Lactonase
 From Wikipedia - Reading time: 7 min
From Wikipedia - Reading time: 7 min
| acyl-L-homoserine-lactone lactonohydrolase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 3.1.1.81 | ||||||||
| CAS no. | 389867-43-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
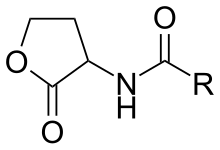
Lactonase (EC 3.1.1.81, acyl-homoserine lactonase; systematic name N-acyl-L-homoserine-lactone lactonohydrolase) is a metalloenzyme, produced by certain species of bacteria, which targets and inactivates acylated homoserine lactones (AHLs). It catalyzes the reaction
- an N-acyl-L-homoserine lactone + H2O an N-acyl-L-homoserine
Many species of α-, β-, and γ-proteobacteria produce acylated homoserine lactones, small hormone-like molecules commonly used as communication signals between bacterial cells in a population to regulate certain gene expression and phenotypic behaviours.[1] This type of gene regulation is known as quorum sensing.
Other names for these types of enzymes are Quorum-quenching N-acyl-homoserine lactonase, acyl homoserine degrading enzyme, acyl-homoserine lactone acylase, AHL lactonase, AHL-degrading enzyme, AHL-inactivating enzyme, AHLase, AhlD, AhlK, AiiA, AiiA lactonase, AiiA-like protein, AiiB, AiiC, AttM, delactonase, lactonase-like enzyme, N-acyl homoserine lactonase, N-acyl homoserine lactone hydrolase, N-acyl-homoserine lactone lactonase, N-acyl-L-homoserine lactone hydrolase, quorum-quenching lactonase, quorum-quenching N-acyl homoserine lactone hydrolase.[2][3][4][5][6][7][8][9][10][11][12]
Enzyme mechanism
[edit]Lactonase hydrolyzes the ester bond of the homoserine lactone ring of acylated homoserine lactones. In hydrolysing the lactone bond, lactonase prevents these signaling molecules from binding to their target transcriptional regulators, thus inhibiting quorum sensing.[13]
Enzyme Structure
[edit]A dinuclear zinc binding site is conserved in all known lactonases and essential for enzyme activity and protein folding.[14]
Zn1 is tetracoordinated by His104, His106, His169, and the bridging hydroxide ion. Zn2 has five ligands, including Asp191, His235, His109, Asp108, and the bridging hydroxide ion. The metal ions assist in polarizing the lactone bond, increasing the electrophilicity of the lactone ring’s carbonyl carbon. Isotopic labeling studies indicated that the ring opening occurs via an addition elimination reaction with water addition shown below.[15]
Biological Function
[edit]Lactonases are able to interfere with AHL-mediated quorum sensing. Some examples of these lactonases are AiiA produced by Bacillus species, AttM and AiiB produced by Agrobacterium tumefaciens, and QIcA produced by Hyphomicrobiales species.[16]
Lactonases have been reported for Bacillus, Agrobacterium, Rhodococcus, Streptomyces, Arthrobacter, Pseudomonas, and Klebsiella.[17] The Bacillus cereus group (consisting of B. cereus, B. thuringiensis, B. mycoides, and B. anthracis) was found to contain nine genes homologous to the AiiA gene that encode AHL-inactivating enzymes, with the catalytic zinc-binding motif conserved in all cases.[18]
In the phytopathogen A. tumefaciens, AiiB lactonase acts as a fine modulator that essentially delays the release of lactone OC8-HSL and the resultant number of tumors produced by the pathogen. AttM lactonase mediates the degradation of the lactone OC8-HSL in wounded plant tissues.[19]
The primary activity of the anti-atherosclerotic paraoxonase (PON) enzymes is as lactonase.[20] Oxidized polyunsaturated fatty acids (notably in oxidized low-density lipoprotein) form lactone-like structures that are PON substrates.[20]
Ecology
[edit]It is still unclear the ecological effects of lactonase but it has been proposed that since bacteria mostly coexist with other microorganisms in the environment, some bacteria strains could have evolved its feeding strategies and utilize AHLs as their main resource for energy and nitrogen.[21]
Applications
[edit]Understanding the mechanisms and purposes of lactonase activity could lead to potential applied roles for these lactonases to control bacterial infections by inhibiting quorum-sensing activity and bring about profound effects on human health and the environment. However, in both the chemical and enzymatic lactonolysis, the reaction is reversible, complicating direct therapeutic application of lactonases.[22]
