. In effect, the archaea fuse the tails of two phospholipid molecules into a single molecule with two polar heads (a bolaamphiphile); this fusion may make their membranes more rigid and better able to resist harsh environments.[138] For example, the lipids in Ferroplasma are of this type, which is thought to aid this organism's survival in its highly acidic habitat.[139]
Metabolism
[edit]Archaea exhibit a great variety of chemical reactions in their metabolism and use many sources of energy. These reactions are classified into nutritional groups, depending on energy and carbon sources. Some archaea obtain energy from inorganic compounds such as sulfur or ammonia (they are chemotrophs). These include nitrifiers, methanogens and anaerobic methane oxidisers.[140] In these reactions, one compound passes electrons to another (in a redox reaction), releasing energy to fuel the cell's activities. One compound acts as an electron donor and one as an electron acceptor. The energy released is used to generate adenosine triphosphate (ATP) through chemiosmosis, the same basic process that happens in the mitochondrion of eukaryotic cells.[141]
Other groups of archaea use sunlight as a source of energy (they are phototrophs), but oxygen–generating photosynthesis does not occur in any of these organisms.[141] Many basic metabolic pathways are shared among all forms of life; for example, archaea use a modified form of glycolysis (the Entner–Doudoroff pathway) and either a complete or partial citric acid cycle.[142] These similarities to other organisms probably reflect both early origins in the history of life and their high level of efficiency.[143]
| Nutritional type | Source of energy | Source of carbon | Examples |
|---|---|---|---|
| Phototrophs | Sunlight | Organic compounds | Halobacterium |
| Lithotrophs | Inorganic compounds | Organic compounds or carbon fixation | Ferroglobus, Methanobacteria or Pyrolobus |
| Organotrophs | Organic compounds | Organic compounds or carbon fixation | Pyrococcus, Sulfolobus or Methanosarcinales |
Some Methanobacteriati are methanogens (archaea that produce methane as a result of metabolism) living in anaerobic environments, such as swamps. This form of metabolism evolved early, and it is even possible that the first free-living organism was a methanogen.[144] A common reaction involves the use of carbon dioxide as an electron acceptor to oxidize hydrogen. Methanogenesis involves a range of coenzymes that are unique to these archaea, such as coenzyme M and methanofuran.[145] Other organic compounds such as alcohols, acetic acid or formic acid are used as alternative electron acceptors by methanogens. These reactions are common in gut-dwelling archaea. Acetic acid is also broken down into methane and carbon dioxide directly, by acetotrophic archaea. These acetotrophs are archaea in the order Methanosarcinales, and are a major part of the communities of microorganisms that produce biogas.[146]

Other archaea use CO
2 in the atmosphere as a source of carbon, in a process called carbon fixation (they are autotrophs). This process involves either a highly modified form of the Calvin cycle[148] or another metabolic pathway called the 3-hydroxypropionate/ 4-hydroxybutyrate cycle.[149] The Thermoproteota also use the reverse Krebs cycle while the Methanobacteriati also use the reductive acetyl-CoA pathway.[150] Carbon fixation is powered by inorganic energy sources. No known archaea carry out photosynthesis.[151] Archaeal energy sources are extremely diverse, and range from the oxidation of ammonia by the Nitrosopumilales[152][153] to the oxidation of hydrogen sulfide or elemental sulfur by species of Sulfolobus, using either oxygen or metal ions as electron acceptors.[141]
Phototrophic archaea use light to produce chemical energy in the form of ATP. In the Halobacteria, light-activated ion pumps like bacteriorhodopsin and halorhodopsin generate ion gradients by pumping ions out of and into the cell across the plasma membrane. The energy stored in these electrochemical gradients is then converted into ATP by ATP synthase.[110] This process is a form of photophosphorylation. The ability of these light-driven pumps to move ions across membranes depends on light-driven changes in the structure of a retinol cofactor buried in the center of the protein.[154]
Genetics
[edit]Archaea usually have a single circular chromosome,[155] but many euryarchaea have been shown to bear multiple copies of this chromosome.[156] The largest known archaeal genome as of 2002 was 5,751,492 base pairs in Methanosarcina acetivorans.[157] The tiny 490,885 base-pair genome of Nanoarchaeum equitans is one-tenth of this size and the smallest archaeal genome known; it is estimated to contain only 537 protein-encoding genes.[158] Smaller independent pieces of DNA, called plasmids, are also found in archaea. Plasmids may be transferred between cells by physical contact, in a process that may be similar to bacterial conjugation.[159][160]
Archaea are genetically distinct from bacteria and eukaryotes, with up to 15% of the proteins encoded by any one archaeal genome being unique to the domain, although most of these unique genes have no known function.[162] Of the remainder of the unique proteins that have an identified function, most belong to the Methanobacteriati and are involved in methanogenesis. The proteins that archaea, bacteria and eukaryotes share form a common core of cell function, relating mostly to transcription, translation, and nucleotide metabolism.[163] Other characteristic archaeal features are the organization of genes of related function – such as enzymes that catalyze steps in the same metabolic pathway into novel operons, and large differences in tRNA genes and their aminoacyl tRNA synthetases.[163]
Transcription in archaea more closely resembles eukaryotic than bacterial transcription, with the archaeal RNA polymerase being very close to its equivalent in eukaryotes,[155] while archaeal translation shows signs of both bacterial and eukaryotic equivalents.[164] Although archaea have only one type of RNA polymerase, its structure and function in transcription seems to be close to that of the eukaryotic RNA polymerase II, with similar protein assemblies (the general transcription factors) directing the binding of the RNA polymerase to a gene's promoter,[165] but other archaeal transcription factors are closer to those found in bacteria.[166] Post-transcriptional modification is simpler than in eukaryotes, since most archaeal genes lack introns, although there are many introns in their transfer RNA and ribosomal RNA genes,[167] and introns may occur in a few protein-encoding genes.[168][169]
Gene transfer and genetic exchange
[edit]Haloferax volcanii, an extreme halophilic archaeon, forms cytoplasmic bridges between cells that appear to be used for transfer of DNA from one cell to another in either direction.[170]
When the hyperthermophilic archaea Sulfolobus solfataricus[171] and Sulfolobus acidocaldarius[172] are exposed to DNA-damaging UV irradiation or to the agents bleomycin or mitomycin C, species-specific cellular aggregation is induced. Aggregation in S. solfataricus could not be induced by other physical stressors, such as pH or temperature shift,[171] suggesting that aggregation is induced specifically by DNA damage. Ajon et al.[172] showed that UV-induced cellular aggregation mediates chromosomal marker exchange with high frequency in S. acidocaldarius. Recombination rates exceeded those of uninduced cultures by up to three orders of magnitude. Frols et al.[171][173] and Ajon et al.[172] hypothesized that cellular aggregation enhances species-specific DNA transfer between Sulfolobus cells in order to provide increased repair of damaged DNA by means of homologous recombination. This response may be a primitive form of sexual interaction similar to the more well-studied bacterial transformation systems that are also associated with species-specific DNA transfer between cells leading to homologous recombinational repair of DNA damage.[174]
Archaeal viruses
[edit]Archaea are the target of a number of viruses in a diverse virosphere distinct from bacterial and eukaryotic viruses. They have been organized into 15–18 DNA-based families so far, but multiple species remain un-isolated and await classification.[175][176][177] These families can be informally divided into two groups: archaea-specific and cosmopolitan. Archaeal-specific viruses target only archaean species and currently include 12 families. Numerous unique, previously unidentified viral structures have been observed in this group, including: bottle-shaped, spindle-shaped, coil-shaped, and droplet-shaped viruses.[176] While the reproductive cycles and genomic mechanisms of archaea-specific species may be similar to other viruses, they bear unique characteristics that were specifically developed due to the morphology of host cells they infect.[175] Their virus release mechanisms differ from that of other phages. Bacteriophages generally undergo either lytic pathways, lysogenic pathways, or (rarely) a mix of the two.[178] Most archaea-specific viral strains maintain a stable, somewhat lysogenic, relationship with their hosts – appearing as a chronic infection. This involves the gradual, and continuous, production and release of virions without killing the host cell.[179] Prangishyili (2013) noted that it has been hypothesized that tailed archaeal phages originated from bacteriophages capable of infecting haloarchaeal species. If the hypothesis is correct, it can be concluded that other double-stranded DNA viruses that make up the rest of the archaea-specific group are their own unique group in the global viral community. Krupovic et al. (2018) states that the high levels of horizontal gene transfer, rapid mutation rates in viral genomes, and lack of universal gene sequences have led researchers to perceive the evolutionary pathway of archaeal viruses as a network. The lack of similarities among phylogenetic markers in this network and the global virosphere, as well as external linkages to non-viral elements, may suggest that some species of archaea specific viruses evolved from non-viral mobile genetic elements (MGE).[176]
These viruses have been studied in most detail in thermophilics, particularly the orders Sulfolobales and Thermoproteales.[180] Two groups of single-stranded DNA viruses that infect archaea have been recently isolated. One group is exemplified by the Halorubrum pleomorphic virus 1 (Pleolipoviridae) infecting halophilic archaea,[181] and the other one by the Aeropyrum coil-shaped virus (Spiraviridae) infecting a hyperthermophilic (optimal growth at 90–95 °C) host.[182] Notably, the latter virus has the largest currently reported ssDNA genome. Defenses against these viruses may involve RNA interference from repetitive DNA sequences that are related to the genes of the viruses.[183][184]
Reproduction
[edit]Archaea reproduce asexually by binary or multiple fission, fragmentation, or budding; mitosis and meiosis do not occur, so if a species of archaea exists in more than one form, all have the same genetic material.[110] Cell division is controlled in a cell cycle; after the cell's chromosome is replicated and the two daughter chromosomes separate, the cell divides.[185] In the genus Sulfolobus, the cycle has characteristics that are similar to both bacterial and eukaryotic systems. The chromosomes replicate from multiple starting points (origins of replication) using DNA polymerases that resemble the equivalent eukaryotic enzymes.[186]
In Methanobacteriati the cell division protein FtsZ, which forms a contracting ring around the cell, and the components of the septum that is constructed across the center of the cell, are similar to their bacterial equivalents.[185] In cren-[187][188] and thaumarchaea,[189] the cell division machinery Cdv fulfills a similar role. This machinery is related to the eukaryotic ESCRT-III machinery which, while best known for its role in cell sorting, also has been seen to fulfill a role in separation between divided cell, suggesting an ancestral role in cell division.[190]
Both bacteria and eukaryotes, but not archaea, make spores.[191] Some species of Haloarchaea undergo phenotypic switching and grow as several different cell types, including thick-walled structures that are resistant to osmotic shock and allow the archaea to survive in water at low salt concentrations, but these are not reproductive structures and may instead help them reach new habitats.[192]
Behavior
[edit]Communication
[edit]Quorum sensing was originally thought to not exist in Archaea, but recent studies have shown evidence of some species being able to perform cross-talk through quorum sensing. Other studies have shown syntrophic interactions between archaea and bacteria during biofilm growth. Although research is limited in archaeal quorum sensing, some studies have uncovered LuxR proteins in archaeal species, displaying similarities with bacteria LuxR, and ultimately allowing for the detection of small molecules that are used in high density communication. Similarly to bacteria, Archaea LuxR solos have shown to bind to AHLs (lactones) and non-AHLs ligans, which is a large part in performing intraspecies, interspecies, and interkingdom communication through quorum sensing.[193]
