Protist
 From Wikipedia - Reading time: 81 min
From Wikipedia - Reading time: 81 min
| Protists Temporal range:
| |
|---|---|

| |
| Examples of protists. Clockwise from top left: red algae, kelp, ciliate, golden alga, dinoflagellate, metamonad, amoeba, slime mold. | |
| Scientific classification | |
| Domain: | Eukaryota |
| Major subdivisions[2] | |
| Cladistically included but traditionally excluded taxa | |
| |
A protist (/ˈproʊtɪst/ PROH-tist) or protoctist is any eukaryotic organism that is not an animal, land plant, or fungus. Protists do not form a natural group, or clade, but are a paraphyletic grouping of all descendants of the last eukaryotic common ancestor excluding land plants, animals, and fungi.
Protists were historically regarded as a separate taxonomic kingdom known as Protista or Protoctista. With the advent of phylogenetic analysis and electron microscopy studies, the use of Protista as a formal taxon was gradually abandoned. In modern classifications, protists are spread across several eukaryotic clades called supergroups, such as Archaeplastida (photoautotrophs that includes land plants), SAR, Obazoa (which includes fungi and animals), Amoebozoa and "Excavata".
Protists represent an extremely large genetic and ecological diversity in all environments, including extreme habitats. Their diversity, larger than for all other eukaryotes, has only been discovered in recent decades through the study of environmental DNA and is still in the process of being fully described. They are present in all ecosystems as important components of the biogeochemical cycles and trophic webs. They exist abundantly and ubiquitously in a variety of mostly unicellular forms that evolved multiple times independently, such as free-living algae, amoebae and slime moulds, or as important parasites. Together, they compose an amount of biomass that doubles that of animals. They exhibit varied types of nutrition (such as phototrophy, phagotrophy or osmotrophy), sometimes combining them (in mixotrophy). They present unique adaptations not present in multicellular animals, fungi or land plants. The study of protists is termed protistology.
Definition
[edit]
Protists are a diverse group of eukaryotes that are primarily single-celled and microscopic and exhibit a wide variety of shapes and life strategies. They have different life cycles, trophic levels, modes of locomotion, and cellular structures.[4][5] Although most protists are unicellular, there is a considerable range of multicellularity amongst them; some form colonies or multicellular structures visible to the naked eye. The term 'protist' refers to all eukaryotes that are not animals, land plants or fungi, the three traditional eukaryotic kingdoms.[a][13] Because of this definition by exclusion, protists compose a paraphyletic group that includes the ancestors of those three kingdoms.[14]
The names of some protists (called ambiregnal protists), because of their mixture of traits similar to both animals and land plants or fungi (e.g., slime molds and flagellated algae like euglenids), have been published under either or both of the botanical (ICNafp) and the zoological (ICZN) codes of nomenclature.[15][16]
Common types
[edit]Protists display a wide range of distinct morphological types that have been used to classify them for practical purposes, although most of these categories do not represent evolutionary cohesive lineages or clades and have instead evolved independently several times. The most recognizable types are:[17]
- Amoebae. Characterized by their irregular, flexible shapes, these protists move by extending portions of their cytoplasm, known as pseudopodia, to crawl along surfaces.[18] Many groups of amoebae are naked, but testate amoebae and foraminifera grow a shell around their cell made from digested material or surrounding debris. Some, known as radiolarians and heliozoans, have special spherical shapes with microtubule-supported pseudopodia radiating from the cell.[17] Some amoebae are capable of producing stalked multicellular stages that bear spores, often by aggregating together; these are known as slime molds.[19] The main clades containing amoebae are Amoebozoa (including various slime molds and testate amoebae) and Rhizaria (including famous groups such as foraminifera and radiolarians, as well as a few testate amoebae).[20][21] Even some individual amoebae can grow to giant sizes visible to the naked eye.[22][23]
- Flagellates. These protists are equipped with one or more whip-like appendages called cilia, undulipodia or eukaryotic flagella,[b] which enable them to swim or glide freely through the environment. Flagellates are found in all lineages, reflecting that the common ancestor of all living eukaryotes was a flagellate. They usually exhibit two cilia (e.g., in Provora, Telonemia, Stramenopiles, Alveolata, Obazoa and most excavates), but there are a number of flagellate groups with a high number of cilia (such as Hemimastigophora and other excavates).[17] Some groups, such as the well-known ciliates and the parasitic opalinids, have a cell surface covered in rows of cilia that beat rhythmically. A few groups of amoebae have retained their flagella, making them amoeboflagellates.[27]
