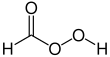
Performic acid
 From Wikipedia - Reading time: 5 min
From Wikipedia - Reading time: 5 min
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methaneperoxoic acid[1] | |||
| Other names
Performic acid
Hydroperoxyformaldehyde Formyl hydroperoxide Permethanoic acid Peroxyformic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.124.147 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH2O3 | |||
| Molar mass | 62.024 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Melting point | −18 °C (0 °F; 255 K)[2] | ||
| Boiling point | 50 °C (122 °F; 323 K) (at 13.3 kPa; 90% pure acid)[2] | ||
| Acidity (pKa) | 7.1[2][3] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Performic acid (PFA) is an organic compound with the formula CH2O3. It is an unstable colorless liquid which can be produced by mixing formic acid with hydrogen peroxide. Owing to its oxidizing and disinfecting action, it is used in the chemical, medical and food industries.
Properties and applications
[edit]Performic acid is a colorless liquid soluble in water, alcohols, ether, benzene, chloroform and other organic solvents.[4][5] Its strong oxidizing properties are used for cleaving disulfide bonds in protein mapping,[6] as well as for epoxidation, hydroxylation[7] and oxidation reactions in organic synthesis.[5] In the medical and food industries, performic acid is commonly used to disinfect equipment. It is effective against viruses, bacterial spores, algae, microscopic fungi and mycobacteria, as well as other microorganisms such as zooplankton.
The popularity of performic acid as a sterilizer originates from the safe nature of its degradation products, mostly carbon dioxide, oxygen and water.[4][8] The disinfecting action of performic acid is also faster than that of the related compounds peracetic acid and hydrogen peroxide.[9] The major drawbacks of performic acid are handling dangers related to its high reactivity, as well as instability, especially upon heating, which means that the acid must be used within about 12 hours of it being synthesised.[9][10][11]
Synthesis
[edit]Performic acid is synthesized by the reaction of formic acid and hydrogen peroxide by the following equilibrium reaction:
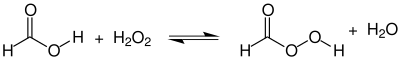
Synthesis of pure performic acid has not been reported, but aqueous solutions up to about 48% can be formed by simply mixing equimolar amounts of concentrated aqueous reactant solutions.[4] Using an excess of either reactant shifts the equilibrium towards the product side. The aqueous product solution can be distilled to increase the concentration of performic acid to about 90%.[4]
This reaction is reversible and can be used for large scale industrial production if accelerated with a catalyst; however, its temperature must be kept below 80–85 °C to avoid an explosion.[12] The catalyst can be nitric, hydrofluoric, phosphoric or sulfuric acid or their salts;[4][13] it can also be an organic compound containing at least one ester group, such as carboxylic acid ester[14] or peracetic acid.[9]
Safety
[edit]Performic acid is non-toxic; it does irritate the skin, but less so than peracetic acid. Concentrated acid (above 50%) is highly reactive; it readily decomposes upon heating, and explodes upon rapid heating to 80–85 °C. It may ignite or explode at room temperature when combined with flammable substances, such as formaldehyde, benzaldehyde, or aniline, and explodes violently upon addition of metal powders.[4] For this reason, spilled performic acid is diluted with cold water and collected with neutral, non-flammable inorganic absorbents, such as vermiculite.[5]
References
[edit]- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 749. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b c Elvers, B. et al. (ed.) (1991) Ullmann's Encyclopedia of Industrial Chemistry, 5th ed. Vol. A19, Wiley, p. 206
- ^ F. A. Carroll Perspectives on Structure and Mechanism in Organic Chemistry, Wiley-Interscience, 2010, ISBN 0-470-27610-X p. 416
- ^ a b c d e f Swern, Daniel (1949). "Organic Peracids". Chemical Reviews. 45: 1–68. doi:10.1021/cr60140a001.
In the absence of catalysts, performic acid explodes when heated rapidly to 80–85°C.
- ^ a b c Pradyot Patnaik A comprehensive guide to the hazardous properties of chemical substances, Wiley-Interscience, 2007, ISBN 0-471-71458-5, p. 128
- ^ Simpson, R. J. (2007). "Performic Acid Oxidation of Proteins". Cold Spring Harbor Protocols. 2007 (3): pdb.prot4698. doi:10.1101/pdb.prot4698.
- ^ "trans-1,2-CYCLOHEXANEDIOL". Organic Syntheses. 28: 35. 1948. doi:10.15227/orgsyn.028.0035.
- ^ Gehr, R; Chen, D; Moreau, M (2009). "Performic acid (PFA): tests on an advanced primary effluent show promising disinfection performance" (PDF). Water Science and Technology. 59 (1): 89–96. doi:10.2166/wst.2009.761. PMID 19151490. Archived from the original (PDF) on 2010-11-16.
- ^ a b c Preuss, A., Fuchs, R., Huss, M. & Schneider, R. 2001 Aqueous Disinfecting Agent Containing Performic Acid and Peracetic Acid Process for Production and Process for Use Thereof U.S. patent 6,211,237, Issue date: April 3, 2001
- ^ Bydzovska, O. & Merka, V. (1981). "Disinfecting Properties of Performic Acid Against Bacteriophage (X 174 as a Model of Small Envelope-free Viruses". J. Hygiene, Epidemiology Microbiology and Immunology. 25 (4): 414–423. PMID 6459365.
- ^ Ripin, D.H.B.; et al. (2007). "Execution of a Performic Acid Oxidation on Multikilogram Scale". Org. Process Res. Dev. 11 (4): 762–765. doi:10.1021/op700039r.
- ^ Elvers, B. et al. (ed.) (1991) Ullmann's Encyclopedia of Industrial Chemistry, 5th ed. Vol. A12, Wiley, p. 16
- ^ English, James; Gregory, J. Delafield (1947). "Performic Acid Hydroxylation of a,p-Unsaturated Acids and Esters". Journal of the American Chemical Society. 69: 2120. doi:10.1021/ja01201a016.
- ^ Matilla, T. and Aksela, R. 2000 Method for the Preparation of Aqueous Solutions Containing Performic Acid as Well as Their Use. U.S. patent 6,049,002, Issue date: April 11, 2000
 KSF
KSF