Ecology
[edit]Habitats
[edit]
Archaea exist in a broad range of habitats, are recognized as a major part of global ecosystems,[20] and may represent about 20% of microbial cells in the oceans.[194] However, the first-discovered archaeans were extremophiles.[140] Indeed, some archaea survive high temperatures, often above 100 °C (212 °F), as found in geysers, black smokers, and oil wells. Other common habitats include very cold habitats and highly saline, acidic, or alkaline water, but archaea include mesophiles that grow in mild conditions, in swamps and marshland, sewage, the oceans, the intestinal tract of animals, and soils.[8][20] Similar to PGPR, Archaea are considered a source of plant growth promotion as well.[8]
Extremophile archaea are members of four main physiological groups. These are the halophiles, thermophiles, alkaliphiles, and acidophiles.[195] These groups are not comprehensive or phylum-specific, nor are they mutually exclusive, since some archaea belong to several groups. Nonetheless, they are a useful starting point for classification.[196]
Halophiles, including the genus Halobacterium, live in extremely saline environments such as salt lakes and outnumber their bacterial counterparts at salinities greater than 20–25%.[140] Thermophiles grow best at temperatures above 45 °C (113 °F), in places such as hot springs; hyperthermophilic archaea grow optimally at temperatures greater than 80 °C (176 °F).[197] The archaeal Methanopyrus kandleri Strain 116 can even reproduce at 122 °C (252 °F), the highest recorded temperature of any organism.[198]
Other archaea exist in very acidic or alkaline conditions.[195] For example, one of the most extreme archaean acidophiles is Picrophilus torridus, which grows at pH 0, which is equivalent to thriving in 1.2 molar sulfuric acid.[199]
This resistance to extreme environments has made archaea the focus of speculation about the possible properties of extraterrestrial life.[200] Some extremophile habitats are not dissimilar to those on Mars,[201] leading to the suggestion that viable microbes could be transferred between planets in meteorites.[202]
Recently, several studies have shown that archaea exist not only in mesophilic and thermophilic environments but are also present, sometimes in high numbers, at low temperatures as well. For example, archaea are common in cold oceanic environments such as polar seas.[203] Even more significant are the large numbers of archaea found throughout the world's oceans in non-extreme habitats among the plankton community (as part of the picoplankton).[204] Although these archaea can be present in extremely high numbers (up to 40% of the microbial biomass), almost none of these species have been isolated and studied in pure culture.[205] Consequently, our understanding of the role of archaea in ocean ecology is rudimentary, so their full influence on global biogeochemical cycles remains largely unexplored.[206] Some marine Thermoproteota are capable of nitrification, suggesting these organisms may affect the oceanic nitrogen cycle,[152] although these oceanic Thermoproteota may also use other sources of energy.[207]
Vast numbers of archaea are also found in the sediments that cover the sea floor, with these organisms making up the majority of living cells at depths over 1 meter below the ocean bottom.[208][209] It has been demonstrated that in all oceanic surface sediments (from 1,000- to 10,000-m water depth), the impact of viral infection is higher on archaea than on bacteria and virus-induced lysis of archaea accounts for up to one-third of the total microbial biomass killed, resulting in the release of ~0.3 to 0.5 gigatons of carbon per year globally.[210]
Role in chemical cycling
[edit]Archaea recycle elements such as carbon, nitrogen, and sulfur through their various habitats.[211] Archaea carry out many steps in the nitrogen cycle. This includes both reactions that remove nitrogen from ecosystems (such as nitrate-based respiration and denitrification) as well as processes that introduce nitrogen (such as nitrate assimilation and nitrogen fixation).[212][213]
Researchers recently discovered archaeal involvement in ammonia oxidation reactions. These reactions are particularly important in the oceans.[153][214] The archaea also appear crucial for ammonia oxidation in soils. They produce nitrite, which other microbes then oxidize to nitrate. Plants and other organisms consume the latter.[215]
In the sulfur cycle, archaea that grow by oxidizing sulfur compounds release this element from rocks, making it available to other organisms, but the archaea that do this, such as Sulfolobus, produce sulfuric acid as a waste product, and the growth of these organisms in abandoned mines can contribute to acid mine drainage and other environmental damage.[216]
In the carbon cycle, methanogen archaea remove hydrogen and play an important role in the decay of organic matter by the populations of microorganisms that act as decomposers in anaerobic ecosystems, such as sediments, marshes, and sewage-treatment works.[217]
Interactions with other organisms
[edit]
The well-characterized interactions between archaea and other organisms are either mutual or commensal. There are no clear examples of known archaeal pathogens or parasites,[218][219] but some species of methanogens have been suggested to be involved in infections in the mouth,[220][221] and Nanoarchaeum equitans may be a parasite of another species of archaea, since it only survives and reproduces within the cells of the Crenarchaeon Ignicoccus hospitalis,[158] and appears to offer no benefit to its host.[222]
Mutualism
[edit]Mutualism is an interaction between individuals of different species that results in positive (beneficial) effects on per capita reproduction and/or survival of the interacting populations. One well-understood example of mutualism is the interaction between protozoa and methanogenic archaea in the digestive tracts of animals that digest cellulose, such as ruminants and termites.[223] In these anaerobic environments, protozoa break down plant cellulose to obtain energy. This process releases hydrogen as a waste product, but high levels of hydrogen reduce energy production. When methanogens convert hydrogen to methane, protozoa benefit from more energy.[224]
In anaerobic protozoa, such as Plagiopyla frontata, Trimyema, Heterometopus and Metopus contortus, archaea reside inside the protozoa and consume hydrogen produced in their hydrogenosomes.[225][226][227][228][229] Archaea associate with larger organisms, too. For example, the marine archaean Cenarchaeum symbiosum is an endosymbiont of the sponge Axinella mexicana.[230]
Commensalism
[edit]Some archaea are commensals, benefiting from an association without helping or harming the other organism. For example, the methanogen Methanobrevibacter smithii is by far the most common archaean in the human flora, making up about one in ten of the prokaryotes in the human gut.[231] In termites and in humans, these methanogens may in fact be mutualists, interacting with other microbes in the gut to aid digestion.[232] Archaean communities associate with a range of other organisms, such as on the surface of corals,[233] and in the region of soil that surrounds plant roots (the rhizosphere).[234][235]
Parasitism
[edit]Although Archaea do not have a historical reputation of being pathogens, Archaea are often found with similar genomes to more common pathogens like E. coli,[236] showing metabolic links and evolutionary history with today's pathogens. Archaea have been inconsistently detected in clinical studies because of the lack of categorization of Archaea into more specific species.[237]
Significance in technology and industry
[edit]Extremophile archaea, particularly those resistant either to heat or to extremes of acidity and alkalinity, are a source of enzymes that function under these harsh conditions.[238][239] These enzymes have found many uses. For example, thermostable DNA polymerases, such as the Pfu DNA polymerase from Pyrococcus furiosus, revolutionized molecular biology by allowing the polymerase chain reaction to be used in research as a simple and rapid technique for cloning DNA. In industry, amylases, galactosidases and pullulanases in other species of Pyrococcus that function at over 100 °C (212 °F) allow food processing at high temperatures, such as the production of low lactose milk and whey.[240] Enzymes from these thermophilic archaea also tend to be very stable in organic solvents, allowing their use in environmentally friendly processes in green chemistry that synthesize organic compounds.[239] This stability makes them easier to use in structural biology. Consequently, the counterparts of bacterial or eukaryotic enzymes from extremophile archaea are often used in structural studies.[241]
In contrast with the range of applications of archaean enzymes, the use of the organisms themselves in biotechnology is less developed. Methanogenic archaea are a vital part of sewage treatment, since they are part of the community of microorganisms that carry out anaerobic digestion and produce biogas.[242] In mineral processing, acidophilic archaea display promise for the extraction of metals from ores, including gold, cobalt and copper.[243]
Archaea host a new class of potentially useful antibiotics. A few of these archaeocins have been characterized, but hundreds more are believed to exist, especially within Haloarchaea and Sulfolobus. These compounds differ in structure from bacterial antibiotics, so they may have novel modes of action. In addition, they may allow the creation of new selectable markers for use in archaeal molecular biology.[244]
See also
[edit]- Aerobic methane production
- Earliest known life forms
- List of Archaea genera
- List of sequenced archaeal genomes
- Nuclear localization sequence
- Stirrup protein domain
- The Surprising Archaea (book)
- Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya
- Unique properties of hyperthermophilic archaea
- Branching order of bacterial phyla (Genome Taxonomy Database, 2018)
References
[edit]- ^ a b Göker, Markus; Oren, Aharon (22 January 2024). "Valid publication of names of two domains and seven kingdoms of prokaryotes". International Journal of Systematic and Evolutionary Microbiology. 74 (1). doi:10.1099/ijsem.0.006242. PMID 38252124. – this article represents the valid publication of the name Archaea. Although Woese proposed the name in 1990, the name was not valid until this article from 2024, hence the date.
- ^ [Archaea, not assigned to family] in LPSN; Parte, Aidan C.; Sardà Carbasse, Joaquim; Meier-Kolthoff, Jan P.; Reimer, Lorenz C.; Göker, Markus (1 November 2020). "List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ". International Journal of Systematic and Evolutionary Microbiology. 70 (11): 5607–5612. doi:10.1099/ijsem.0.004332.
- ^ a b c d e f Parte, Aidan C.; Sardà Carbasse, Joaquim; Meier-Kolthoff, Jan P.; Reimer, Lorenz C.; Göker, Markus (1 November 2020). "List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ". International Journal of Systematic and Evolutionary Microbiology. 70 (11): 5607–5612. doi:10.1099/ijsem.0.004332. ISSN 1466-5026. PMC 7723251. PMID 32701423.
- ^ Pace NR (May 2006). "Time for a change". Nature. 441 (7091): 289. Bibcode:2006Natur.441..289P. doi:10.1038/441289a. PMID 16710401. S2CID 4431143.
- ^ van Wolferen, Marleen; Pulschen, Andre Arashiro; Baum, Buzz; Gribaldo, Simonetta; Albers, Sonja-Verena (November 2022). "The cell biology of archaea". Nature Microbiology. 7 (11): 1744–1755. doi:10.1038/s41564-022-01215-8. ISSN 2058-5276.
- ^ Stoeckenius W (October 1981). "Walsby's square bacterium: fine structure of an orthogonal procaryote". Journal of Bacteriology. 148 (1): 352–60. doi:10.1128/JB.148.1.352-360.1981. PMC 216199. PMID 7287626.
- ^ "Archaea Basic Biology". March 2018.
- ^ a b c d Chow C, Padda KP, Puri A, Chanway CP (September 2022). "An Archaic Approach to a Modern Issue: Endophytic Archaea for Sustainable Agriculture". Current Microbiology. 79 (11) 322. doi:10.1007/s00284-022-03016-y. PMID 36125558. S2CID 252376815.
- ^ Bang C, Schmitz RA (September 2015). "Archaea associated with human surfaces: not to be underestimated". FEMS Microbiology Reviews. 39 (5): 631–48. doi:10.1093/femsre/fuv010. PMID 25907112.
- ^ Moissl-Eichinger C, Pausan M, Taffner J, Berg G, Bang C, Schmitz RA (January 2018). "Archaea Are Interactive Components of Complex Microbiomes". Trends in Microbiology. 26 (1): 70–85. doi:10.1016/j.tim.2017.07.004. PMID 28826642.
- ^ Staley JT (November 2006). "The bacterial species dilemma and the genomic-phylogenetic species concept". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 361 (1475): 1899–909. doi:10.1098/rstb.2006.1914. PMC 1857736. PMID 17062409.
- ^ Zuckerkandl E, Pauling L (March 1965). "Molecules as documents of evolutionary history". Journal of Theoretical Biology. 8 (2): 357–66. Bibcode:1965JThBi...8..357Z. doi:10.1016/0022-5193(65)90083-4. PMID 5876245.
- ^ Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil PA, Hugenholtz P (November 2018). "A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life". Nature Biotechnology. 36 (10): 996–1004. doi:10.1038/nbt.4229. PMID 30148503. S2CID 52093100.