- Algae. They are the photosynthetic protists, and can be found in most of the main clades, completely intermingled with heterotrophic protists which are traditionally called protozoa.[28] Algae exhibit the most diverse range of morphologies, from single flagellated or coccoid cells (e.g., cryptophytes, haptophytes, dinoflagellates, chromerids, many green algae, ochrophytes, euglenophytes) to amoeboid cells (chlorarachniophytes) to colonial and multicellular macroscopic forms (e.g., red algae, some green algae, and some ochrophytes such as kelp).[29]
- Fungus-like protists. Several clades of protists have evolved an appearance similar to fungi through hyphae-like structures and a saprophytic nutrition. They have evolved multiple times, often very distantly from true fungi (e.g., the oomycetes, labyrinthulomycetes and hyphochytrids, in Stramenopiles; the myxomycetes, in Amoebozoa; the phytomyxeans, in Rhizaria; the perkinsozoans, in Alveolata).[30][31]
- Sporozoa. This category traditionally included parasitic protists that reproduced via spores (the apicomplexans, microsporidians, myxozoans and ascetosporeans).[6] Its current use is restricted to the apicomplexans,[32] such as Plasmodium falciparum, the cause of malaria.[33]
Diversity
[edit]
The species diversity of protists is severely underestimated by traditional methods that differentiate species based on morphological characteristics. The number of described protist species is very low (ranging from 26,000[35] to over 76,000)[c] in comparison to the diversity of land plants, animals and fungi, which are historically and biologically well-known and studied. The predicted number of species also varies greatly, ranging from 140,000 to 1,600,000, and in several groups the number of predicted species is arbitrarily doubled. Most of these predictions are highly subjective. Molecular techniques such as environmental DNA barcoding have revealed a vast diversity of undescribed protists that accounts for the majority of eukaryotic sequences or operational taxonomic units (OTUs), dwarfing those from land plants, animals and fungi.[34] As such, it is considered that protists dominate eukaryotic diversity.[37]
| Protist phylogeny |
| One possible topology for the eukaryotic tree of life, with uncertain positions of ancyromonads, excavates, provorans and hemimastigotes.[38][3][39][40] Excavate groups are shown in green. 1Includes land plants. 2Includes animals and fungi. |
The evolutionary relationships of protists have been explained through molecular phylogenetics, the sequencing of entire genomes and transcriptomes, and electron microscopy studies of the flagellar apparatus and cytoskeleton. New major lineages of protists and novel biodiversity continue to be discovered, resulting in dramatic changes to the eukaryotic tree of life. The newest classification systems of eukaryotes do not recognize the formal taxonomic ranks (kingdom, phylum, class, order...) and instead only recognize clades of related organisms, making the classification more stable in the long term and easier to update. In this new cladistic scheme, the protists are divided into various branches informally named supergroups. Most photosynthetic eukaryotes fall under the Diaphoretickes clade, which contains the supergroups Archaeplastida (which includes land plants) and SAR (including, Stramenopiles, Alveolata and Rhizaria), as well as the phyla Telonemia, Cryptista and Haptista.[17][41] The animals and fungi fall into the Amorphea supergroup, which contains the phylum Amoebozoa and several other protist lineages. Various groups of eukaryotes with primitive cell architecture are collectively known as the "Excavata".[2]
"Excavata"
[edit]"Excavata" is a group that encompasses diverse protists, mostly flagellates, ranging from aerobic and anaerobic predators to phototrophs and heterotrophs.[42]: 597 The common name 'excavate' refers to the common characteristic of a ventral groove in the cell used for suspension feeding, which is considered to be an ancestral trait present in the last eukaryotic common ancestor.[43] The "Excavata" is composed of three clades: Discoba, Metamonada and Malawimonadida, each including 'typical excavates' that are free-living phagotrophic flagellates with the characteristic ventral groove.[44] According to most phylogenetic analyses, this group is paraphyletic,[40] with some analyses placing the root of the eukaryote tree within Metamonada.[45]
