Carbocation
 From Wikipedia - Reading time: 8 min
From Wikipedia - Reading time: 8 min

A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium CH+
3, methanium CH+
5, acylium ions RCO+, and vinyl C
2H+
3 cations.[2]
Until the early 1970s, carbocations were called carbonium ions.[3] In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. This nomenclature was proposed by G. A. Olah.[4] Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. However, others have more narrowly defined the term 'carbonium ion' as formally protonated or alkylated alkanes (CR+
5, where R is H or alkyl), to the exclusion of non-classical carbocations like the 2-norbornyl cation.[5]
Definitions
[edit]According to the IUPAC, a carbocation is any cation containing an even number of electrons in which a significant portion of the positive charge resides on a carbon atom.[6] Prior to the observation of five-coordinate carbocations by Olah and coworkers, carbocation and carbonium ion were used interchangeably. Olah proposed a redefinition of carbonium ion as a carbocation featuring any type of three-center two-electron bonding, while a carbenium ion was newly coined to refer to a carbocation containing only two-center two-electron bonds with a three-coordinate positive carbon. Subsequently, others have used the term carbonium ion more narrowly to refer to species that are derived (at least formally) from electrophilic attack of H+ or R+ on an alkane, in analogy to other main group onium species, while a carbocation that contains any type of three-centered bonding is referred to as a non-classical carbocation. In this usage, 2-norbornyl cation is not a carbonium ion, because it is formally derived from protonation of an alkene (norbornene) rather than an alkane, although it is a non-classical carbocation due to its bridged structure. The IUPAC acknowledges the three divergent definitions of carbonium ion and urges care in the usage of this term. For the remainder of this article, the term carbonium ion will be used in this latter restricted sense, while non-classical carbocation will be used to refer to any carbocation with C–C and/or C–H σ-bonds delocalized by bridging.
Structure and properties
[edit]Carbonium ions
[edit]
Carbonium ions can be thought of as protonated or alkylated alkanes. They feature delocalized 3c-2e bonds. For this reason, they are often referred to as non-classical ions. A well studied example, albeit of no practical value, is the 2-norbornyl cation. Like carbenium ions, carbonium ions are often invoked as intermediates in the upgrading of hydrocarbons in refineries.
Carbenium ions
[edit]At least in a formal sense, carbenium ions are derived from the protonation (addition of H+) or alkylation (addition of R+) of a carbene or alkene. Thus, in at least one of their resonance depictions, they possess a carbon atom bearing a formal positive charge that is surrounded by a sextet of electrons (six valence electrons) instead of the usual octet required to fill the valence shell of carbon (octet rule). Therefore, carbenium ions (and carbocations in general) are often reactive, seeking to fill the octet of valence electrons as well as regain a neutral charge. In accord with VSEPR and Bent's rule, unless geometrically constrained to be pyramidal (e.g., 1-adamantyl cation), 3-coordinate carbon in carbenium ions are usually trigonal planar, with a pure p character empty orbital as its lowest unoccupied molecular orbital (LUMO) and CH/CC bonds formed from C(sp2) orbitals. A prototypical example is the methyl cation, CH+3.
History
[edit]The history of carbocations dates back to 1891 when G. Merling[8] reported that he added bromine to tropylidene (cycloheptatriene) and then heated the product to obtain a crystalline, water-soluble material, C
7H
7Br. He did not suggest a structure for it; however, Doering and Knox[9] convincingly showed that it was tropylium (cycloheptatrienylium) bromide. This ion is predicted to be aromatic by Hückel's rule.
In 1902, Norris and Kehrman independently discovered that colorless triphenylmethanol gives deep-yellow solutions in concentrated sulfuric acid. Triphenylmethyl chloride similarly formed orange complexes when treated with aluminium and tin chlorides. In 1902, Adolf von Baeyer recognized the salt-like character of the compounds formed. He dubbed the relationship between color and salt formation halochromy, of which malachite green is a prime example. The trityl carbocation (shown below) is indeed a stable carbocationic system, for example in the form of trityl hexafluorophosphate.[10]
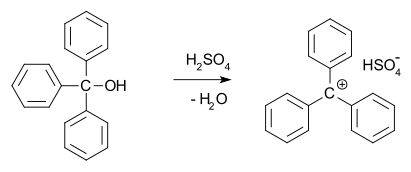
Carbocations are reactive intermediates in many organic reactions. This idea, first proposed by Julius Stieglitz in 1899,[11] was further developed by Hans Meerwein in his 1922 study[12][13] of the Wagner–Meerwein rearrangement. Carbocations were also found to be involved in the SN1 reaction, the E1 reaction, and in rearrangement reactions such as the Whitmore 1,2 shift. The chemical establishment was reluctant to accept the notion of a carbocation and for a long time the Journal of the American Chemical Society refused articles that mentioned them.
An NMR spectrum of a carbocation was first reported by Doering et al.[14] in 1958. It was the heptamethylbenzenium ion, made by treating hexamethylbenzene with methyl chloride and aluminium chloride. The stable 7-norbornadienyl cation was prepared by Story et al. in 1960[15] by reacting norbornadienyl chloride with silver tetrafluoroborate in sulfur dioxide at −80 °C. The NMR spectrum established that it was non-classically bridged (the first stable non-classical ion observed).