- ^ a b c d Woese CR, Fox GE (November 1977). "Phylogenetic structure of the prokaryotic domain: the primary kingdoms". Proceedings of the National Academy of Sciences of the United States of America. 74 (11): 5088–90. Bibcode:1977PNAS...74.5088W. doi:10.1073/pnas.74.11.5088. PMC 432104. PMID 270744.
- ^ a b Woese CR, Kandler O, Wheelis ML (June 1990). "Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya". Proceedings of the National Academy of Sciences of the United States of America. 87 (12): 4576–9. Bibcode:1990PNAS...87.4576W. doi:10.1073/pnas.87.12.4576. PMC 54159. PMID 2112744.
- ^ Sapp J (2009). The new foundations of evolution: on the tree of life. New York: Oxford University Press. ISBN 978-0-19-973438-2.
- ^ "Archaea". Merriam-Webster Online Dictionary. 2008. Retrieved 1 July 2008.
- ^ Magrum LJ, Luehrsen KR, Woese CR (May 1978). "Are extreme halophiles actually "bacteria"?". Journal of Molecular Evolution. 11 (1): 1–8. Bibcode:1978JMolE..11....1M. doi:10.1007/bf01768019. PMID 660662. S2CID 1291732.
- ^ Stetter KO (1996). "Hyperthermophiles in the history of life". Ciba Foundation Symposium. 202: 1–10, discussion 11–8. PMID 9243007.
- ^ a b c DeLong EF (December 1998). "Everything in moderation: archaea as 'non-extremophiles'". Current Opinion in Genetics & Development. 8 (6): 649–54. doi:10.1016/S0959-437X(98)80032-4. PMID 9914204.
- ^ Theron J, Cloete TE (2000). "Molecular techniques for determining microbial diversity and community structure in natural environments". Critical Reviews in Microbiology. 26 (1): 37–57. doi:10.1080/10408410091154174. PMID 10782339. S2CID 5829573.
- ^ Schmidt TM (September 2006). "The maturing of microbial ecology" (PDF). International Microbiology. 9 (3): 217–23. PMID 17061212. Archived from the original (PDF) on 11 September 2008.
- ^ Gevers D, Dawyndt P, Vandamme P, Willems A, Vancanneyt M, Swings J, et al. (November 2006). "Stepping stones towards a new prokaryotic taxonomy". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 361 (1475): 1911–16. doi:10.1098/rstb.2006.1915. PMC 1764938. PMID 17062410.
- ^ a b Robertson CE, Harris JK, Spear JR, Pace NR (December 2005). "Phylogenetic diversity and ecology of environmental Archaea". Current Opinion in Microbiology. 8 (6): 638–642. doi:10.1016/j.mib.2005.10.003. PMID 16236543.
- ^ Göker, Markus; Oren, Aharon (2023). "Valid publication of four additional phylum names". International Journal of Systematic and Evolutionary Microbiology. 73 (9): 006024. doi:10.1099/ijsem.0.006024. ISSN 1466-5034. PMID 37695645.
- ^ Huber H, Hohn MJ, Rachel R, Fuchs T, Wimmer VC, Stetter KO (May 2002). "A new phylum of Archaea represented by a nanosized hyperthermophilic symbiont". Nature. 417 (6884): 63–67. Bibcode:2002Natur.417...63H. doi:10.1038/417063a. PMID 11986665. S2CID 4395094.
- ^ Barns SM, Delwiche CF, Palmer JD, Pace NR (August 1996). "Perspectives on archaeal diversity, thermophily and monophyly from environmental rRNA sequences". Proceedings of the National Academy of Sciences of the United States of America. 93 (17): 9188–93. Bibcode:1996PNAS...93.9188B. doi:10.1073/pnas.93.17.9188. PMC 38617. PMID 8799176.
- ^ Elkins JG, Podar M, Graham DE, Makarova KS, Wolf Y, Randau L, et al. (June 2008). "A korarchaeal genome reveals insights into the evolution of the Archaea". Proceedings of the National Academy of Sciences of the United States of America. 105 (23): 8102–07. Bibcode:2008PNAS..105.8102E. doi:10.1073/pnas.0801980105. PMC 2430366. PMID 18535141.
- ^ Baker BJ, Tyson GW, Webb RI, Flanagan J, Hugenholtz P, Allen EE, Banfield JF (December 2006). "Lineages of acidophilic archaea revealed by community genomic analysis". Science. 314 (5807): 1933–35. Bibcode:2006Sci...314.1933B. doi:10.1126/science.1132690. PMID 17185602. S2CID 26033384.
- ^ Baker BJ, Comolli LR, Dick GJ, Hauser LJ, Hyatt D, Dill BD, et al. (May 2010). "Enigmatic, ultrasmall, uncultivated Archaea". Proceedings of the National Academy of Sciences of the United States of America. 107 (19): 8806–11. Bibcode:2010PNAS..107.8806B. doi:10.1073/pnas.0914470107. PMC 2889320. PMID 20421484.
- ^ Guy L, Ettema TJ (December 2011). "The archaeal 'TACK' superphylum and the origin of eukaryotes". Trends in Microbiology. 19 (12): 580–87. doi:10.1016/j.tim.2011.09.002. PMID 22018741.
- ^ a b Zaremba-Niedzwiedzka K, Caceres EF, Saw JH, Bäckström D, Juzokaite L, Vancaester E, et al. (January 2017). "Asgard archaea illuminate the origin of eukaryotic cellular complexity" (PDF). Nature. 541 (7637): 353–58. Bibcode:2017Natur.541..353Z. doi:10.1038/nature21031. OSTI 1580084. PMID 28077874. S2CID 4458094.
- ^ Nina Dombrowski, Jun-Hoe Lee, Tom A Williams, Pierre Offre, Anja Spang (2019). Genomic diversity, lifestyles and evolutionary origins of DPANN archaea. Nature.
- ^ Petitjean C, Deschamps P, López-García P, Moreira D (December 2014). "Rooting the domain archaea by phylogenomic analysis supports the foundation of the new kingdom Proteoarchaeota". Genome Biology and Evolution. 7 (1): 191–204. doi:10.1093/gbe/evu274. PMC 4316627. PMID 25527841.
- ^ a b Williams TA, Szöllősi GJ, Spang A, Foster PG, Heaps SE, Boussau B, et al. (June 2017). "Integrative modeling of gene and genome evolution roots the archaeal tree of life". Proceedings of the National Academy of Sciences of the United States of America. 114 (23): E4602 – E4611. Bibcode:2017PNAS..114E4602W. doi:10.1073/pnas.1618463114. PMC 5468678. PMID 28533395.
- ^ a b Castelle CJ, Banfield JF (2018). "Major New Microbial Groups Expand Diversity and Alter our Understanding of the Tree of Life". Cell. 172 (6): 1181–1197. doi:10.1016/j.cell.2018.02.016. PMID 29522741.
- ^ a b "GTDB release 10-RS226". Genome Taxonomy Database. Retrieved 1 May 2025.
- ^ a b "ar53_r226.sp_label". Genome Taxonomy Database. Retrieved 1 May 2025.
- ^ a b "Taxon History". Genome Taxonomy Database. Retrieved 1 May 2025.
- ^ Seitz KW, Dombrowski N, Eme L, Spang A, Lombard J, Sieber JR, et al. (April 2019). "Asgard archaea capable of anaerobic hydrocarbon cycling". Nature Communications. 10 (1) 1822. Bibcode:2019NatCo..10.1822S. doi:10.1038/s41467-019-09364-x. PMC 6478937. PMID 31015394.
- ^ de Queiroz K (May 2005). "Ernst Mayr and the modern concept of species". Proceedings of the National Academy of Sciences of the United States of America. 102 (Supplement 1): 6600–6007. Bibcode:2005PNAS..102.6600D. doi:10.1073/pnas.0502030102. PMC 1131873. PMID 15851674.
- ^ Eppley JM, Tyson GW, Getz WM, Banfield JF (September 2007). "Genetic exchange across a species boundary in the archaeal genus ferroplasma". Genetics. 177 (1): 407–16. doi:10.1534/genetics.107.072892. PMC 2013692. PMID 17603112.
- ^ Papke RT, Zhaxybayeva O, Feil EJ, Sommerfeld K, Muise D, Doolittle WF (August 2007). "Searching for species in haloarchaea". Proceedings of the National Academy of Sciences of the United States of America. 104 (35): 14092–97. Bibcode:2007PNAS..10414092P. doi:10.1073/pnas.0706358104. PMC 1955782. PMID 17715057.
- ^ Kunin V, Goldovsky L, Darzentas N, Ouzounis CA (July 2005). "The net of life: reconstructing the microbial phylogenetic network". Genome Research. 15 (7): 954–59. doi:10.1101/gr.3666505. PMC 1172039. PMID 15965028.
- ^ Hugenholtz P (2002). "Exploring prokaryotic diversity in the genomic era". Genome Biology. 3 (2): REVIEWS0003. doi:10.1186/gb-2002-3-2-reviews0003. PMC 139013. PMID 11864374.
- ^ Rappé MS, Giovannoni SJ (2003). "The uncultured microbial majority" (PDF). Annual Review of Microbiology. 57: 369–94. doi:10.1146/annurev.micro.57.030502.090759. PMID 14527284. S2CID 10781051. Archived from the original (PDF) on 2 March 2019.
- ^ Oren A, Garrity GM (2021). "Valid publication of the names of forty-two phyla of prokaryotes". Int J Syst Evol Microbiol. 71 (10): 5056. doi:10.1099/ijsem.0.005056. PMID 34694987. S2CID 239887308.
- ^ Göker, Markus; Oren, Aharon (11 September 2023). "Valid publication of four additional phylum names". International Journal of Systematic and Evolutionary Microbiology. 73 (9). doi:10.1099/ijsem.0.006024. PMID 37695645.
- ^ Valentin-Alvarado, Luis E.; Appler, Kathryn E.; De Anda, Valerie; Schoelmerich, Marie C.; West-Roberts, Jacob; Kivenson, Veronika; Crits-Christoph, Alexander; Ly, Lynn; Sachdeva, Rohan; Greening, Chris; Savage, David F.; Baker, Brett J.; Banfield, Jillian F. (31 July 2024). "Asgard archaea modulate potential methanogenesis substrates in wetland soil". Nature Communications. 15 (1): 6384. doi:10.1038/s41467-024-49872-z. ISSN 2041-1723. PMC 11291895. PMID 39085194.
- ^ "Age of the Earth". U.S. Geological Survey. 1997. Archived from the original on 23 December 2005. Retrieved 10 January 2006.
- ^ Dalrymple GB (2001). "The age of the Earth in the twentieth century: a problem (mostly) solved". Special Publications, Geological Society of London. 190 (1): 205–21. Bibcode:2001GSLSP.190..205D. doi:10.1144/GSL.SP.2001.190.01.14. S2CID 130092094.
- ^ Manhesa G, Allègre CJ, Dupréa B, Hamelin B (1980). "Lead isotope study of basic-ultrabasic layered complexes: Speculations about the age of the earth and primitive mantle characteristics". Earth and Planetary Science Letters. 47 (3): 370–82. Bibcode:1980E&PSL..47..370M. doi:10.1016/0012-821X(80)90024-2.
- ^ de Duve C (October 1995). "The Beginnings of Life on Earth". American Scientist. Archived from the original on 6 June 2017. Retrieved 15 January 2014.
- ^ Timmer J (4 September 2012). "3.5 billion year old organic deposits show signs of life". Ars Technica. Retrieved 15 January 2014.
