Phosphenium
 From Wikipedia - Reading time: 7 min
From Wikipedia - Reading time: 7 min
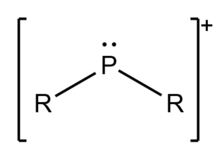
Phosphenium ions, not to be confused with phosphonium or phosphirenium, are divalent cations of phosphorus of the form [PR2]+. Phosphenium ions have long been proposed as reaction intermediates.
Synthesis
[edit]Legacy methods
[edit]The first cyclic phosphenium compounds were reported in 1972 by Suzanne Fleming and coworkers.[1] Acyclic phosphenium compounds were synthesized by Fleming's thesis advisor Robert Parry in 1976.[2]

Methods
[edit]Several methods exist for the preparation of two-coordinate phosphorus ions. A common method involves halide abstraction from halophosphines:[3]
- R2PCl + AlCl3 → [R2P+][AlCl−
4]
Protonolysis of tris(dimethylamino)phosphine affords the phosphenium salt:[4]
- P(NMe2)3 + 2 HOTf → [P(NMe2)2]OTf + [HNMe2]OTf
Weakly coordinating anions are desirable. Triflic acid is often used.[3]
N-heterocyclic phosphenium (NHP) have also been reported.[5] Reaction of PI3 with the α-diimine yields the NHP cation by reduction of the diimine and oxidation of iodine.

Structure and bonding
[edit]According to X-ray crystallography, [(i-Pr2N)2P]+ is nearly planar consistent with sp2-hybridized phosphorus center.[6] The planarity of the nitrogen center is consistent with the resonance of the lone pair of the nitrogen atom as a pi bond to the empty phosphorus 3p orbital perpendicular to the N−P−N plane. An idealized sp2 phosphorus center would expect an N−P−N angle of 120°. The tighter N−P−N angle observed in the crystal structure can be interpreted as the result of repulsion between the phosphorus lone pair with the bulky i-Pr2N ligands, as the P(NH
2)+
2 and PH+
2 molecules have bond angles closer to 110° and 90°, respectively.[3][6]

Calculations also show that the analogy to carbenes is lessened by strongly π-donating substituents. With NH2 substituents, the phosphenium cation assumes allyl character.[7] Generalized Valence Bond (GVB) calculations of the phosphenium ions as having a singlet ground state, singlet-triplet separation increases with increasing electronegativity of the ligands.[3][8][9] The singlet-triplet separation for PH+
2 and PF+
2 were calculated to be 20.38 and 84.00 kcal/mol, respectively. Additionally, the triplet state of the phosphenium ion displays a greater bond angle at the phosphorus. For example, the calculated bond angle of the singlet state of PH+
2 is approximately 94° compared to 121.5° in the triplet state. Calculated bond lengths between the two states are not significantly impacted.[9]
Reactivity
[edit]Phosphenium is isoelectronic with singlet (Fisher) carbenes and are therefore expected to be Lewis acidic. Adducts are produced by combining [P(NMe2)2]+ and P(NMe2)3:[2]
- P(NMe2)2]+ + P(NMe2)3 → [(Me2N)3P−P(NMe2)2]+
Being electrophilic, they undergo C−H insertion reactions.[10]
Reactions with dienes
[edit]Phosphenium intermediates are invoked as intermediates in the McCormack reaction, a method for the synthesis of organophosphorus heterocycles. An illustrative reaction involves phenyldichlorophosphine and isoprene:[11]
Isolated phosphenium salts undergo this reaction readily.[12]
There are few examples of reactions catalyzed by phosphenium. In 2018, Rei Kinjo and coworkers reported the hydroboration of pyridines by the NHP salt, 1,3,2-diazaphosphenium triflate. The NHP is proposed to act as a hydride transfer reagent in this reaction.[13]

Coordination chemistry
[edit]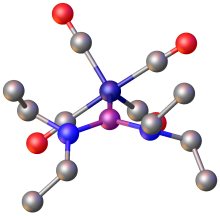
4 salt.