Pseudomonas aeruginosa, is an AHL-producing bacteria an opportunistic pathogen that infects immuno-compromised patients,[23] and is found in lung infections of cystic fibrosis patients. P. aeruginosa relies on quorum sensing via production of lactones N-butanoyl-L-homoserine (C4-HSL) and N-(3-oxododecanoyl)-l-HSL (3-oxo-C12-HSL) to regulate swarming, toxin and protease production, and proper biofilm formation. The absence of one or more components of the quorum-sensing system results in a significant reduction in virulence of the pathogen.[24]
Erwinia carotovora is a plant pathogen that causes soft rot in a number of crops such as potatoes and carrots [25] by using N-hexanoyl-l-HSL (C6-HSL) quorum sensing to evade the plant's defense systems and coordinate its production of pectate lyase during the infection process.[26]
Plants expressing AHL-Lactonase were shown to demonstrate enhanced resistance to infection from the pathogen Erwinia carotovora. Expression of virulence genes in E. Carotovora is regulated by N-(3-oxohexanoyl)-L-homoserine lactone (OHHL). Presumably, OHHL-hydrolysis via lactonase reduced OHHL levels, inhibiting the quorum-sensing systems driving virulence gene expression.[18]
See also
[edit]- 1,4-lactonase
- 2-pyrone-4,6-dicarboxylate lactonase
- 3-oxoadipate enol-lactonase
- Actinomycin lactonase
- Deoxylimonate A-ring-lactonase
- Gluconolactonase
- L-rhamnono-1,4-lactonase
- Limonin-D-ring-lactonase
- Steroid-lactonase
- Triacetate-lactonase
- Xylono-1,4-lactonase
References
[edit]- ^ Fuqua, C.; Winans, S. C.; Greenberg, E. P. (1996). "Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators". Annu. Rev. Microbiol. 50: 727–751. doi:10.1146/annurev.micro.50.1.727. PMID 8905097.
- ^ Thomas PW, Stone EM, Costello AL, Tierney DL, Fast W (May 2005). "The quorum-quenching lactonase from Bacillus thuringiensis is a metalloprotein". Biochemistry. 44 (20): 7559–69. doi:10.1021/bi050050m. PMID 15895999.
- ^ Dong YH, Gusti AR, Zhang Q, Xu JL, Zhang LH (April 2002). "Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species". Applied and Environmental Microbiology. 68 (4): 1754–9. doi:10.1128/AEM.68.4.1754-1759.2002. PMC 123891. PMID 11916693.
- ^ Wang LH, Weng LX, Dong YH, Zhang LH (April 2004). "Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase)". The Journal of Biological Chemistry. 279 (14): 13645–51. doi:10.1074/jbc.M311194200. PMID 14734559.
- ^ Dong YH, Xu JL, Li XZ, Zhang LH (March 2000). "AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora". Proceedings of the National Academy of Sciences of the United States of America. 97 (7): 3526–31. doi:10.1073/pnas.060023897. PMC 16273. PMID 10716724.
- ^ Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH (June 2001). "Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase". Nature. 411 (6839): 813–7. doi:10.1038/35081101. PMID 11459062. S2CID 4324448.
- ^ Lee SJ, Park SY, Lee JJ, Yum DY, Koo BT, Lee JK (August 2002). "Genes encoding the N-acyl homoserine lactone-degrading enzyme are widespread in many subspecies of Bacillus thuringiensis". Applied and Environmental Microbiology. 68 (8): 3919–24. doi:10.1128/aem.68.8.3919-3924.2002. PMC 124016. PMID 12147491.
- ^ Park SY, Lee SJ, Oh TK, Oh JW, Koo BT, Yum DY, Lee JK (June 2003). "AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria". Microbiology. 149 (Pt 6): 1541–50. doi:10.1099/mic.0.26269-0. PMID 12777494.
- ^ Ulrich RL (October 2004). "Quorum quenching: enzymatic disruption of N-acylhomoserine lactone-mediated bacterial communication in Burkholderia thailandensis". Applied and Environmental Microbiology. 70 (10): 6173–80. doi:10.1128/AEM.70.10.6173-6180.2004. PMC 522112. PMID 15466564.
- ^ Kim MH, Choi WC, Kang HO, Lee JS, Kang BS, Kim KJ, Derewenda ZS, Oh TK, Lee CH, Lee JK (December 2005). "The molecular structure and catalytic mechanism of a quorum-quenching N-acyl-L-homoserine lactone hydrolase". Proceedings of the National Academy of Sciences of the United States of America. 102 (49): 17606–11. doi:10.1073/pnas.0504996102. PMC 1295591. PMID 16314577.
- ^ Liu D, Lepore BW, Petsko GA, Thomas PW, Stone EM, Fast W, Ringe D (August 2005). "Three-dimensional structure of the quorum-quenching N-acyl homoserine lactone hydrolase from Bacillus thuringiensis". Proceedings of the National Academy of Sciences of the United States of America. 102 (33): 11882–7. doi:10.1073/pnas.0505255102. PMC 1187999. PMID 16087890.