- ^ Ohtomo Y, Kakegawa T, Ishida A, Nagase T, Rosingm MT (8 December 2013). "Evidence for biogenic graphite in early Archaean Isua metasedimentary rocks". Nature Geoscience. 7 (1): 25. Bibcode:2014NatGe...7...25O. doi:10.1038/ngeo2025.
- ^ Borenstein S (13 November 2013). "Oldest fossil found: Meet your microbial mom". Associated Press. Retrieved 15 November 2013.
- ^ Noffke N, Christian D, Wacey D, Hazen RM (December 2013). "Microbially induced sedimentary structures recording an ancient ecosystem in the ca. 3.48 billion-year-old Dresser Formation, Pilbara, Western Australia". Astrobiology. 13 (12): 1103–24. Bibcode:2013AsBio..13.1103N. doi:10.1089/ast.2013.1030. PMC 3870916. PMID 24205812.
- ^ Borenstein S (19 October 2015). "Hints of life on what was thought to be desolate early Earth". Excite. Yonkers, NY: Mindspark Interactive Network. Associated Press. Retrieved 20 October 2015.
- ^ Bell EA, Boehnke P, Harrison TM, Mao WL (November 2015). "Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 112 (47). National Academy of Sciences: 14518–21. Bibcode:2015PNAS..11214518B. doi:10.1073/pnas.1517557112. PMC 4664351. PMID 26483481.
- ^ Schopf JW (June 2006). "Fossil evidence of Archaean life". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 361 (1470): 869–85. doi:10.1098/rstb.2006.1834. PMC 1578735. PMID 16754604.
- ^ Chappe B, Albrecht P, Michaelis W (July 1982). "Polar lipids of archaebacteria in sediments and petroleums". Science. 217 (4554): 65–66. Bibcode:1982Sci...217...65C. doi:10.1126/science.217.4554.65. PMID 17739984. S2CID 42758483.
- ^ Brocks JJ, Logan GA, Buick R, Summons RE (August 1999). "Archean molecular fossils and the early rise of eukaryotes". Science. 285 (5430): 1033–36. Bibcode:1999Sci...285.1033B. CiteSeerX 10.1.1.516.9123. doi:10.1126/science.285.5430.1033. PMID 10446042.
- ^ Rasmussen B, Fletcher IR, Brocks JJ, Kilburn MR (October 2008). "Reassessing the first appearance of eukaryotes and cyanobacteria". Nature. 455 (7216): 1101–4. Bibcode:2008Natur.455.1101R. doi:10.1038/nature07381. PMID 18948954. S2CID 4372071.
- ^ Hahn J, Haug P (1986). "Traces of Archaebacteria in ancient sediments". System Applied Microbiology. 7 (Archaebacteria '85 Proceedings): 178–83. Bibcode:1986SyApM...7..178H. doi:10.1016/S0723-2020(86)80002-9.
- ^ Wang M, Yafremava LS, Caetano-Anollés D, Mittenthal JE, Caetano-Anollés G (November 2007). "Reductive evolution of architectural repertoires in proteomes and the birth of the tripartite world". Genome Research. 17 (11): 1572–85. doi:10.1101/gr.6454307. PMC 2045140. PMID 17908824.
- ^ Woese CR, Gupta R (January 1981). "Are archaebacteria merely derived 'prokaryotes'?". Nature. 289 (5793): 95–96. Bibcode:1981Natur.289...95W. doi:10.1038/289095a0. PMID 6161309. S2CID 4343245.
- ^ a b c Woese C (June 1998). "The universal ancestor". Proceedings of the National Academy of Sciences of the United States of America. 95 (12): 6854–59. Bibcode:1998PNAS...95.6854W. doi:10.1073/pnas.95.12.6854. PMC 22660. PMID 9618502.
- ^ a b Kandler OT (August 1998). "The early diversification of life and the origin of the three domains: a proposal.". In Wiegel J, Adams WW (eds.). Thermophiles: the keys to molecular evolution and the origin of life. Athens: Taylor and Francis. pp. 19–31. ISBN 978-1-4822-7304-5.
- ^ Gribaldo S, Brochier-Armanet C (June 2006). "The origin and evolution of Archaea: a state of the art". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 361 (1470): 1007–22. doi:10.1098/rstb.2006.1841. PMC 1578729. PMID 16754611.
- ^ a b Woese CR (March 1994). "There must be a prokaryote somewhere: microbiology's search for itself". Microbiological Reviews. 58 (1): 1–9. doi:10.1128/MMBR.58.1.1-9.1994. PMC 372949. PMID 8177167.
- ^ Information is from Willey JM, Sherwood LM, Woolverton CJ. Microbiology 7th ed. (2008), Ch. 19 pp. 474–475, except where noted.
- ^ Heimerl T, Flechsler J, Pickl C, Heinz V, Salecker B, Zweck J, Wanner G, Geimer S, Samson RY, Bell SD, Huber H, Wirth R, Wurch L, Podar M, Rachel R (13 June 2017). "A Complex Endomembrane System in the Archaeon Ignicoccus hospitalis Tapped by Nanoarchaeum equitans". Frontiers in Microbiology. 8: 1072. doi:10.3389/fmicb.2017.01072. PMC 5468417. PMID 28659892.
- ^ Jurtshuk P (1996). "Bacterial Metabolism". Medical Microbiology (4th ed.). Galveston (TX): University of Texas Medical Branch at Galveston. ISBN 9780963117212. PMID 21413278.
- ^ Howland JL (2000). The Surprising Archaea: Discovering Another Domain of Life. Oxford: Oxford University Press. pp. 25–30. ISBN 978-0-19-511183-5.
- ^ a b c Cavicchioli R (January 2011). "Archaea—timeline of the third domain". Nature Reviews. Microbiology. 9 (1): 51–61. doi:10.1038/nrmicro2482. PMID 21132019. S2CID 21008512.
- ^ Gupta RS, Shami A (February 2011). "Molecular signatures for the Crenarchaeota and the Thaumarchaeota". Antonie van Leeuwenhoek. 99 (2): 133–57. doi:10.1007/s10482-010-9488-3. PMID 20711675. S2CID 12874800.
- ^ Gao B, Gupta RS (March 2007). "Phylogenomic analysis of proteins that are distinctive of Archaea and its main subgroups and the origin of methanogenesis". BMC Genomics. 8: 86. doi:10.1186/1471-2164-8-86. PMC 1852104. PMID 17394648.
- ^ Gupta RS, Naushad S, Baker S (March 2015). "Phylogenomic analyses and molecular signatures for the class Halobacteria and its two major clades: a proposal for division of the class Halobacteria into an emended order Halobacteriales and two new orders, Haloferacales ord. nov. and Natrialbales ord. nov., containing the novel families Haloferacaceae fam. nov. and Natrialbaceae fam. nov". International Journal of Systematic and Evolutionary Microbiology. 65 (Pt 3): 1050–69. doi:10.1099/ijs.0.070136-0. PMID 25428416.
- ^ Deppenmeier U (2002). The unique biochemistry of methanogenesis. Progress in Nucleic Acid Research and Molecular Biology. Vol. 71. pp. 223–83. doi:10.1016/s0079-6603(02)71045-3. ISBN 978-0-12-540071-8. PMID 12102556.
- ^ Martin W, Russell MJ (January 2003). "On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 358 (1429): 59–85. doi:10.1098/rstb.2002.1183. PMC 1693102. PMID 12594918.
- ^ Jordan SF, Nee E, Lane N (December 2019). "Isoprenoids enhance the stability of fatty acid membranes at the emergence of life potentially leading to an early lipid divide". Interface Focus. 9 (6): 20190067. doi:10.1098/rsfs.2019.0067. PMC 6802135. PMID 31641436.
- ^ Ciccarelli FD, Doerks T, von Mering C, Creevey CJ, Snel B, Bork P (March 2006). "Toward automatic reconstruction of a highly resolved tree of life". Science. 311 (5765): 1283–1287. Bibcode:2006Sci...311.1283C. CiteSeerX 10.1.1.381.9514. doi:10.1126/science.1123061. PMID 16513982. S2CID 1615592.
- ^ Koonin EV, Mushegian AR, Galperin MY, Walker DR (August 1997). "Comparison of archaeal and bacterial genomes: Computer analysis of protein sequences predicts novel functions and suggests a chimeric origin for the archaea". Molecular Microbiology. 25 (4): 619–637. doi:10.1046/j.1365-2958.1997.4821861.x. PMID 9379893. S2CID 36270763.
- ^ a b c d e Gupta RS (December 1998). "Protein phylogenies and signature sequences: A reappraisal of evolutionary relationships among archaebacteria, eubacteria, and eukaryotes". Microbiology and Molecular Biology Reviews. 62 (4): 1435–1491. doi:10.1128/MMBR.62.4.1435-1491.1998. PMC 98952. PMID 9841678.
- ^ Koch AL (April 2003). "Were Gram-positive rods the first bacteria?". Trends in Microbiology. 11 (4): 166–170. doi:10.1016/S0966-842X(03)00063-5. PMID 12706994.
- ^ a b c Gupta RS (August 1998). "What are archaebacteria: Life's third domain or monoderm prokaryotes related to gram-positive bacteria? A new proposal for the classification of prokaryotic organisms". Molecular Microbiology. 29 (3): 695–707. doi:10.1046/j.1365-2958.1998.00978.x. PMID 9723910. S2CID 41206658.
- ^ Gogarten JP (November 1994). "Which is the most conserved group of proteins? Homology-orthology, paralogy, xenology, and the fusion of independent lineages". Journal of Molecular Evolution. 39 (5): 541–543. Bibcode:1994JMolE..39..541G. doi:10.1007/bf00173425. PMID 7807544. S2CID 44922755.
- ^ Brown JR, Masuchi Y, Robb FT, Doolittle WF (June 1994). "Evolutionary relationships of bacterial and archaeal glutamine synthetase genes". Journal of Molecular Evolution. 38 (6): 566–576. Bibcode:1994JMolE..38..566B. doi:10.1007/BF00175876. PMID 7916055. S2CID 21493521.
- ^ Katz LA (September 2015). "Recent events dominate inter-domain lateral gene transfers between prokaryotes and eukaryotes and, with the exception of endosymbiotic gene transfers, few ancient transfer events persist". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 370 (1678): 20140324. doi:10.1098/rstb.2014.0324. PMC 4571564. PMID 26323756.
- ^ a b c Gupta RS (2000). "The natural evolutionary relationships among prokaryotes". Critical Reviews in Microbiology. 26 (2): 111–131. CiteSeerX 10.1.1.496.1356. doi:10.1080/10408410091154219. PMID 10890353. S2CID 30541897.
- ^ Gupta RS (2005). "Molecular sequences and the early history of life". In Sapp J (ed.). Microbial Phylogeny and Evolution: Concepts and controversies. New York, NY: Oxford University Press. pp. 160–183.
- ^ Cavalier-Smith T (January 2002). "The neomuran origin of archaebacteria, the negibacterial root of the universal tree and bacterial megaclassification". International Journal of Systematic and Evolutionary Microbiology. 52 (Pt 1): 7–76. doi:10.1099/00207713-52-1-7. PMID 11837318.
- ^ Valas RE, Bourne PE (February 2011). "The origin of a derived superkingdom: How a gram-positive bacterium crossed the desert to become an archaeon". Biology Direct. 6: 16. doi:10.1186/1745-6150-6-16. PMC 3056875. PMID 21356104.
- ^ Skophammer RG, Herbold CW, Rivera MC, Servin JA, Lake JA (September 2006). "Evidence that the root of the tree of life is not within the Archaea". Molecular Biology and Evolution. 23 (9): 1648–1651. doi:10.1093/molbev/msl046. PMID 16801395.