Phosphenium ions serve as ligands in coordination chemistry.[2] [(R2N)2PFe(CO)4]+ was prepared by two methods: the first being the abstraction of a fluoride ion from (R2N)2(F)PFe(CO)4 by PF5. The second method is the direct substitution reaction of Fe(CO)5 by the phosphenium ion [P(NR2)]+.[14] Related complexes exist of the type Fe(CO)4L, where L = [(Me2N)2P]+, [(Et2N)2P]+, [(Me2N)(Cl)P]+, and [(en)P]+ (en = C2H4(NH2)2).[3]

N-heterocyclic phosphenium-transition metal complexes are anticipated due to their isoelectronicity to N-heterocyclic carbenes. In 2004, Martin Nieger and coworkers synthesized two Cobalt-NHP complexes. Experimental and computation analysis of the complexes confirmed the expected L→M σ donation and the M→L π backbonding, though the phosphenium was observed to have reduced σ donor ability. It was suggested that this is due to the greater s orbital-character of the phosphorus lone pair compared to the lone pair of the analogous carbene.[15] Additional studies of NHP ligands by Christine Thomas and coworkers in 2012, likened the phosphenium to nitrosyl.[16] Nitrosyl is well known for its redox non-innocence, coordinating in either a bent or linear geometry that possess different L–M bonding modes. It was observed that NHPs in complex with a transition metal may have either a planar or pyramidal geometry about the phosphorus, reminiscent of the linear versus bent geometries of nitrosyl. Highly electron-rich metal complexes were observed to have pyramidal phosphorus, while less electron-rich metals showed greater phosphenium character at the phosphorus. Pyramidal phosphorus indicates significant lone pair character at phosphorus, suggesting that the L→M σ donation and the M→L π backbonding interactions have been replaced with M→L σ donation, formally oxidizing the metal center by two electrons.[16]

Additional reading
[edit]Cycloadditions
[edit]- Cowley, A. H.; Kemp, R. A.; Lasch, J. G.; Norman, N. C.; Stewart, C. A.; Whittlesey, B. R.; Wright, T. C. (1986-03-01). "Reactivity of phosphenium ions toward 1,3- and 1,4-dienes". Inorganic Chemistry. 25 (6): 740–749. doi:10.1021/ic00226a007. ISSN 0020-1669.
- SooHoo, Carlton K.; Baxter, S. G. (1983-11-01). "Phosphenium ions as dienophiles". Journal of the American Chemical Society. 105 (25): 7443–7444. doi:10.1021/ja00363a039. ISSN 0002-7863.
- Thomas, Michael G.; Schultz, Charles W.; Parry, R. W. (1977-05-01). "Synthesis and characterization of dicoordinate phosphorus cations. Compounds of the type [(R2N)2P]+[Y]− and their congeners". Inorganic Chemistry. 16 (5): 994–1001. doi:10.1021/ic50171a005. ISSN 0020-1669.
Adducts
[edit]- Baxter, S. G.; Collins, R. L.; Cowley, A. H.; Sena, S. F. (1983-11-01). "Ferrocenyl-substituted phosphenium cations and phosphide anions". Inorganic Chemistry. 22 (23): 3475–3479. doi:10.1021/ic00165a022. ISSN 0020-1669.
- Cowley, A. H.; Lattman, M.; Wilburn, J. C. (1981-09-01). "NMR study of the reactions of phosphorus(III) halides with halide ion acceptors. Two-coordinate phosphorus cations with bulky ligands". Inorganic Chemistry. 20 (9): 2916–2919. doi:10.1021/ic50223a034. ISSN 0020-1669.
Electrophilic reactions
[edit]- Cowley, A. H.; Kemp, R. A.; Stewart, C. A. (1982-06-01). "Reaction of stannocene and plumbocene with phosphenium ions: oxidative addition of carbon-hydrogen bonds to low-coordination number main group species". Journal of the American Chemical Society. 104 (11): 3239–3240. doi:10.1021/ja00375a061. ISSN 0002-7863.