Discoba includes three major groups: Jakobida, Euglenozoa and Percolozoa.[d] Jakobida are a small group (~20 species) of free-living heterotrophic flagellates, with two cilia, that primarily eat bacteria through suspension feeding; most are aquatic aerobes, with some anaerobic species, found in marine, brackish or fresh water.[47] They are best known for their bacterial-like mitochondrial genomes.[17] Euglenozoa is a rich (>2,000 species)[48] group of flagellates with very different lifestyles, including: the free-living heterotrophic (both osmo- and phagotrophic)[42] and photosynthetic euglenids (e.g., the euglenophytes, with chloroplasts originated from green algae); the free-living and parasitic kinetoplastids (such as Trypanosoma); the deep-sea anaerobic symbiontids; and the elusive diplonemids.[49] Percolozoa[d] (~150 species) are a collection of amoebae, flagellates and amoeboflagellates with complex life cycles, among which are some slime molds (acrasids).[17][46] The two clades Euglenozoa and Percolozoa are sister taxa, united under the name Discicristata, in reference to their mitochondrial cristae shaped like discs.[9] The species Tsukubamonas globosa is a free-living flagellate whose precise position within Discoba is not yet settled, but is probably more closely related to Discicristata than to Jakobida.[47]
The metamonads (Metamonada) are a phylum of completely anaerobic or microaerophilic protozoa, primarily flagellates. Some are gut symbionts of animals such as termites, others are free-living, and others are parasitic. They include three main clades: Fornicata, Parabasalia and Preaxostyla.[17] Fornicata (>140 species)[36] encompasses the diplomonads, with two nuclei (e.g., Giardia), and several smaller groups of free-living, commensal and parasitic protists (e.g., Carpediemonas, retortamonads).[17] Parabasalia (>460 species)[36] is a varied group of anaerobic, mostly endobiotic organisms, ranging from small parasites (like Trichomonas) to giant intestinal symbionts with numerous flagella and nuclei found in wood-eating termites and cockroaches.[17] Preaxostyla (~140 species) includes the anaerobic and endobiotic oxymonads, with modified (or completely lost)[50][51] mitochondria, and two genera of free-living microaerophilic bacterivorous flagellates Trimastix and Paratrimastix, with typical excavate morphology.[51][52] Two genera of anaerobic flagellates of recent description and unique cell architecture, Barthelona and Skoliomonas, are closely related to the Fornicata.[53]
The malawimonads (Malawimonadida) are a small group (three species) of freshwater or marine suspension-feeding bacterivorous flagellates[54] with typical excavate appearance, closely resembling Jakobida and some metamonads but not phylogenetically close to either in most analyses.[17]
-
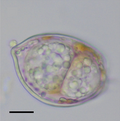
Giardia, a genus of intestinal parasites that cause giardiasis
-
Trichomonas vaginalis, the causative agent of trichomoniasis
-
Trypanosoma cruzi, the causative agent of Chagas disease
-
Euglena, a genus of photosynthetic euglenids
-
Malawimonas cells, with typical excavate architecture
Diaphoretickes
[edit]Diaphoretickes includes nearly all photosynthetic eukaryotes. The SAR supergroup gathers a colossal diversity of protists. It includes Stramenopiles, Alveolata and Stramenopiles.[55] Another highly diverse clade within Diaphoretickes is Archaeplastida, which houses land plants and a variety of algae. In addition, three smaller groups, Telonemia, Haptista and Cryptista, also belong to Diaphoretickes.[2] TSAR is a possible clade that would comprise Telonemia and SAR,[56] although Telonemia may branch with Haptista instead of SAR.[57][58] Telonemia shares some cellular similarities with the SAR supergroup.[55]
Stramenopiles
[edit]The stramenopiles, also known as Heterokonta, are characterized by the presence of two cilia, one of which bears many short, straw-like hairs (mastigonemes). They include one clade of phototrophs and numerous clades of heterotrophs, present in virtually all habitats. Stramenopiles include two usually well-supported clades, Bigyra and Gyrista, although the monophyly of Bigyra is being questioned.[59] Branching outside both Bigyra and Gyrista is a single species of enigmatic heterotrophic flagellates, Platysulcus tardus.[59] Much of the diversity of heterotrophic stramenopiles is still uncharacterized, known almost entirely from lineages of genetic sequences known as MASTs (MArine STramenopiles),[59] of which only a few species have been described.[60][61]