- ^ a b Latorre A, Durban A, Moya A, Pereto J (2011). "The role of symbiosis in eukaryotic evolution". In Gargaud M, López-Garcìa P, Martin H (eds.). Origins and Evolution of Life: An astrobiological perspective. Cambridge: Cambridge University Press. pp. 326–339. ISBN 978-0-521-76131-4. Archived from the original on 24 March 2019. Retrieved 27 August 2017.
- ^ Eme L, Spang A, Lombard J, Stairs CW, Ettema TJG (November 2017). "Archaea and the origin of eukaryotes". Nature Reviews. Microbiology. 15 (12): 711–723. doi:10.1038/nrmicro.2017.133. PMID 29123225. S2CID 8666687.
- ^ Lake JA (January 1988). "Origin of the eukaryotic nucleus determined by rate-invariant analysis of rRNA sequences". Nature. 331 (6152): 184–86. Bibcode:1988Natur.331..184L. doi:10.1038/331184a0. PMID 3340165. S2CID 4368082.
- ^ Nelson KE, Clayton RA, Gill SR, Gwinn ML, Dodson RJ, Haft DH, et al. (May 1999). "Evidence for lateral gene transfer between Archaea and bacteria from genome sequence of Thermotoga maritima". Nature. 399 (6734): 323–29. Bibcode:1999Natur.399..323N. doi:10.1038/20601. PMID 10360571. S2CID 4420157.
- ^ Gouy M, Li WH (May 1989). "Phylogenetic analysis based on rRNA sequences supports the archaebacterial rather than the eocyte tree". Nature. 339 (6220): 145–47. Bibcode:1989Natur.339..145G. doi:10.1038/339145a0. PMID 2497353. S2CID 4315004.
- ^ Yutin N, Makarova KS, Mekhedov SL, Wolf YI, Koonin EV (August 2008). "The deep archaeal roots of eukaryotes". Molecular Biology and Evolution. 25 (8): 1619–30. doi:10.1093/molbev/msn108. PMC 2464739. PMID 18463089.
- ^ Williams TA, Foster PG, Cox CJ, Embley TM (December 2013). "An archaeal origin of eukaryotes supports only two primary domains of life". Nature. 504 (7479): 231–36. Bibcode:2013Natur.504..231W. doi:10.1038/nature12779. PMID 24336283. S2CID 4461775.
- ^ Zimmer C (6 May 2015). "Under the Sea, a Missing Link in the Evolution of Complex Cells". The New York Times. Retrieved 6 May 2015.
- ^ Spang A, Saw JH, Jørgensen SL, Zaremba-Niedzwiedzka K, Martijn J, Lind AE, van Eijk R, Schleper C, Guy L, Ettema TJ (May 2015). "Complex archaea that bridge the gap between prokaryotes and eukaryotes". Nature. 521 (7551): 173–179. Bibcode:2015Natur.521..173S. doi:10.1038/nature14447. PMC 4444528. PMID 25945739.
- ^ NCBI taxonomy page on Archaea
- ^ Seitz KW, Lazar CS, Hinrichs KU, Teske AP, Baker BJ (July 2016). "Genomic reconstruction of a novel, deeply branched sediment archaeal phylum with pathways for acetogenesis and sulfur reduction". The ISME Journal. 10 (7): 1696–705. Bibcode:2016ISMEJ..10.1696S. doi:10.1038/ismej.2015.233. PMC 4918440. PMID 26824177.
- ^ MacLeod F, Kindler GS, Wong HL, Chen R, Burns BP (2019). "Asgard archaea: Diversity, function, and evolutionary implications in a range of microbiomes". AIMS Microbiology. 5 (1): 48–61. doi:10.3934/microbiol.2019.1.48. PMC 6646929. PMID 31384702.
- ^ Zimmer C (15 January 2020). "This Strange Microbe May Mark One of Life's Great Leaps – A organism living in ocean muck offers clues to the origins of the complex cells of all animals and plants". The New York Times. Retrieved 16 January 2020.
- ^ Imachi H, Nobu MK, Nakahara N, Morono Y, Ogawara M, Takaki Y, et al. (January 2020). "Isolation of an archaeon at the prokaryote-eukaryote interface". Nature. 577 (7791): 519–525. Bibcode:2020Natur.577..519I. doi:10.1038/s41586-019-1916-6. PMC 7015854. PMID 31942073.
- ^ Tirumalai MR, Sivaraman RV, Kutty LA, Song EL, Fox GE (September 2023). "Ribosomal Protein Cluster Organization in Asgard Archaea". Archaea. 2023: 1–16. Bibcode:2023Archa202312414T. doi:10.1155/2023/5512414. PMC 10833476. PMID 38314098.
- ^ a b c d Krieg N (2005). Bergey's Manual of Systematic Bacteriology. US: Springer. pp. 21–26. ISBN 978-0-387-24143-2.
- ^ Barns S, Burggraf S (1997). "Crenarchaeota". The Tree of Life Web Project. Version 01 January 1997.
- ^ Walsby AE (1980). "A square bacterium". Nature. 283 (5742): 69–71. Bibcode:1980Natur.283...69W. doi:10.1038/283069a0. S2CID 4341717.
- ^ Hara F, Yamashiro K, Nemoto N, Ohta Y, Yokobori S, Yasunaga T, et al. (March 2007). "An actin homolog of the archaeon Thermoplasma acidophilum that retains the ancient characteristics of eukaryotic actin". Journal of Bacteriology. 189 (5): 2039–45. doi:10.1128/JB.01454-06. PMC 1855749. PMID 17189356.
- ^ Trent JD, Kagawa HK, Yaoi T, Olle E, Zaluzec NJ (May 1997). "Chaperonin filaments: the archaeal cytoskeleton?". Proceedings of the National Academy of Sciences of the United States of America. 94 (10): 5383–88. Bibcode:1997PNAS...94.5383T. doi:10.1073/pnas.94.10.5383. PMC 24687. PMID 9144246.
- ^ Hixon WG, Searcy DG (1993). "Cytoskeleton in the archaebacterium Thermoplasma acidophilum? Viscosity increase in soluble extracts". Bio Systems. 29 (2–3): 151–60. Bibcode:1993BiSys..29..151H. doi:10.1016/0303-2647(93)90091-P. PMID 8374067.
- ^ a b Golyshina OV, Pivovarova TA, Karavaiko GI, Kondratéva TF, Moore ER, Abraham WR, et al. (May 2000). "Ferroplasma acidiphilum gen. nov., sp. nov., an acidophilic, autotrophic, ferrous-iron-oxidizing, cell-wall-lacking, mesophilic member of the Ferroplasmaceae fam. nov., comprising a distinct lineage of the Archaea". International Journal of Systematic and Evolutionary Microbiology. 50 (3): 997–1006. doi:10.1099/00207713-50-3-997. PMID 10843038.
- ^ Hall-Stoodley L, Costerton JW, Stoodley P (February 2004). "Bacterial biofilms: from the natural environment to infectious diseases". Nature Reviews. Microbiology. 2 (2): 95–108. doi:10.1038/nrmicro821. PMID 15040259. S2CID 9107205.
- ^ Kuwabara T, Minaba M, Iwayama Y, Inouye I, Nakashima M, Marumo K, et al. (November 2005). "Thermococcus coalescens sp. nov., a cell-fusing hyperthermophilic archaeon from Suiyo Seamount". International Journal of Systematic and Evolutionary Microbiology. 55 (Pt 6): 2507–14. doi:10.1099/ijs.0.63432-0. PMID 16280518.
- ^ Nickell S, Hegerl R, Baumeister W, Rachel R (January 2003). "Pyrodictium cannulae enter the periplasmic space but do not enter the cytoplasm, as revealed by cryo-electron tomography". Journal of Structural Biology. 141 (1): 34–42. doi:10.1016/S1047-8477(02)00581-6. PMID 12576018.
- ^ Horn C, Paulmann B, Kerlen G, Junker N, Huber H (August 1999). "In vivo observation of cell division of anaerobic hyperthermophiles by using a high-intensity dark-field microscope". Journal of Bacteriology. 181 (16): 5114–18. doi:10.1128/JB.181.16.5114-5118.1999. PMC 94007. PMID 10438790.
- ^ Rudolph C, Wanner G, Huber R (May 2001). "Natural communities of novel archaea and bacteria growing in cold sulfurous springs with a string-of-pearls-like morphology". Applied and Environmental Microbiology. 67 (5): 2336–44. Bibcode:2001ApEnM..67.2336R. doi:10.1128/AEM.67.5.2336-2344.2001. PMC 92875. PMID 11319120.
- ^ a b Thomas NA, Bardy SL, Jarrell KF (April 2001). "The archaeal flagellum: a different kind of prokaryotic motility structure". FEMS Microbiology Reviews. 25 (2): 147–74. doi:10.1111/j.1574-6976.2001.tb00575.x. PMID 11250034.
- ^ Rachel R, Wyschkony I, Riehl S, Huber H (March 2002). "The ultrastructure of Ignicoccus: evidence for a novel outer membrane and for intracellular vesicle budding in an archaeon". Archaea. 1 (1): 9–18. doi:10.1155/2002/307480. PMC 2685547. PMID 15803654.
- ^ Sára M, Sleytr UB (February 2000). "S-Layer proteins". Journal of Bacteriology. 182 (4): 859–68. doi:10.1128/JB.182.4.859-868.2000. PMC 94357. PMID 10648507.
- ^ Engelhardt H, Peters J (December 1998). "Structural research on surface layers: a focus on stability, surface layer homology domains, and surface layer-cell wall interactions". Journal of Structural Biology. 124 (2–3): 276–302. doi:10.1006/jsbi.1998.4070. PMID 10049812.
- ^ a b Kandler O, König H (April 1998). "Cell wall polymers in Archaea (Archaebacteria)". Cellular and Molecular Life Sciences. 54 (4): 305–08. doi:10.1007/s000180050156. PMC 11147200. PMID 9614965. S2CID 13527169.
- ^ Howland JL (2000). The Surprising Archaea: Discovering Another Domain of Life. Oxford: Oxford University Press. p. 32. ISBN 978-0-19-511183-5.
- ^ Gophna U, Ron EZ, Graur D (July 2003). "Bacterial type III secretion systems are ancient and evolved by multiple horizontal-transfer events". Gene. 312: 151–63. doi:10.1016/S0378-1119(03)00612-7. PMID 12909351.
- ^ Nguyen L, Paulsen IT, Tchieu J, Hueck CJ, Saier MH (April 2000). "Phylogenetic analyses of the constituents of Type III protein secretion systems". Journal of Molecular Microbiology and Biotechnology. 2 (2): 125–44. PMID 10939240.
- ^ Ng SY, Chaban B, Jarrell KF (2006). "Archaeal flagella, bacterial flagella and type IV pili: a comparison of genes and posttranslational modifications". Journal of Molecular Microbiology and Biotechnology. 11 (3–5): 167–91. doi:10.1159/000094053. PMID 16983194. S2CID 30386932.
- ^ Bardy SL, Ng SY, Jarrell KF (February 2003). "Prokaryotic motility structures". Microbiology. 149 (Pt 2): 295–304. doi:10.1099/mic.0.25948-0. PMID 12624192. S2CID 20751743.
- ^ a b Koga Y, Morii H (March 2007). "Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations". Microbiology and Molecular Biology Reviews. 71 (1): 97–120. doi:10.1128/MMBR.00033-06. PMC 1847378. PMID 17347520.
- ^ Youssefian S, Rahbar N, Van Dessel S (May 2018). "Thermal conductivity and rectification in asymmetric archaeal lipid membranes". The Journal of Chemical Physics. 148 (17): 174901. Bibcode:2018JChPh.148q4901Y. doi:10.1063/1.5018589. PMID 29739208.