- Cowley, A. H.; Mehrotra, S. K. (1983-04-01). "Ring methyl to phosphorus hydrogen shifts in pentamethylcyclopentadienyl-substituted phosphorus cations: parallel between main-group and transition-metal chemistry". Journal of the American Chemical Society. 105 (7): 2074–2075. doi:10.1021/ja00345a072. ISSN 0002-7863.
Coordination complexes
[edit]- Day, V. W.; Tavanaiepour, I.; Abdel-Meguid, S. S.; Kirner, J. F.; Goh, L. Y.; Muetterties, E. L. (1982). "Modes of Phosphite Reactions with Transition-Metal Complexes. Crystal Structure of (η5-C5H5)Cr[P(O)(OCH3)2](CO)2[P(OCH3)3]; and [(CH3O)2PMo{P(OCH3)3]5}+(PF−
6)". Inorganic Chemistry. 21: 657–663. doi:10.1021/ic00132a038. - Hutchins, L. D.; Paine, R. T.; Campana, C. F. (1980). "Structure and Bonding in a Phosphenium Ion-Metal complex, CH3NCH2CH2N(CH3)PMo(η5-C5H5)(CO)2. An Example of a Molybdenum-Phosphorus Multiple Bond". Journal of the American Chemical Society. 102 (13): 4521–4523. doi:10.1021/ja00533a039. ISSN 0002-7863.
- Light, R. W.; Paine, R. T. (1978). "Interaction of the Dicoordinate Phosphorus Cation 1,3-Dimethyl-1,3,2-Diazaphospholidide with Transition Metal Nucleophiles". Journal of the American Chemical Society. 100 (7): 2230–2231. doi:10.1021/ja00475a043. ISSN 0002-7863.
- Dubois, Donn A.; Duesler, Eileen N.; Paine, Robert T. (1983). "Synthesis and Structure of a Bimetallic Diphosphenium ion Complex Containing a Diazadiphosphetidine Ring". Organometallics. 2 (12): 1903–1905. doi:10.1021/om50006a044. ISSN 0276-7333.
- Hutchins, Larry D.; Duesler, Eileen N.; Paine, Robert T. (1984). "Synthesis and Characterization of Metallophosphenium Ion Complexes Derived from Aminohalophosphites. Crystal and Molecular Structure of [cyclo]Mo(η5-C5H5)(CO)2(POCH2CH2NCMe3)". Organometallics. 3 (3): 399–403. doi:10.1021/om00081a013. ISSN 0276-7333.
References
[edit]- ^ a b Fleming, Suzanne.; Lupton, Mary Kathryn.; Jekot, Kathleen. (1972-10-01). "Synthesis of a cyclic fluorodialkylaminophosphine and its coordination with boron acids. Formation of a unique dialkylaminophosphine cation". Inorganic Chemistry. 11 (10): 2534–2540. doi:10.1021/ic50116a050. ISSN 0020-1669.
- ^ a b c d Schultz, C. W.; Parry, R. W. (1976-12-01). "Structure of [2((CH3)2N)2PCl]·AlCl3, ((CH3)2N)3P·((CH3)2N)2PCl·AlCl3, and related species-diphosphorus cations". Inorganic Chemistry. 15: 3046–3050. doi:10.1021/ic50166a022. ISSN 0020-1669.
- ^ a b c d e f g Cowley, A. H.; Kemp, R. A. (1985-10-01). "Synthesis and reaction chemistry of stable two-coordinate phosphorus cations (phosphenium ions)". Chemical Reviews. 85 (5): 367–382. doi:10.1021/cr00069a002. ISSN 0009-2665.
- ^ Dahl, Otto (1982-01-01). "Reactions of aminophosphines with trifluormethanesulfonic acid: phosphenium ion (two-coordinate phosphorus ion) or tricovalent phosphorus products?". Tetrahedron Letters. 23 (14): 1493–1496. doi:10.1016/s0040-4039(00)87141-5. ISSN 0040-4039.
- ^ a b Reeske, Gregor; Cowley, Alan H. (2007-02-01). "One-Step Redox Route to N-Heterocyclic Phosphenium Ions". Inorganic Chemistry. 46 (4): 1426–1430. doi:10.1021/ic061956z. ISSN 0020-1669. PMID 17291126.