The phylum Gyrista includes the photosynthetic Ochrophyta or Heterokontophyta (>23,000 species),[48] which contain chloroplasts originated from a red alga. Among these are many lineages of algae that encompass a wide range of structures and morphologies. The three most diverse ochrophyte classes are: the diatoms, unicellular or colonial organisms encased in silica cell walls (frustules) that exhibit widely different shapes and ornamentations and comprise much of the marine phytoplankton;[17][62] the brown algae, filamentous or 'truly' multicellular (with differentiated tissues) macroalgae that constitute the basis of many temperate and cold marine ecosystems, such as kelp forests;[63] and the golden algae, unicellular or colonial flagellates that are mostly present in freshwater habitats.[64] Inside Gyrista, the sister clade to Ochrophyta are the predominantly osmotrophic and filamentous pseudofungi (>1,200 species),[65] which include three distinct lineages: the parasitic oomycetes or water moulds (e.g., Phytophthora), which encompass most of the pseudofungi species; the less diverse non-parasitic hyphochytrids that maintain a fungus-like lifestyle; and the bigyromonads, a group of bacterivorous or eukaryovorous phagotrophs.[59] A small group of heliozoan-like heterotrophic amoebae, Actinophryida, has an uncertain position, either within or as the sister taxon of Ochrophyta.[66]
The little studied phylum Bigyra is an assemblage of exclusively heterotrophic organisms, most of which are free-living. It includes the labyrinthulomycetes, among which are single-celled amoeboid phagotrophs, mixotrophs, and fungus-like filamentous heterotrophs that create slime networks to move and absorb nutrients, as well as some parasites and a few testate amoebae (Amphitremida). Also included in Bigyra are the bicosoecids, phagotrophic flagellates that consume bacteria, and the closely related Placidozoa, which consists of several groups of heterotrophic flagellates (e.g., the deep-sea halophilic Placididea) as well as the intestinal commensals known as Opalinata (e.g., the human parasite Blastocystis, and the highly unusual opalinids, composed of giant cells with numerous nuclei and cilia, originally misclassified as ciliates).[59]
-
Macrocystis pyrifera, the giant kelp
-
Cafeteria, a genus of bicosoecids
-
Opalina cell covered in numerous rows of cilia
Alveolata
[edit]The alveolates (Alveolata) are characterized by the presence of cortical alveoli, cytoplasmic sacs underlying the cell membrane of unknown physiological function.[42]: 599 Among them are three of the most well-known groups of protists: apicomplexans, dinoflagellates and ciliates. The ciliates (Ciliophora) are a highly diverse (>8,000 species) and probably the most thoroughly studied[17] group of protists. They are mostly free-living microbes characterized by large cells covered in rows of cilia and containing two kinds of nuclei, micronucleus and macronucleus. Free-living ciliates are usually the top heterotrophs and predators in microbial food webs, feeding on bacteria and smaller eukaryotes, present in a variety of ecosystems, although a few species are kleptoplastic. Others are parasitic of numerous animals.[67] Ciliates have a basal position in the evolution of alveolates, together with a few species of heterotrophic flagellates with two cilia collectively known as colponemids.[68]
The remaining alveolates are grouped under the clade Myzozoa, whose common ancestor acquired chloroplasts through a secondary endosymbiosis from a red alga.[69] One branch of Myzozoa contains the apicomplexans and their closest relatives, a small clade of flagellates known as Chrompodellida where phototrophic and heterotrophic flagellates, called chromerids and colpodellids respectively, are evolutionarily intermingled.[69] In contrast, the apicomplexans (Apicomplexa) are a large (>6,000 species) and highly specialized group of obligate parasites who have all secondarily lost their photosynthetic ability (e.g., Plasmodium). Their adult stages absorb nutrients from the host through the cell membrane, and they reproduce between hosts via sporozoites, which exhibit an organelle complex (the apicoplast) evolved from non-photosynthetic chloroplasts.[70][42]: 600
The other branch of Myzozoa contains the dinoflagellates and their closest relatives, the perkinsids (Perkinsozoa), a small group (26 species) of aquatic intracellular parasites which have lost their photosynthetic ability similarly to apicomplexans.[69] They reproduce through flagellated spores that infect dinoflagellates, molluscs and fish.[71] In contrast, the dinoflagellates (Dinoflagellata) are a highly diversified (~4,500 species)[72] group of aquatic algae that have mostly retained their chloroplasts, although many lineages have lost their own and instead either live as heterotrophs or reacquire new chloroplasts from other sources, including tertiary endosymbiosis and kleptoplasty.[73] Most dinoflagellates are free-living and compose an important portion of phytoplankton, as well as a major cause of harmful algal blooms due to their toxicity; some live as symbionts of corals, allowing the creation of coral reefs. Dinoflagellates exhibit a diversity of cellular structures, such as complex eyelike ocelli, specialized vacuoles, bioluminescent organelles, and a wall surrounding the cell known as the theca.[72]