- ^ De Rosa M, Gambacorta A, Gliozzi A (March 1986). "Structure, biosynthesis, and physicochemical properties of archaebacterial lipids". Microbiological Reviews. 50 (1): 70–80. doi:10.1128/MMBR.50.1.70-80.1986. PMC 373054. PMID 3083222.
- ^ Balleza D, Garcia-Arribas AB, Sot J, Ruiz-Mirazo K, Goñi FM (September 2014). "Ether- versus ester-linked phospholipid bilayers containing either linear or branched apolar chains". Biophysical Journal. 107 (6): 1364–74. Bibcode:2014BpJ...107.1364B. doi:10.1016/j.bpj.2014.07.036. PMC 4167531. PMID 25229144.
- ^ Damsté JS, Schouten S, Hopmans EC, van Duin AC, Geenevasen JA (October 2002). "Crenarchaeol: The characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeota". Journal of Lipid Research. 43 (10): 1641–51. doi:10.1194/jlr.M200148-JLR200. PMID 12364548.
- ^ Koga Y, Morii H (November 2005). "Recent advances in structural research on ether lipids from archaea including comparative and physiological aspects". Bioscience, Biotechnology, and Biochemistry. 69 (11): 2019–34. doi:10.1271/bbb.69.2019. PMID 16306681. S2CID 42237252.
- ^ Hanford MJ, Peeples TL (January 2002). "Archaeal tetraether lipids: unique structures and applications". Applied Biochemistry and Biotechnology. 97 (1): 45–62. doi:10.1385/ABAB:97:1:45. PMID 11900115. S2CID 22334666.
- ^ Macalady JL, Vestling MM, Baumler D, Boekelheide N, Kaspar CW, Banfield JF (October 2004). "Tetraether-linked membrane monolayers in Ferroplasma spp: a key to survival in acid". Extremophiles. 8 (5): 411–19. doi:10.1007/s00792-004-0404-5. PMID 15258835. S2CID 15702103.
- ^ a b c Valentine DL (April 2007). "Adaptations to energy stress dictate the ecology and evolution of the Archaea". Nature Reviews. Microbiology. 5 (4): 316–23. doi:10.1038/nrmicro1619. PMID 17334387. S2CID 12384143.
- ^ a b c Schäfer G, Engelhard M, Müller V (September 1999). "Bioenergetics of the Archaea". Microbiology and Molecular Biology Reviews. 63 (3): 570–620. doi:10.1128/MMBR.63.3.570-620.1999. PMC 103747. PMID 10477309.
- ^ Zillig W (December 1991). "Comparative biochemistry of Archaea and Bacteria". Current Opinion in Genetics & Development. 1 (4): 544–51. doi:10.1016/S0959-437X(05)80206-0. PMID 1822288.
- ^ Romano AH, Conway T (1996). "Evolution of carbohydrate metabolic pathways". Research in Microbiology. 147 (6–7): 448–55. doi:10.1016/0923-2508(96)83998-2. PMID 9084754.
- ^ Koch AL (1998). How did bacteria come to be?. Advances in Microbial Physiology. Vol. 40. pp. 353–99. doi:10.1016/S0065-2911(08)60135-6. ISBN 978-0-12-027740-7. PMID 9889982.
- ^ DiMarco AA, Bobik TA, Wolfe RS (1990). "Unusual coenzymes of methanogenesis". Annual Review of Biochemistry. 59: 355–94. doi:10.1146/annurev.bi.59.070190.002035. PMID 2115763.
- ^ Klocke M, Nettmann E, Bergmann I, Mundt K, Souidi K, Mumme J, et al. (August 2008). "Characterization of the methanogenic Archaea within two-phase biogas reactor systems operated with plant biomass". Systematic and Applied Microbiology. 31 (3): 190–205. Bibcode:2008SyApM..31..190K. doi:10.1016/j.syapm.2008.02.003. PMID 18501543.
- ^ Based on PDB 1FBB. Data published in Subramaniam S, Henderson R (August 2000). "Molecular mechanism of vectorial proton translocation by bacteriorhodopsin". Nature. 406 (6796): 653–57. Bibcode:2000Natur.406..653S. doi:10.1038/35020614. PMID 10949309. S2CID 4395278.
- ^ Mueller-Cajar O, Badger MR (August 2007). "New roads lead to Rubisco in archaebacteria". BioEssays. 29 (8): 722–24. doi:10.1002/bies.20616. PMID 17621634.
- ^ Berg IA, Kockelkorn D, Buckel W, Fuchs G (December 2007). "A 3-hydroxypropionate/ 4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea". Science. 318 (5857): 1782–86. Bibcode:2007Sci...318.1782B. doi:10.1126/science.1149976. PMID 18079405. S2CID 13218676.
- ^ Thauer RK (December 2007). "Microbiology. A fifth pathway of carbon fixation". Science. 318 (5857): 1732–33. doi:10.1126/science.1152209. PMID 18079388. S2CID 83805878.
- ^ Bryant DA, Frigaard NU (November 2006). "Prokaryotic photosynthesis and phototrophy illuminated". Trends in Microbiology. 14 (11): 488–96. doi:10.1016/j.tim.2006.09.001. PMID 16997562.
- ^ a b Könneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (September 2005). "Isolation of an autotrophic ammonia-oxidizing marine archaeon". Nature. 437 (7058): 543–46. Bibcode:2005Natur.437..543K. doi:10.1038/nature03911. PMID 16177789. S2CID 4340386.
- ^ a b Francis CA, Beman JM, Kuypers MM (May 2007). "New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation". The ISME Journal. 1 (1): 19–27. Bibcode:2007ISMEJ...1...19F. doi:10.1038/ismej.2007.8. PMID 18043610.
- ^ Lanyi JK (2004). "Bacteriorhodopsin". Annual Review of Physiology. 66: 665–88. doi:10.1146/annurev.physiol.66.032102.150049. PMID 14977418.
- ^ a b Allers T, Mevarech M (January 2005). "Archaeal genetics – the third way" (PDF). Nature Reviews Genetics. 6 (1): 58–73. doi:10.1038/nrg1504. PMID 15630422. S2CID 20229552. Archived from the original (PDF) on 20 July 2018. Retrieved 12 September 2019.
- ^ Hildenbrand C, Stock T, Lange C, Rother M, Soppa J (February 2011). "Genome copy numbers and gene conversion in methanogenic archaea". Journal of Bacteriology. 193 (3): 734–43. doi:10.1128/JB.01016-10. PMC 3021236. PMID 21097629.
- ^ Galagan JE, Nusbaum C, Roy A, Endrizzi MG, Macdonald P, FitzHugh W, et al. (April 2002). "The genome of M. acetivorans reveals extensive metabolic and physiological diversity". Genome Research. 12 (4): 532–42. doi:10.1101/gr.223902. PMC 187521. PMID 11932238.
- ^ a b Waters E, Hohn MJ, Ahel I, Graham DE, Adams MD, Barnstead M, et al. (October 2003). "The genome of Nanoarchaeum equitans: insights into early archaeal evolution and derived parasitism". Proceedings of the National Academy of Sciences of the United States of America. 100 (22): 12984–88. Bibcode:2003PNAS..10012984W. doi:10.1073/pnas.1735403100. PMC 240731. PMID 14566062.
- ^ Schleper C, Holz I, Janekovic D, Murphy J, Zillig W (August 1995). "A multicopy plasmid of the extremely thermophilic archaeon Sulfolobus effects its transfer to recipients by mating". Journal of Bacteriology. 177 (15): 4417–26. doi:10.1128/jb.177.15.4417-4426.1995. PMC 177192. PMID 7635827.
- ^ Sota M, Top EM (2008). "Horizontal Gene Transfer Mediated by Plasmids". Plasmids: Current Research and Future Trends. Caister Academic Press. ISBN 978-1-904455-35-6.
- ^ Xiang X, Chen L, Huang X, Luo Y, She Q, Huang L (July 2005). "Sulfolobus tengchongensis spindle-shaped virus STSV1: virus-host interactions and genomic features". Journal of Virology. 79 (14): 8677–86. doi:10.1128/JVI.79.14.8677-8686.2005. PMC 1168784. PMID 15994761.
- ^ Graham DE, Overbeek R, Olsen GJ, Woese CR (March 2000). "An archaeal genomic signature". Proceedings of the National Academy of Sciences of the United States of America. 97 (7): 3304–08. Bibcode:2000PNAS...97.3304G. doi:10.1073/pnas.050564797. PMC 16234. PMID 10716711.
- ^ a b Gaasterland T (October 1999). "Archaeal genomics". Current Opinion in Microbiology. 2 (5): 542–47. doi:10.1016/S1369-5274(99)00014-4. PMID 10508726.
- ^ Dennis PP (June 1997). "Ancient ciphers: translation in Archaea". Cell. 89 (7): 1007–10. doi:10.1016/S0092-8674(00)80288-3. PMID 9215623. S2CID 18862794.
- ^ Werner F (September 2007). "Structure and function of archaeal RNA polymerases". Molecular Microbiology. 65 (6): 1395–404. doi:10.1111/j.1365-2958.2007.05876.x. PMID 17697097. S2CID 28078184.
- ^ Aravind L, Koonin EV (December 1999). "DNA-binding proteins and evolution of transcription regulation in the archaea". Nucleic Acids Research. 27 (23): 4658–70. doi:10.1093/nar/27.23.4658. PMC 148756. PMID 10556324.
- ^ Lykke-Andersen J, Aagaard C, Semionenkov M, Garrett RA (September 1997). "Archaeal introns: splicing, intercellular mobility and evolution". Trends in Biochemical Sciences. 22 (9): 326–31. doi:10.1016/S0968-0004(97)01113-4. PMID 9301331.
- ^ Watanabe Y, Yokobori S, Inaba T, Yamagishi A, Oshima T, Kawarabayasi Y, et al. (January 2002). "Introns in protein-coding genes in Archaea". FEBS Letters. 510 (1–2): 27–30. Bibcode:2002FEBSL.510...27W. doi:10.1016/S0014-5793(01)03219-7. PMID 11755525. S2CID 27294545.
- ^ Yoshinari S, Itoh T, Hallam SJ, DeLong EF, Yokobori S, Yamagishi A, et al. (August 2006). "Archaeal pre-mRNA splicing: a connection to hetero-oligomeric splicing endonuclease". Biochemical and Biophysical Research Communications. 346 (3): 1024–32. Bibcode:2006BBRC..346.1024Y. doi:10.1016/j.bbrc.2006.06.011. PMID 16781672.
- ^ Rosenshine I, Tchelet R, Mevarech M (September 1989). "The mechanism of DNA transfer in the mating system of an archaebacterium". Science. 245 (4924): 1387–89. Bibcode:1989Sci...245.1387R. doi:10.1126/science.2818746. PMID 2818746.
- ^ a b c Fröls S, Ajon M, Wagner M, Teichmann D, Zolghadr B, Folea M, et al. (November 2008). "UV-inducible cellular aggregation of the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by pili formation". Molecular Microbiology. 70 (4): 938–52. doi:10.1111/j.1365-2958.2008.06459.x. PMID 18990182. S2CID 12797510.
- ^ a b c Ajon M, Fröls S, van Wolferen M, Stoecker K, Teichmann D, Driessen AJ, et al. (November 2011). "UV-inducible DNA exchange in hyperthermophilic archaea mediated by type IV pili" (PDF). Molecular Microbiology. 82 (4): 807–17. doi:10.1111/j.1365-2958.2011.07861.x. PMID 21999488. S2CID 42880145.
- ^ Fröls S, White MF, Schleper C (February 2009). "Reactions to UV damage in the model archaeon Sulfolobus solfataricus". Biochemical Society Transactions. 37 (Pt 1): 36–41. doi:10.1042/BST0370036. PMID 19143598.