- ^ Yang F, Wang LH, Wang J, Dong YH, Hu JY, Zhang LH (July 2005). "Quorum quenching enzyme activity is widely conserved in the sera of mammalian species". FEBS Letters. 579 (17): 3713–7. doi:10.1016/j.febslet.2005.05.060. PMID 15963993.
- ^ Dong, Y.; Wang, L.; Xu, J.; Zhang, H.; Zhang, X.; Zhang, L. (2001). "Quencing quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase". Nature. 411 (6839): 813–817. Bibcode:2001Natur.411..813D. doi:10.1038/35081101. PMID 11459062. S2CID 4324448.
- ^ Thomas P. W.; Stone E. M.; Costello A. L.; Tierney D. L.; Fast W. (2005). "The quorum-quenching lactonase from Bacillus thuringiensis is a metalloprotein". Biochemistry. 44 (20): 7559–7569. doi:10.1021/bi050050m. PMID 15895999.
- ^ Momb J.; Wang C.; Liu D.; Thomas P. W.; Petsko G. A.; Guo H.; Ringe D; Fast W. (2008). "Mechanism of the quorum-quenching lactonae (AiiA) from Bacillus thuringiensis. 2. Substrate modeling and active site mutations". Biochemistry. 47 (29): 7715–7725. doi:10.1021/bi8003704. PMC 2646874. PMID 18627130.
- ^ Riaz K.; Elmerich, C.; Moreira, D.; Raffoux A.; Dessaux Y.; Faure D. (2008). "A metagenomic analysis of soil bacteria extends the diversity of quorum-quenching lactonases". Environmental Microbiology. 10 (3): 560–570. doi:10.1111/j.1462-2920.2007.01475.x. PMID 18201196.
- ^ Schipper C.; Hornung C.; Bijtenhoorn P.; Quitschau M.; Grond S.; Streit W. R. (2009). "Metagenome-derived clones encoding two novel lactonase family proteins involved in biofilm inhibition in Pseudomonas aeruginosa". Applied and Environmental Microbiology. 75 (1): 224–233. doi:10.1128/aem.01389-08. PMC 2612230. PMID 18997026.
- ^ a b Dong Y. H.; Gusti A. R.; Zhang Q.; Xu J. L.; Zhang, L. H. (2002). "Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species". Applied and Environmental Microbiology. 68 (4): 1754–1759. doi:10.1128/AEM.68.4.1754-1759.2002. PMC 123891. PMID 11916693.
- ^ Haudecoeur E.; Tannieres M.; Cirou A.; Raffoux A; Dessaux Y.; Faure D. (2009). "Different regulation and roles of lactonases AiiB and AttM in Agrobacterium tumefaciens C58". Molecular Plant-Microbe Interactions. 22 (5): 529–537. doi:10.1094/MPMI-22-5-0529. PMID 19348571.
- ^ a b Chistiakov DA, Melnichenko AA, Orekhov AN, Bobryshev YV (2017). "Paraoxonase and atherosclerosis-related cardiovascular diseases". Biochimie. 132: 19–27. doi:10.1016/j.biochi.2016.10.010. PMID 27771368.
- ^ Leadbetter, J. R. G.; Greenberg, E. P. (2000). "Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus". J. Bacteriol. 182 (24): 6921–6926. doi:10.1128/JB.182.24.6921-6926.2000. PMC 94816. PMID 11092851.
- ^ Rasmussen T. B.; Givskov M. (2006). "Quorum-sensing inhibitors as anti-pathogenic drugs". International Journal of Medical Microbiology. 296 (2–3): 149–161. doi:10.1016/j.ijmm.2006.02.005. PMID 16503194.
- ^ Whitehead, N. A.; Barnard A. M. L.; Slater H.; Simpson, N. J. L.; Salmond, G. P. C. (2001). "Quorum sensing in Gram-negative bacteria". FEMS Microbiol. Rev. 25 (4): 365–404. doi:10.1111/j.1574-6976.2001.tb00583.x. PMID 11524130..
- ^ Smith R. S.; Iglewski B. H. (2003). "P. aeruginosa quorum-sensing systems and virulence". Current Opinion in Microbiology. 6 (1): 56–60. doi:10.1016/S1369-5274(03)00008-0. PMID 12615220.
- ^ Pirhonen, M.; Flegom, D.; Heikinheimo, R.; Palva, E. T. (1993). "A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora". EMBO J. 12 (6): 2467–2476. doi:10.1002/j.1460-2075.1993.tb05901.x. PMC 413482. PMID 8508772.
- ^ Von Bodman S. B.; Bauer W. D.; Coplin D. L. (2003). "Quorum-sensing in plant-pathogenic bacteria". Annual Review of Phytopathology. 41: 455–482. doi:10.1146/annurev.phyto.41.052002.095652. PMID 12730390.
 KSF
KSF