-
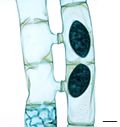
Paramecium, a well-studied genus of ciliates[74]
-
Vitrella brassicaformis, a photosynthetic chromerid, relative of apicomplexans
-
Plasmodium falciparum, the causative agent of malaria, infecting blood cells
-
Dinovorax, a perkinsid that infects dinoflagellates
-
Alexandrium dinoflagellates, responsible for certain harmful algal blooms
Rhizaria
[edit]Rhizaria is a lineage of morphologically diverse organisms, composed almost entirely of unicellular heterotrophic amoebae, flagellates and amoeboflagellates,[17] commonly with reticulose (net-like) or filose (thread-like) pseudopodia for feeding and locomotion.[75][42]: 604 It was the last supergroup to be described, because it lacks any defining characteristic and was discovered exclusively through molecular phylogenetics.[76] Three major clades are included, namely the phyla Cercozoa, Endomyxa and Retaria.[2]
Retaria contains the most familiar rhizarians: forams and radiolarians, two groups of large free-living marine amoebae with pseudopodia supported by microtubules, many of which are macroscopic.[17] The radiolarians (Radiolaria) are a diverse group (>1,000 living species) of amoebae, often bearing delicate and intricate siliceous skeletons.[77] The forams (Foraminifera) are also diverse (>6,700 living species),[78] and most of them are encased in multichambered tests constructed from calcium carbonate or agglutinated mineral particles.[17] Both groups have a rich fossil record, with tens of thousands of described fossil species.[78][79]
Cercozoa (also known as Filosa) is an assemblage of free-living protists with very different morphologies. Cercozoan amoeboflagellates are important predators of other microbes in terrestrial habitats and the plant microbiota (e.g., cercomonads and paracercomonads and glissomonads, collectively known as class SARcomonadea),[80] and a few can generate slime molds (e.g., Helkesea).[81] Many cercozoans are testate or scale-bearing amoebae, namely the elusive Kraken and the two classes Imbricatea (e.g., the euglyphids) and Thecofilosea.[80] Thecofilosea also contains the Phaeodaria (~400–500 species), a group of skeleton-bearing marine amoebae previously classified as radiolarians,[79] and both classes include some non-scaly naked flagellates (e.g., spongomonads in Imbricatea and thaumatomonads in Thecofilosea).[82] Among the basal-branching cercozoans are the pseudopodia-lacking thecate flagellates of Metromonadea, the heliozoan-like Granofilosea[82] and the photosynthetic chlorarachniophytes, whose chloroplasts originated from a secondary endosymbiosis with a green alga.[17]
Endomyxa contains two major clades of parasitic protists: Ascetosporea are sporozoan-type parasites of marine invertebrates,[83] while Phytomyxea are obligate pathogens of plants and algae, divided into the terrestrial plasmodiophorids and the marine phagomyxids.[84] Also included in Endomyxa are the order of predatory amoebae Vampyrellida (48 species)[85] and two genera of marine amoebae, the thecate Gromia and the naked Filoreta.[2]
Besides these three phyla, Rhizaria includes numerous enigmatic and understudied lineages of uncertain evolutionary position. One such clade is the Gymnosphaerida, which includes heliozoan-type protists.[86] Several clades labeled as Novel Clades (NC) are entirely composed of environmental DNA from uncultured protists, although a few have slowly been resolved over the decades with the description of new taxa (e.g., Tremulida and Aquavolonida, formerly NC11 and NC10 respectively, with a deep-branching position in Rhizaria).[87]
-
Globorotalia, a genus of forams visible to the naked eye
-
Cladococcus cell, showing the intricate radiolarian skeleton
-
Chlorarachnion, a genus of photosynthetic cercozoans
-
Euglypha, a prominent genus of testate amoebae
-
Haplosporidium species infect a variety of invertebrates
Haptista and Cryptista
[edit]Haptista and Cryptista are two similar phyla of single-celled protists previously thought to be closely related, and collectively known as Hacrobia.[88] However, the monophyly of Hacrobia was disproven, as the two groups originated independently.[89] Molecular analyses place Cryptista next to Archaeplastida, forming the hypothesized CAM clade,[39] and Haptista next to the Telonemia and the SAR clade[40] (Telonemia may either be the sister group to SAR, forming the hypothesized TSAR clade,[90] or to Haptista, forming a common sister clade to SAR[91][39][92]). Within the CAM clade, the closest relative of Cryptista is the species Microheliella maris, together composing the clade Pancryptista.[39]