- ^ Bernstein H, Bernstein C (2017). "Sexual Communication in Archaea, the Precursor to Eukaryotic Meiosis". In Witzany G (ed.). Biocommunication of Archaea. Springer Nature. pp. 301–117. doi:10.1007/978-3-319-65536-9_7. ISBN 978-3-319-65535-2.
- ^ a b Blohs M, Moissl-Eichinger C, Mahnert A, Spang A, Dombrowski N, Krupovic M, Klingl A (January 2019). "Archaea – An Introduction". In Schmidt TM (ed.). Encyclopedia of Microbiology (Fourth ed.). Academic Press. pp. 243–252. doi:10.1016/B978-0-12-809633-8.20884-4. ISBN 978-0-12-811737-8. S2CID 164553336.
- ^ a b c Krupovic M, Cvirkaite-Krupovic V, Iranzo J, Prangishvili D, Koonin EV (January 2018). "Viruses of archaea: Structural, functional, environmental and evolutionary genomics". Virus Research. 244: 181–193. doi:10.1016/j.virusres.2017.11.025. PMC 5801132. PMID 29175107.
- ^ Pietilä MK, Demina TA, Atanasova NS, Oksanen HM, Bamford DH (June 2014). "Archaeal viruses and bacteriophages: comparisons and contrasts". Trends in Microbiology. 22 (6): 334–44. doi:10.1016/j.tim.2014.02.007. PMID 24647075.
- ^ Principi N, Silvestri E, Esposito S (2019). "Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections". Frontiers in Pharmacology. 10: 513. doi:10.3389/fphar.2019.00513. PMC 6517696. PMID 31139086.
- ^ Prangishvili D (1 January 2013). "Viruses of the Archaea". In Maloy S, Hughes K (eds.). Brenner's Encyclopedia of Genetics. Brenner's Encyclopedia of Genetics (Second Edition). Academic Press. pp. 295–298. doi:10.1016/B978-0-12-374984-0.01627-2. ISBN 978-0-08-096156-9. Retrieved 16 March 2020.
- ^ Prangishvili D, Garrett RA (April 2004). "Exceptionally diverse morphotypes and genomes of crenarchaeal hyperthermophilic viruses" (PDF). Biochemical Society Transactions. 32 (Pt 2): 204–08. doi:10.1042/BST0320204. PMID 15046572. S2CID 20018642.
- ^ Pietilä MK, Roine E, Paulin L, Kalkkinen N, Bamford DH (April 2009). "An ssDNA virus infecting archaea: a new lineage of viruses with a membrane envelope". Molecular Microbiology. 72 (2): 307–19. doi:10.1111/j.1365-2958.2009.06642.x. PMID 19298373. S2CID 24894269.
- ^ Mochizuki T, Krupovic M, Pehau-Arnaudet G, Sako Y, Forterre P, Prangishvili D (August 2012). "Archaeal virus with exceptional virion architecture and the largest single-stranded DNA genome". Proceedings of the National Academy of Sciences of the United States of America. 109 (33): 13386–91. Bibcode:2012PNAS..10913386M. doi:10.1073/pnas.1203668109. PMC 3421227. PMID 22826255.
- ^ Mojica FJ, Díez-Villaseñor C, García-Martínez J, Soria E (February 2005). "Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements". Journal of Molecular Evolution. 60 (2): 174–82. Bibcode:2005JMolE..60..174M. doi:10.1007/s00239-004-0046-3. PMID 15791728. S2CID 27481111.
- ^ Makarova KS, Grishin NV, Shabalina SA, Wolf YI, Koonin EV (March 2006). "A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action". Biology Direct. 1 7. doi:10.1186/1745-6150-1-7. PMC 1462988. PMID 16545108.
- ^ a b Bernander R (August 1998). "Archaea and the cell cycle". Molecular Microbiology. 29 (4): 955–61. doi:10.1046/j.1365-2958.1998.00956.x. PMID 9767564. S2CID 34816545.
- ^ Kelman LM, Kelman Z (September 2004). "Multiple origins of replication in archaea". Trends in Microbiology. 12 (9): 399–401. doi:10.1016/j.tim.2004.07.001. PMID 15337158.
- ^ Lindås AC, Karlsson EA, Lindgren MT, Ettema TJ, Bernander R (December 2008). "A unique cell division machinery in the Archaea". Proceedings of the National Academy of Sciences of the United States of America. 105 (48): 18942–46. Bibcode:2008PNAS..10518942L. doi:10.1073/pnas.0809467105. PMC 2596248. PMID 18987308.
- ^ Samson RY, Obita T, Freund SM, Williams RL, Bell SD (December 2008). "A role for the ESCRT system in cell division in archaea". Science. 322 (5908): 1710–13. Bibcode:2008Sci...322.1710S. doi:10.1126/science.1165322. PMC 4121953. PMID 19008417.
- ^ Pelve EA, Lindås AC, Martens-Habbena W, de la Torre JR, Stahl DA, Bernander R (November 2011). "Cdv-based cell division and cell cycle organization in the thaumarchaeon Nitrosopumilus maritimus". Molecular Microbiology. 82 (3): 555–66. doi:10.1111/j.1365-2958.2011.07834.x. PMID 21923770. S2CID 1202516.
- ^ Caspi Y, Dekker C (2018). "Dividing the Archaeal Way: The Ancient Cdv Cell-Division Machinery". Frontiers in Microbiology. 9: 174. doi:10.3389/fmicb.2018.00174. PMC 5840170. PMID 29551994.
- ^ Onyenwoke RU, Brill JA, Farahi K, Wiegel J (October 2004). "Sporulation genes in members of the low G+C Gram-type-positive phylogenetic branch ( Firmicutes)". Archives of Microbiology. 182 (2–3): 182–92. Bibcode:2004ArMic.182..182O. doi:10.1007/s00203-004-0696-y. PMID 15340788. S2CID 34339306.
- ^ Kostrikina NA, Zvyagintseva IS, Duda VI (1991). "Cytological peculiarities of some extremely halophilic soil archaeobacteria". Arch. Microbiol. 156 (5): 344–49. Bibcode:1991ArMic.156..344K. doi:10.1007/BF00248708. S2CID 13316631.
- ^ Rajput A, Kumar M (2017). "Computational Exploration of Putative LuxR Solos in Archaea and Their Functional Implications in Quorum Sensing". Frontiers in Microbiology. 8: 798. doi:10.3389/fmicb.2017.00798. PMC 5413776. PMID 28515720.
- ^ DeLong EF, Pace NR (August 2001). "Environmental diversity of bacteria and archaea". Systematic Biology. 50 (4): 470–78. CiteSeerX 10.1.1.321.8828. doi:10.1080/106351501750435040. PMID 12116647.
- ^ a b Pikuta EV, Hoover RB, Tang J (2007). "Microbial extremophiles at the limits of life". Critical Reviews in Microbiology. 33 (3): 183–209. doi:10.1080/10408410701451948. PMID 17653987. S2CID 20225471.
- ^ Adam PS, Borrel G, Brochier-Armanet C, Gribaldo S (November 2017). "The growing tree of Archaea: new perspectives on their diversity, evolution and ecology". The ISME Journal. 11 (11): 2407–2425. Bibcode:2017ISMEJ..11.2407A. doi:10.1038/ismej.2017.122. PMC 5649171. PMID 28777382.
- ^ Madigan MT, Martino JM (2006). Brock Biology of Microorganisms (11th ed.). Pearson. p. 136. ISBN 978-0-13-196893-6.
- ^ Takai K, Nakamura K, Toki T, Tsunogai U, Miyazaki M, Miyazaki J, Hirayama H, Nakagawa S, Nunoura T, Horikoshi K (August 2008). "Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation". Proceedings of the National Academy of Sciences of the United States of America. 105 (31): 10949–54. Bibcode:2008PNAS..10510949T. doi:10.1073/pnas.0712334105. PMC 2490668. PMID 18664583.
- ^ Ciaramella M, Napoli A, Rossi M (February 2005). "Another extreme genome: how to live at pH 0". Trends in Microbiology. 13 (2): 49–51. doi:10.1016/j.tim.2004.12.001. PMID 15680761.
- ^ Javaux EJ (2006). "Extreme life on Earth--past, present and possibly beyond". Research in Microbiology. 157 (1): 37–48. doi:10.1016/j.resmic.2005.07.008. PMID 16376523.
- ^ Nealson KH (January 1999). "Post-Viking microbiology: new approaches, new data, new insights" (PDF). Origins of Life and Evolution of the Biosphere. 29 (1): 73–93. Bibcode:1999OLEB...29...73N. doi:10.1023/A:1006515817767. PMID 11536899. S2CID 12289639. Archived from the original (PDF) on 16 October 2019. Retrieved 24 June 2008.
- ^ Davies PC (1996). "The Transfer of Viable Microorganisms Between Planets". In Bock GR, Goode JA (eds.). Ciba Foundation Symposium 202 – Evolution of Hydrothermal Ecosystems on Earth (And Mars?). Novartis Foundation Symposia. Vol. 202. pp. 304–14, discussion 314–17. doi:10.1002/9780470514986.ch16. ISBN 9780470514986. PMID 9243022.
- ^ López-García P, López-López A, Moreira D, Rodríguez-Valera F (July 2001). "Diversity of free-living prokaryotes from a deep-sea site at the Antarctic Polar Front". FEMS Microbiology Ecology. 36 (2–3): 193–202. Bibcode:2001FEMME..36..193L. doi:10.1016/s0168-6496(01)00133-7. PMID 11451524.
- ^ Karner MB, DeLong EF, Karl DM (January 2001). "Archaeal dominance in the mesopelagic zone of the Pacific Ocean". Nature. 409 (6819): 507–10. Bibcode:2001Natur.409..507K. doi:10.1038/35054051. PMID 11206545. S2CID 6789859.
- ^ Giovannoni SJ, Stingl U (September 2005). "Molecular diversity and ecology of microbial plankton". Nature. 437 (7057): 343–48. Bibcode:2005Natur.437..343G. doi:10.1038/nature04158. PMID 16163344. S2CID 4349881.
- ^ DeLong EF, Karl DM (September 2005). "Genomic perspectives in microbial oceanography". Nature. 437 (7057): 336–42. Bibcode:2005Natur.437..336D. doi:10.1038/nature04157. PMID 16163343. S2CID 4400950.
- ^ Agogué H, Brink M, Dinasquet J, Herndl GJ (December 2008). "Major gradients in putatively nitrifying and non-nitrifying Archaea in the deep North Atlantic" (PDF). Nature. 456 (7223): 788–91. Bibcode:2008Natur.456..788A. doi:10.1038/nature07535. PMID 19037244. S2CID 54566989.
- ^ Teske A, Sørensen KB (January 2008). "Uncultured archaea in deep marine subsurface sediments: have we caught them all?". The ISME Journal. 2 (1): 3–18. Bibcode:2008ISMEJ...2....3T. doi:10.1038/ismej.2007.90. hdl:10379/14139. PMID 18180743.
- ^ Lipp JS, Morono Y, Inagaki F, Hinrichs KU (August 2008). "Significant contribution of Archaea to extant biomass in marine subsurface sediments". Nature. 454 (7207): 991–94. Bibcode:2008Natur.454..991L. doi:10.1038/nature07174. PMID 18641632. S2CID 4316347.
- ^ Danovaro R, Dell'Anno A, Corinaldesi C, Rastelli E, Cavicchioli R, Krupovic M, Noble RT, Nunoura T, Prangishvili D (October 2016). "Virus-mediated archaeal hecatomb in the deep seafloor". Science Advances. 2 (10): e1600492. Bibcode:2016SciA....2E0492D. doi:10.1126/sciadv.1600492. PMC 5061471. PMID 27757416.