- ^ a b c Cowley, Alan H.; Cushner, Mike C.; Szobota, John S. (1978-11-01). "Static and dynamic stereochemistry of dicoordinate phosphorus cations". Journal of the American Chemical Society. 100 (24): 7784–7786. doi:10.1021/ja00492a087. ISSN 0002-7863.
- ^ Gudat, Dietrich (1998-08-01). "Cation Stabilities, Electrophilicities, and "Carbene Analogue" Character of Low Coordinate Phosphorus Cations". European Journal of Inorganic Chemistry. 1998 (8): 1087–1094. doi:10.1002/(sici)1099-0682(199808)1998:8<1087::aid-ejic1087>3.0.co;2-3. ISSN 1099-0682.
- ^ Harrison, James F.; Liedtke, Richard C.; Liebman, Joel F. (1979-11-01). "The multiplicity of substituted acyclic carbenes and related molecules". Journal of the American Chemical Society. 101 (24): 7162–7168. doi:10.1021/ja00518a006. ISSN 0002-7863.
- ^ a b Harrison, James F. (1981-12-01). "Electronic structure of the phosphenium ions PH+
2, HPF+, and PF+
2". Journal of the American Chemical Society. 103 (25): 7406–7413. doi:10.1021/ja00415a002. ISSN 0002-7863. - ^ Nakazawa, Hiroshi; Buhro, William E.; Bertrand, Guy; Gladysz, J. A. (1984-10-01). "Reactions of phosphorus electrophiles with [(η5-C5Me5)Fe(CO)2]−; spectroscopic evidence for a phosphinidene complex". Inorganic Chemistry. 23 (22): 3431–3433. doi:10.1021/ic00190a001. ISSN 0020-1669.
- ^ W. B. McCormack (1963). "3-Methyl-1-Phenylphospholene oxide". Organic Syntheses. 43: 73. doi:10.15227/orgsyn.043.0073.
- ^ Cowley, A. H.; Kemp, R. A.; Lasch, J. G.; Norman, N. C.; Stewart, C. A. (1983-11-01). "Reaction of phosphenium ions with 1,3-dienes: a rapid synthesis of phosphorus-containing five-membered rings". Journal of the American Chemical Society. 105 (25): 7444–7445. doi:10.1021/ja00363a040. ISSN 0002-7863.
- ^ a b Rao, Bin; Chong, Che Chang; Kinjo, Rei (2018-01-05). "Metal-Free Regio- and Chemoselective Hydroboration of Pyridines Catalyzed by 1,3,2-Diazaphosphenium Triflate". Journal of the American Chemical Society. 140 (2): 652–656. doi:10.1021/jacs.7b09754. ISSN 0002-7863. PMID 29303259.
- ^ Montemayor, R. G.; Sauer, Dennis T.; Fleming, Suzanne; Bennett, Dennis W.; Thomas, Michael G.; Parry, Robert W. (1978-03-01). "Iron carbonyl complexes containing positively charged phosphorus ligands". Journal of the American Chemical Society. 100 (7): 2231–2233. doi:10.1021/ja00475a044. ISSN 0002-7863.
- ^ a b Burck, Sebastian; Daniels, Jörg; Gans-Eichler, Timo; Gudat, Dietrich; Nättinen, Kalle; Nieger, Martin (2005-06-01). "N-Heterocyclic Phosphenium, Arsenium, and Stibenium Ions as Ligands in Transition Metal Complexes: A Comparative Experimental and Computational Study". Zeitschrift für Anorganische und Allgemeine Chemie. 631 (8): 1403–1412. doi:10.1002/zaac.200400538. ISSN 0044-2313.
- ^ a b Pan, Baofei; Xu, Zhequan; Bezpalko, Mark W.; Foxman, Bruce M.; Thomas, Christine M. (2012-03-14). "N-Heterocyclic Phosphenium Ligands as Sterically and Electronically-Tunable Isolobal Analogues of Nitrosyls". Inorganic Chemistry. 51 (7): 4170–4179. doi:10.1021/ic202581v. ISSN 0020-1669. PMID 22416761.
 KSF
KSF