The phylum Haptista includes two distinct clades with mineralized scales: haptophytes and centrohelids.[17] The haptophytes (Haptophyta) are a group of over 500 living species[48] of flagellated or coccoid algae that have acquired chloroplasts from a secondary endosymbiosis. They are mostly marine, comprise an important portion of oceanic plankton, and include the coccolithophores, whose calcified scales ('coccoliths') contribute to the formation of sedimentary rocks and the biogeochemical cycles of carbon and calcium. Some species are capable of forming toxic blooms.[93] The centrohelids (Centroplasthelida) are a small (~95 species)[94] but widespread group of heterotrophic heliozoan-type amoebae, usually covered in scale-bearing mucous, that form an important component of benthic food webs of aquatic habitats, both marine and freshwater.[95]
The phylum Cryptista is a clade of three distinct groups of unicellular protists: cryptomonads, katablepharids, and the species Palpitomonas bilix.[2] The cryptomonads (>100 species), also known as cryptophytes, are flagellated algae found in aquatic habitats of diverse salinity, characterized by extrusive organelles or extrusomes called ejectisomes. Their chloroplasts, of red algal origin, contain a nucleomorph, a remnant of the eukaryotic nucleus belonging to the endosymbiotic red alga.[96] The katablepharids, the closest relatives of cryptomonads, are heterotrophic flagellates with two cilia, also characterized by ejectisomes.[88][2] The species Palpitomonas bilix is the most basal-branching member of Cryptista, a marine heterotrophic flagellate with two cilia, but unlike the remaining members it lacks ejectisomes.[97]
-
Raphidiophrys, a centrohelid heliozoan
-
Coccolithophore covered in coccoliths
-
Cryptomonas, common algae in fresh waters worldwide
-
Roombia truncata, filled with rows of ejectisomes
Archaeplastida
[edit]Archaeplastida is the clade containing those photosynthetic groups whose plastids were likely obtained through a single event of primary endosymbiosis with a cyanobacterium. It contains land plants (Embryophyta) and a big portion of the diversity of algae, most of which are the green algae, from which plants evolved, and the red algae.[98] A third lineage of algae, the glaucophytes (25 species),[48] contains rare and obscure species found in surfaces of freshwater and terrestrial habitats.[98]
The red algae or Rhodophyta (>7,100 species) are a group of diverse morphologies, ranging from single cells to multicellular filaments to giant pseudoparenchymatous thalli, all without flagella. They lack chlorophyll and only harvest light energy through phycobiliproteins. Their life cycles are varied and may include two or three generations. They are present in terrestrial, freshwater and primarily marine habitats, from the intertidal zone to deep waters; some are calcified and are vital components of marine ecosystems such as coral reefs.[99] Closely related to the red algae are two small lineages of non-photosynthetic predatory flagellates: the freshwater and marine Rhodelphidia (3 species),[100] which still retain genetic evidence of relic plastids;[101] and the marine Picozoa (1 species), which lack any remains of plastids. The evolutionary position of Picozoa may indicate that there have been two separate events of primary endosymbiosis, as opposed to one.[102]
The green algae, unlike the monophyletic glaucophytes and rhodophytes, are a paraphyletic group from which land plants evolved. Together they compose the Chloroplastida or Viridiplantae clade.[2] The earliest branching member is the phylum Prasinodermophyta (ten species), whose members are exclusively marine coccoid cells or small macroscopic thalli.[103] The remaining green algae are distributed in two major clades. One clade is the phylum Chlorophyta (>7,900 species),[48] which includes numerous lineages of scaly unicellular flagellate algae known collectively as prasinophytes along with the Prasinodermophyta, but also includes a variety of morphologies such as coccoids, palmelloids, colonies, and macroscopic filamentous, foliose or tubular thalli, present in aquatic and terrestrial habitats.[2] The opposed clade is Streptophyta, which contains the land plants and a paraphyletic group of green algae collectively known as phylum Charophyta, composed of several classes: Zygnematophyceae (>4,300 species),[48] containing unicellular, colonial and filamentous flagella-lacking organisms found almost exclusively in freshwater habitats;[104] Charophyceae (450 living species),[48] also known as stoneworts, consisting of complex multicellular thalli only found in freshwater habitats;[105] Klebsormidiophyceae (52 species), with unbranched filamentous thalli; Coleochaetophyceae (36 species), containing branched filamentous thalli; Mesostigmatophyceae, composed of a single species of scaly flagellates; and Chlorokybophyceae (five species), with sarcinoid forms.[106][48]