- ^ Liu X, Pan J, Liu Y, Li M, Gu JD (October 2018). "Diversity and distribution of Archaea in global estuarine ecosystems". The Science of the Total Environment. 637–638: 349–358. Bibcode:2018ScTEn.637..349L. doi:10.1016/j.scitotenv.2018.05.016. PMID 29753224. S2CID 21697690.
- ^ Cabello P, Roldán MD, Moreno-Vivián C (November 2004). "Nitrate reduction and the nitrogen cycle in archaea". Microbiology. 150 (Pt 11): 3527–46. doi:10.1099/mic.0.27303-0. PMID 15528644.
- ^ Mehta MP, Baross JA (December 2006). "Nitrogen fixation at 92 °C by a hydrothermal vent archaeon". Science. 314 (5806): 1783–86. Bibcode:2006Sci...314.1783M. doi:10.1126/science.1134772. PMID 17170307. S2CID 84362603.
- ^ Coolen MJ, Abbas B, van Bleijswijk J, Hopmans EC, Kuypers MM, Wakeham SG, et al. (April 2007). "Putative ammonia-oxidizing Crenarchaeota in suboxic waters of the Black Sea: A basin-wide ecological study using 16S ribosomal and functional genes and membrane lipids". Environmental Microbiology. 9 (4): 1001–16. Bibcode:2007EnvMi...9.1001C. doi:10.1111/j.1462-2920.2006.01227.x. hdl:1912/2034. PMID 17359272.
- ^ Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (August 2006). "Archaea predominate among ammonia-oxidizing prokaryotes in soils". Nature. 442 (7104): 806–09. Bibcode:2006Natur.442..806L. doi:10.1038/nature04983. PMID 16915287. S2CID 4380804.
- ^ Baker BJ, Banfield JF (May 2003). "Microbial communities in acid mine drainage". FEMS Microbiology Ecology. 44 (2): 139–52. Bibcode:2003FEMME..44..139B. doi:10.1016/S0168-6496(03)00028-X. PMID 19719632.
- ^ Schimel J (August 2004). "Playing scales in the methane cycle: from microbial ecology to the globe". Proceedings of the National Academy of Sciences of the United States of America. 101 (34): 12400–01. Bibcode:2004PNAS..10112400S. doi:10.1073/pnas.0405075101. PMC 515073. PMID 15314221.
- ^ Eckburg PB, Lepp PW, Relman DA (February 2003). "Archaea and their potential role in human disease". Infection and Immunity. 71 (2): 591–96. doi:10.1128/IAI.71.2.591-596.2003. PMC 145348. PMID 12540534.
- ^ Cavicchioli R, Curmi PM, Saunders N, Thomas T (November 2003). "Pathogenic archaea: do they exist?". BioEssays. 25 (11): 1119–28. doi:10.1002/bies.10354. hdl:1959.4/39571. PMID 14579252.
- ^ Lepp PW, Brinig MM, Ouverney CC, Palm K, Armitage GC, Relman DA (April 2004). "Methanogenic Archaea and human periodontal disease". Proceedings of the National Academy of Sciences of the United States of America. 101 (16): 6176–81. Bibcode:2004PNAS..101.6176L. doi:10.1073/pnas.0308766101. PMC 395942. PMID 15067114.
- ^ Vianna ME, Conrads G, Gomes BP, Horz HP (April 2006). "Identification and quantification of archaea involved in primary endodontic infections". Journal of Clinical Microbiology. 44 (4): 1274–82. doi:10.1128/JCM.44.4.1274-1282.2006. PMC 1448633. PMID 16597851.
- ^ Jahn U, Gallenberger M, Paper W, Junglas B, Eisenreich W, Stetter KO, et al. (March 2008). "Nanoarchaeum equitans and Ignicoccus hospitalis: new insights into a unique, intimate association of two archaea". Journal of Bacteriology. 190 (5): 1743–50. doi:10.1128/JB.01731-07. PMC 2258681. PMID 18165302.
- ^ Chaban B, Ng SY, Jarrell KF (February 2006). "Archaeal habitats—from the extreme to the ordinary". Canadian Journal of Microbiology. 52 (2): 73–116. doi:10.1139/w05-147. PMID 16541146.
- ^ Schink B (June 1997). "Energetics of syntrophic cooperation in methanogenic degradation". Microbiology and Molecular Biology Reviews. 61 (2): 262–80. doi:10.1128/mmbr.61.2.262-280.1997. PMC 232610. PMID 9184013.
- ^ Lewis WH, Sendra KM, Embley TM, Esteban GF (2018). "Morphology and Phylogeny of a New Species of Anaerobic Ciliate, Trimyema finlayi n. sp., with Endosymbiotic Methanogens". Frontiers in Microbiology. 9: 140. doi:10.3389/fmicb.2018.00140. PMC 5826066. PMID 29515525.
- ^ Anaerobic ciliates as a model group for studying the biodiversityand symbioses in anoxic environments (PDF) (Thesis). 2020.
- ^ Wrede C, Dreier A, Kokoschka S, Hoppert M (2012). "Archaea in symbioses". Archaea. 2012: 596846. doi:10.1155/2012/596846. PMC 3544247. PMID 23326206.
- ^ Lange M, Westermann P, Ahring BK (February 2005). "Archaea in protozoa and metazoa". Applied Microbiology and Biotechnology. 66 (5): 465–474. doi:10.1007/s00253-004-1790-4. PMID 15630514. S2CID 22582800.
- ^ van Hoek AH, van Alen TA, Sprakel VS, Leunissen JA, Brigge T, Vogels GD, Hackstein JH (February 2000). "Multiple acquisition of methanogenic archaeal symbionts by anaerobic ciliates". Molecular Biology and Evolution. 17 (2): 251–258. doi:10.1093/oxfordjournals.molbev.a026304. PMID 10677847.
- ^ Preston CM, Wu KY, Molinski TF, DeLong EF (June 1996). "A psychrophilic crenarchaeon inhabits a marine sponge: Cenarchaeum symbiosum gen. nov., sp. nov". Proceedings of the National Academy of Sciences of the United States of America. 93 (13): 6241–6246. Bibcode:1996PNAS...93.6241P. doi:10.1073/pnas.93.13.6241. PMC 39006. PMID 8692799.
- ^ Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. (June 2005). "Diversity of the human intestinal microbial flora". Science. 308 (5728): 1635–38. Bibcode:2005Sci...308.1635E. doi:10.1126/science.1110591. PMC 1395357. PMID 15831718.
- ^ Samuel BS, Gordon JI (June 2006). "A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism". Proceedings of the National Academy of Sciences of the United States of America. 103 (26): 10011–16. Bibcode:2006PNAS..10310011S. doi:10.1073/pnas.0602187103. PMC 1479766. PMID 16782812.
- ^ Wegley L, Yu Y, Breitbart M, Casas V, Kline DI, Rohwer F (2004). "Coral-associated Archaea". Marine Ecology Progress Series. 273: 89–96. Bibcode:2004MEPS..273...89W. doi:10.3354/meps273089.
- ^ Chelius MK, Triplett EW (April 2001). "The Diversity of Archaea and Bacteria in Association with the Roots of Zea mays L". Microbial Ecology. 41 (3): 252–263. Bibcode:2001MicEc..41..252C. doi:10.1007/s002480000087. JSTOR 4251818. PMID 11391463. S2CID 20069511.
- ^ Simon HM, Dodsworth JA, Goodman RM (October 2000). "Crenarchaeota colonize terrestrial plant roots". Environmental Microbiology. 2 (5): 495–505. Bibcode:2000EnvMi...2..495S. doi:10.1046/j.1462-2920.2000.00131.x. PMID 11233158.
- ^ Gophna U, Charlebois RL, Doolittle WF (May 2004). "Have archaeal genes contributed to bacterial virulence?". Trends in Microbiology. 12 (5): 213–219. doi:10.1016/j.tim.2004.03.002. PMID 15120140.
- ^ Shiffman ME, Charalambous BM (2012). "The search for archaeal pathogens". Reviews in Medical Microbiology. 23 (3): 45–51. doi:10.1097/MRM.0b013e328353c7c9. ISSN 0954-139X. S2CID 88300156.
- ^ Breithaupt H (November 2001). "The hunt for living gold. The search for organisms in extreme environments yields useful enzymes for industry". EMBO Reports. 2 (11): 968–71. doi:10.1093/embo-reports/kve238. PMC 1084137. PMID 11713183.
- ^ a b Egorova K, Antranikian G (December 2005). "Industrial relevance of thermophilic Archaea". Current Opinion in Microbiology. 8 (6): 649–55. doi:10.1016/j.mib.2005.10.015. PMID 16257257.
- ^ Synowiecki J, Grzybowska B, Zdziebło A (2006). "Sources, properties and suitability of new thermostable enzymes in food processing". Critical Reviews in Food Science and Nutrition. 46 (3): 197–205. doi:10.1080/10408690590957296. PMID 16527752. S2CID 7208835.
- ^ Jenney FE, Adams MW (January 2008). "The impact of extremophiles on structural genomics (and vice versa)". Extremophiles. 12 (1): 39–50. doi:10.1007/s00792-007-0087-9. PMID 17563834. S2CID 22178563.
- ^ Schiraldi C, Giuliano M, De Rosa M (September 2002). "Perspectives on biotechnological applications of archaea". Archaea. 1 (2): 75–86. doi:10.1155/2002/436561. PMC 2685559. PMID 15803645.
- ^ Norris PR, Burton NP, Foulis NA (April 2000). "Acidophiles in bioreactor mineral processing". Extremophiles. 4 (2): 71–76. doi:10.1007/s007920050139. PMID 10805560. S2CID 19985179.
- ^ Shand RF, Leyva KJ (2008). "Archaeal Antimicrobials: An Undiscovered Country". In Blum P (ed.). Archaea: New Models for Prokaryotic Biology. Caister Academic Press. ISBN 978-1-904455-27-1.
Further reading
[edit]- Howland JL (2000). The Surprising Archaea: Discovering Another Domain of Life. Oxford University. ISBN 978-0-19-511183-5.
- Martinko JM, Madigan MT (2005). Brock Biology of Microorganisms (11th ed.). Englewood Cliffs, N.J: Prentice Hall. ISBN 978-0-13-144329-7.
- Garrett RA, Klenk H (2005). Archaea: Evolution, Physiology and Molecular Biology. WileyBlackwell. ISBN 978-1-4051-4404-9.
- Cavicchioli R (2007). Archaea: Molecular and Cellular Biology. American Society for Microbiology. ISBN 978-1-55581-391-8.
- Blum P, ed. (2008). Archaea: New Models for Prokaryotic Biology. Caister Academic Press. ISBN 978-1-904455-27-1.
- Lipps G (2008). "Archaeal Plasmids". Plasmids: Current Research and Future Trends. Caister Academic Press. ISBN 978-1-904455-35-6.
- Sapp J (2009). The New Foundations of Evolution: On the Tree of Life. New York: Oxford University Press. ISBN 978-0-19-538850-3.
- Schaechter M (2009). Archaea (Overview) in The Desk Encyclopedia of Microbiology (2nd ed.). San Diego and London: Elsevier Academic Press. ISBN 978-0-12-374980-2.
External links
[edit]General
[edit]- Introduction to the Archaea, ecology, systematics and morphology
- Oceans of Archaea – E.F. DeLong, ASM News, 2003
Classification
[edit]- NCBI taxonomy page on Archaea
- Genera of the domain Archaea – list of Prokaryotic names with Standing in Nomenclature
- Shotgun sequencing finds nanoorganisms – discovery of the ARMAN group of archaea
Genomics
[edit]- Browse any completed archaeal genome at UCSC
- Comparative Analysis of Archaeal Genomes Archived 16 February 2013 at the Wayback Machine (at DOE's IMG system)
 KSF
KSF