Fluorination by sulfur tetrafluoride
 From Wikipedia - Reading time: 4 min
From Wikipedia - Reading time: 4 min
Fluorination by sulfur tetrafluoride produces organofluorine compounds from oxygen-containing organic functional groups using sulfur tetrafluoride. The reaction has broad scope, and SF4 is an inexpensive reagent. It is however hazardous gas whose handling requires specialized apparatus.[1][2] Thus, for many laboratory scale fluorinations diethylaminosulfur trifluoride ("DAST") is used instead.[3]
Main functional group conversions
[edit]Carboxylic acids, amides, esters, and carboxylate salts convert to the trifluoromethyl derivatives, although conditions vary widely:
- SF4 + RCO2H → SO2 + RCF3 + HF
For carboxlic acids, the first step gives the acyl fluorides, in keeping with the tendency of SF4 to fluorinate acidic hydroxyl groups:
- SF4 + RCO2H → SOF2 + RC(O)F + HF
Similarly SF4 converts sulfonic acids to sulfonyl fluorides:
- SF4 + RSO3H → SOF2 + RSO2F + HF
Aldehydes and ketones convert to geminal difluorides:
- SF4 + R2CO → SF2O + R2CF2
Alcohols convert to alkyl fluorides, although this conversion works best with acidic alcohols, such as fluorinated alcohols:[4]
- SF4 + R3COH → SF2O + R3CF + HF
Mechanism
[edit]The mechanism of fluorination by SF4 is assumed to resemble chlorination by phosphorus pentachloride.[1] Hydrogen fluoride, a useful solvent for these reactions, activates SF4:
- SF4 + HF ⇌ SF+3 + HF−2
Species of the type ROSF3 are often invoked as intermediates. In the case of aldehydes and ketones, SF4 is thought to initially add across the double bond to give R2CFOSF3.[4]
Examples
[edit]A solution of sulfur tetrafluoride in hydrogen fluoride converts hydroxy-containing amino acids to the fluoro amino acids:[5]

When vicinal diols are combined with SF4, difluorination occurs with inversion of configuration at only one of the alcohols. This was demonstrated in the synthesis of meso-difluorosuccinate from (L)-tartrate and the synthesis of (D)- and (L)-difluorosuccinate from meso-tartrate.[6]

Carbonyl compounds generally react with SF4 to yield geminal difluorides. Reaction times tend to be on the order of hours and yields are moderate.[7] Fluorination of lactones can provide heterocyclic fluorides, although ring opening has been observed for γ-butyrolactone. The six-membered lactide does not experience ring opening.[8]

Fluorination opens epoxides to give either geminal or vicinal difluorides in most cases. Monoarylepoxides give geminal products with migration of the aryl group. Yields are low for sterically hindered di- and trisubstituted epoxides. Epoxides substituted with an ester group give vicinal difluorides via an alkoxysulfur trifluoride intermediate.[9]
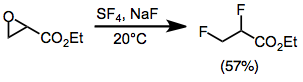
Carboxylic acids react with SF4 to afford trifluoromethyl compounds:[10]
- C6H13CO2H + 2 SF4 → C6H13CF3 + 2 SOF2 + HF
The formation of the trifluoromethyl derivative sometimes competes with formation of tetrafluoroalkyl ethers, which arise from the reaction between difluoromethyl cation and acyl fluoride.[11][12]
Sulfur tetrafluoride can be used to fluorinate polymers efficiently. This often has a profound effect on polymer properties—fluorination of polyvinyl alcohol, for instance, improves its resistance to strong acids and bases.[13]
A prostaglandin bearing a trifluoromethyl group at C-16 is prepared using sulfur tetrafluoride.[14]

Related reagents
[edit]For small scale reactions, SF4 can be inconvenient since it is a gas and stainless steel reaction vessels are required. Many transformations require elevated temperatures. The reaction generates hydrogen fluoride. These concerns have led to interest in alternative fluorinating reagents.[1] Selenium tetrafluoride, a liquid at room temperature, behaves similarly to SF4. Diethylaminosulfur trifluoride (DAST) is a derivative of SF4 that is easier to handle, albeit more expensive.[3]
References
[edit]- ^ a b c Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 1299, ISBN 978-0-471-72091-1
- ^ Wang, Chia-Lin J. (1985). "Fluorination by Sulfur Tetrafluoride". Organic Reactions. pp. 319–400. doi:10.1002/0471264180.or034.02. ISBN 978-0-471-26418-7.
- ^ a b Fauq, Abdul H.; Singh, Rajendra P.; Meshri, Dayal T. (2006). "Diethylaminosulfurtrifluoride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd175.pub2. ISBN 0-471-93623-5.
- ^ a b Boswell, G. A.; Ripka, W. C.; Scribner, R. M.; Tullock, C. W. (2011). "Fluorination by Sulfur Tetrafluoride". Organic Reactions. pp. 1–124. doi:10.1002/0471264180.or021.01. ISBN 978-0-471-26418-7.
- ^ Kollonitsch, J.; Marburg, S.; Perkins, Leroy (1975). "Selective Fluorination of Hydroxy Amines and Hydroxy Amino Acids with Sulfur Tetrafluoride in Liquid Hydrogen Fluoride". The Journal of Organic Chemistry. 40 (25): 3808–3809. doi:10.1021/jo00913a900.
- ^ Bell, M.; Hudlicky, M. J. Fluorine Chem. 1980, 15, 191.
- ^ Mobbs, H. J. Fluorine Chem. 1971, 1, 361.
- ^ Muratov, N.; Burmakov, I.; Kunshenko, V.; Alekseeva, A.; Yagupol'skii, M. J. Org. Chem. USSR (Engl. Transl.) 1982, 18, 1220.
- ^ Yagupol'skii, M.; Golikov, I.; Alekseeva, A.; Aleksandrov, M. J. Org. Chem. USSR (Engl. Transl.) 1971, 7, 737.
- ^ Hasek, W. R. (1961). "1,1,1-Trifluoroheptane". Organic Syntheses. 41: 104. doi:10.15227/orgsyn.041.0104.
- ^ Dmowski, W.; Kolinski, A. Rocz. Chem. 1974, 48, 1697.
- ^ Dmowski, W.; Kolinski, A. Pol. J. Chem. 1978, 52, 547.
- ^ Bezsolitsen, P.; Gorbunov, N.; Nazarov, A.; Khardin, P. Vysokomol. Soedin., Ser. A 1972, 14, 950 [C.A., 77, 75710e (1972)].
- ^ Holland, G. W.; Jernow, J. L.; Rosen, P. U.S. Pat. 4,256,911 (1981) [C.A., 89, 146500x (1978)].
 KSF
KSF