-
Cyanophora, a glaucophyte genus
-
Corallina officinalis, a coralline red alga
-
Volvox, a colonial chlorophyte
-
Spirogyra, a filamentous streptophyte, during conjugation
-
Chara, a complex plant-like streptophyte with reproductive structures
Amorphea
[edit]Amorphea is a group of exclusively heterotrophic organisms. It contains the fungi and animals, as well as most slime moulds, many amoebae and some flagellates.[107] Many of its protist members exhibit complex life cycles with different levels of multicellularity.[108] Amorphea is roughly equivalent to the concept of 'unikonts', meaning 'single cilium', although it currently contains several organisms with more cilia.[109] It is defined as the smallest clade containing the groups Amoebozoa (containing mostly slime moulds and amoebae) and Opisthokonta (containing fungi, animals, and their closest relatives).[107][2] The closest relatives of Opisthokonta are two small lineages of single-celled protists with two cilia: the flagellate Apusomonadida (28 species)[110] and the amoeboflagellate anaerobic Breviatea (four species).[17] Together with opisthokonts, these two groups form the clade Obazoa, the sister clade to Amoebozoa.[109]
The phylum Amoebozoa (2,400 species)[34] is a large group of morphologically diverse phagotrophic protists, mostly amoebae. A considerable portion of amoebozoans are lobose amoebae, meaning they produce round, blunt-ended pseudopods.[111] It includes the 'archetypal' amoebae, known as the naked lobose amoebae or 'gymnamoebae'[112] (such as Amoeba itself),[113] among which is a genus of sorocarp-forming slime moulds, Copromyxa.[114] Some gymnamoebae are important pathogens to animals (e.g., Acanthamoeba).[115] Other relevant lobose amoebae are the Arcellinida, a diverse order of testate amoebae and one of the most conspicuous protist groups overall.[116] The remaining, non-lobose amoebozoans include the Eumycetozoa or 'true slime moulds', comprising the sorocarp-producing bacterivorous dictyostelids and the sporocarp-producing omnivorous myxogastrids and protosporangiids.[2] Due to the fungus-like appearance of their fruiting bodies, eumycetozoans are often studied by mycologists.[17] Closely related to the eumycetozoans are two lineages: the Variosea, a heterogeneous assortment of amoeboid, reticulate or flagellated organisms[113] (including some sorocarp-producing organisms);[117] and the anaerobic Archamoebae, some of which live as intestinal symbionts of some animals (e.g., Entamoeba).[2]
Opisthokonta includes the animal and fungal kingdoms,[a] as well as their closest protist relatives. The branch leading to the fungi is known as Nucletmycea or Holomycota, while the branch leading to the animals is called Holozoa.[118] The Holomycota includes the closest relatives of fungi, the nucleariids, a small group (~50 species) of free-living naked or scale-bearing phagotrophic amoebae with filose pseudopodia, some of which can aggregate into slime moulds.[119] Within the wider definition of fungi, three groups are studied as protists by some authors: Aphelida (15 species),[12] Rozellida (27 species)[120] and Microsporidia (~1,300 species),[121] collectively known as Opisthosporidia, as opposed to the 'true' or osmotrophic fungi. Both aphelids and rozellids are single-celled phagotrophic flagellates that feed in an endobiotic manner, penetrating the cells of their respective hosts. Microsporidians are obligate intracellular parasites that feed through osmotrophy, much like true fungi. Aphelids and true fungi are closest relatives, and generally feed on cellulose-walled organisms (many algae and plants). Conversely, rozellids and microsporidians form a separate clade, and generally feed on chitin-walled organisms (fungi and animals).[122]
The Holozoa includes various lineages with complex life cycles involving different cell types and associated with the origin of animal multicellularity.[17] The closest relatives to animals are the choanoflagellates (~360 species), free-living flagellates that feed through a collar of microvilli surrounding a larger cilium and often form colonies.[123] The Ichthyosporea (>40 species), otherwise known as mesomycetozoans, are a group of fungus-like pathogenic holozoans specialized in infecting fish and other animals.[124] The Filasterea (six species) are a heterogeneous group of free-living, endosymbiotic, or parasitic amoebae or flagellates.[125] Lastly, the Pluriformea are two species of free-living holozoans with life cycles that include multicellular aggregates.[126] An elusive flagellate species Tunicaraptor unikontum has an uncertain evolutionary position among these holozoan groups.[127]
-
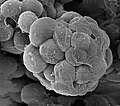
Amoeba, the archetypal amoebae
-
Physarum polycephalum, a true slime mould
