Perfluorosulfonic acids
 From Wikipedia - Reading time: 2 min
From Wikipedia - Reading time: 2 min
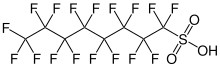
Perfluorosulfonic acids (PFSAs) are chemical compounds of the formula CnF(2n+1)SO3H and thus belong to the family of perfluorinated and polyfluorinated alkyl compounds (PFASs). The simplest example of a perfluorosulfonic acid is the trifluoromethanesulfonic acid. Perfluorosulfonic acids with six or more perfluorinated carbon atoms, i.e. from perfluorohexanesulfonic acid onwards, are referred to as long-chain.[1]

Properties
[edit]Perfluorosulfonic acids are organofluoroanalogues of conventional alkanesulfonic acids, but they are several pKA units stronger (and are therefore strong acids). Their perfluoroalkyl chain has a highly hydrophobic character.
Use
[edit]Perfluorooctanesulfonic acid, for example, has been used in hard chromium plating. Since the early 2000's 6:2 fluorotelomersulfonic acid has been used as a replacement for perfluorooctanesulfonic acid.[2]
Regulation
[edit]Perfluorooctanesulfonic acid was included in Annex B of the Stockholm Convention in 2009 and subsequently in the EU POPs Regulation.[3]
Perfluorohexanesulfonic acid was included in Annex B of the Stockholm Convention in 2022.[4]
Examples
[edit]| Name | Abbreviation | Structural formula | Molecular weight (g/mol) | CAS No. |
|---|---|---|---|---|
| Perfluoropropanesulfonic acid | PFPrS | C3F7SO3H | 250.09 | 423-41-6 |
| Perfluorobutanesulfonic acid | PFBS | C4F9SO3H | 300.10 | 375-73-5 |
| Perfluoropentanesulfonic acid | PFPS | C5F11SO3H | 350.11 | 2706-91-4 |
| Perfluorohexanesulfonic acid | PFHxS | C6F13SO3H | 400.12 | 355-46-4 |
| Perfluoroheptanesulfonic acid | PFHpS | C7F15SO3H | 450.12 | 375-92-8 |
| Perfluorooctanesulfonic acid | PFOS | C8F17SO3H | 500.13 | 1763-23-1 |
| Perfluorononanesulfonic acid | PFNS | C9F19SO3H | 550.14 | 68259-12-1 |
| Perfluorodecanesulfonic acid | PFDS | C10F21SO3H | 600.15 | 335-77-3 |
See also
[edit]- Perfluoro(4-ethylcyclohexane)sulfonic acid, a cyclic analogue
Literature
[edit]- OECD, ed. (2022), "3. Perfluoroalkane sulfonic (a) and sulfinic (b) acids", Fact Cards of Major Groups of Per- and Polyfluoroalkyl Substances (PFASs), OECD Environment, Health and Safety Publications 68 Series on Risk Management, pp. 31–41
References
[edit]- ^ "Terminology | FluoroCouncil".
- ^ Bohannon, Meredith E; Narizzano, Allison M; Guigni, Blas A; East, Andrew G; Quinn, Michael J (2023). "Next-generation PFAS 6:2 fluorotelomer sulfonate reduces plaque formation in exposed white-footed mice". Toxicological Sciences. 192 (1): 97–105. doi:10.1093/toxsci/kfad006.
- ^ Regulation (EU) 2019/1021 of the European Parliament and of the Council of 20 June 2019 on persistent organic pollutants
- ^ "UN experts recommend elimination of additional hazardous chemicals to protect human health and the environment".
 KSF
KSF