In 1962, Olah directly observed the tert-butyl carbocation by nuclear magnetic resonance as a stable species on dissolving tert-butyl fluoride in magic acid. The NMR spectrum of the norbornyl cation was reported by Schleyer et al.[16] It was shown to rapidly undergo proton-scrambling .[17]
See also
[edit]References
[edit]- ^ Scholz, Franziska; Himmel, Daniel; Scherer, Harald; Krossing, Ingo (2013). "Superacidic or Not…︁? Synthesis, Characterisation, and Acidity of the Room-Temperature Ionic Liquid [C(CH3)3]+ [Al2Br7]−". Chemistry – A European Journal. 19 (1): 109–116. doi:10.1002/chem.201203260. PMID 23180742.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 235, ISBN 978-0-471-72091-1
- ^ Robert B. Grossman (2007-07-31). The Art of Writing Reasonable Organic Reaction Mechanisms. Springer Science & Business Media. pp. 105. ISBN 978-0-387-95468-4.
- ^ Olah, George A. (1972). "Stable carbocations. CXVIII. General concept and structure of carbocations based on differentiation of trivalent (classical) carbenium ions from three-center bound penta- of tetracoordinated (nonclassical) carbonium ions. Role of carbocations in electrophilic reactions". Journal of the American Chemical Society. 94 (3): 808–820. doi:10.1021/ja00758a020.
- ^ Sommer, J.; Jost, R. (2000-01-01). "Carbenium and carbonium ions in liquid- and solid-superacid-catalyzed activation of small alkanes". Pure and Applied Chemistry. 72 (12): 2309–2318. doi:10.1351/pac200072122309. ISSN 1365-3075.
- ^ "Carbocation", IUPAC Compendium of Chemical Terminology, International Union of Applied Chemistry, 2009, doi:10.1351/goldbook.C00817, ISBN 978-0967855097, retrieved 2018-11-03
- ^ Scholz, F.; Himmel, D.; Heinemann, F. W.; Schleyer, P. v R.; Meyer, K.; Krossing, I. (2013-07-05). "Crystal Structure Determination of the Nonclassical 2-Norbornyl Cation". Science. 341 (6141): 62–64. Bibcode:2013Sci...341...62S. doi:10.1126/science.1238849. ISSN 0036-8075. PMID 23828938. S2CID 206549219.
- ^ Merling, G. (1891). "Ueber Tropin". Berichte der Deutschen Chemischen Gesellschaft. 24 (2): 3108–3126. doi:10.1002/cber.189102402151. ISSN 0365-9496.
- ^ Doering, W. von E.; Knox, L. H. (1954). "The Cycloheptatrienylium (Tropylium) Ion". Journal of the American Chemical Society. 76 (12): 3203–3206. doi:10.1021/ja01641a027.
- ^ Urch, C. (2001). "Triphenylmethyl Hexafluorophosphate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt363f. ISBN 0471936235.
- ^ "On the Constitution of the Salts of Imido-Ethers and other Carbimide Derivatives". American Chemical Journal. 21: 101. ISSN 0096-4085.
- ^ Meerwein, H.; Emster, K. van (1922). "About the equilibrium isomerism between bornyl chloride isobornyl chloride and camphene chlorohydrate". Berichte. 55: 2500.
- ^ Rzepa, H. S.; Allan, C. S. M. (2010). "Racemization of Isobornyl Chloride via Carbocations: A Nonclassical Look at a Classic Mechanism". Journal of Chemical Education. 87 (2): 221. Bibcode:2010JChEd..87..221R. doi:10.1021/ed800058c.
- ^ Doering, W. von E.; Saunders, M.; Boyton, H. G.; Earhart, H. W.; Wadley, E. F.; Edwards, W. R.; Laber, G. (1958). "The 1,1,2,3,4,5,6-heptamethylbenzenonium ion". Tetrahedron. 4 (1–2): 178–185. doi:10.1016/0040-4020(58)88016-3.
- ^ Story, Paul R.; Saunders, Martin (1960). "The 7-norbornadienyl carbonium ion". Journal of the American Chemical Society. 82 (23): 6199. doi:10.1021/ja01508a058.
- ^ Schleyer, Paul von R.; Watts, William E.; Fort, Raymond C.; Comisarow, Melvin B.; Olah, George A. (1964). "Stable Carbonium Ions. X.1 Direct Nuclear Magnetic Resonance Observation of the 2-Norbornyl Cation". Journal of the American Chemical Society. 86 (24): 5679–5680. doi:10.1021/ja01078a056.
- ^ Saunders, Martin; Schleyer, Paul von R.; Olah, George A. (1964). "Stable Carbonium Ions. XI.1 The Rate of Hydride Shifts in the 2-Norbornyl Cation". Journal of the American Chemical Society. 86 (24): 5680–5681. doi:10.1021/ja01078a057.
External links
[edit] Media related to Carbocations at Wikimedia Commons
Media related to Carbocations at Wikimedia Commons- Press Release The 1994 Nobel Prize in Chemistry". Nobelprize.org. 9 Jun 2010
 KSF
KSF