-
Nuclearia, filose amoebae related to fungi
-
Creolimax fragrantissima, an ichthyosporean that infects peanut worms
-
A choanoflagellate colony, with cells resembling choanocytes found in sponges
Orphan groups
[edit]Several smaller lineages do not belong to any of the three main supergroups, and instead have a deep-branching "kingdom-level" position in eukaryote evolution. They are usually poorly known groups with limited data and few species, often referred to as "orphan groups".[40] The CRuMs clade, containing the free-swimming Collodictyonidae (seven species) with two to four cilia, the amoeboid Rigifilida (two species) with filose pseudopodia, and the gliding Mantamonadidae (three species)[128] and Glissandridae (two species)[129][130] with two cilia, are the sister clade of Amorphea.[38] The Ancyromonadida (35 species)[131] are aquatic gliding flagellates with two cilia, positioned near Amorphea and CRuMs.[38] The Hemimastigophora (ten species), or hemimastigotes, are predatory flagellates with a distinctive cell morphology and two rows of around a dozen flagella.[132] The Provora (eight species)[133] are predatory flagellates with an unremarkable morphology similar to that of excavates and other flagellates with two cilia. Both Hemimastigophora and Provora were thought to be related to or within Diaphoretickes,[3] although further analyses have placed them in a separate clade along with a mysterious species of predatory protists, Meteora sporadica. This species has a remarkable morphology: they lack flagella, are bilaterally symmetrical, project a pair of lateral "arms" that swing back and forth, and contain a system of motility unlike any other.[40]
There are also many genera of uncertain affiliation among eukaryotes because their DNA has not been sequenced, and consequently their phylogenetic affinities are unknown.[2] One enigmatic heliozoan species is so large that it does not match the description of any known genus, and was consequently transferred to a separate genus Berkeleyaesol with an unclear position, although it probably belongs to Diaphoretickes along with all other heliozoa.[134] The organism Parakaryon is harder to place, as it shares traits from both prokaryotes and eukaryotes.[135]
Biology
[edit]In general, protists have typical eukaryotic cells that follow the same principles of biology described for those cells within the "higher" eukaryotes (animals, fungi and land plants).[136] However, many have evolved a variety of unique physiological adaptations that do not appear in the remaining eukaryotes,[137] and in fact protists encompass almost all of the broad spectrum of biological characteristics expected in eukaryotes.[37]
Nutrition
[edit]Protists display a wide variety of food preferences and feeding mechanisms.[2][138] According to the source of their nutrients, they can be divided into autotrophs (producers, traditionally algae) and heterotrophs (consumers, traditionally protozoa). Autotrophic protists synthesize their own organic compounds from inorganic substrates through the process of photosynthesis, using light as the source of energy;[139]: 217 accordingly, they are also known as phototrophs.[140]
Heterotrophic protists obtain organic molecules synthesized by other organisms, and can be further divided according to the size of their nutrients. Those that feed on soluble molecules[139]: 218 or macromolecules under 0.5 μm in size are called osmotrophs,[138] and they absorb them by diffusion, ciliary pits, transport proteins of the cell membrane, and a type of endocytosis (i.e., invagination of the cell membrane into vacuoles, called endosomes) known as pinocytosis[2] or fluid-phase endocytosis.[138] Those that feed on organic particles over 0.5 μm in size or entire cells are called phagotrophs, and they ingest them through a type of endocytosis known as phagocytosis.[138][139]: 218 Endocytosis is considered one of the most important adaptations in the origin of eukaryotes because it increased the potential food supply, and phagocytosis allowed the endosymbiosis and development of mitochondria and chloroplasts. In both osmotrophs and phagotrophs, endocytosis is often restricted to a specific region of the cell membrane, known as the cytostome, which may be followed by a cytopharynx, a specialized tract supported by microtubules.[138]
Osmotrophy
[edit]Osmotrophic protists acquire soluble nutrients through membrane channels and carriers, but also through different types of pinocytosis. Macropinocytosis involves the folding of membrane into ruffles,[141] which creates large (0.2 to 1.0 μm) vacuoles. Micropinocytosis involves smaller vesicles that are usually formed by clathrin. In both scenarios, the vesicles merge into a digestive vacuole or endosome where digestion takes place.[138] Some osmotrophs, called saprotrophs or lysotrophs, perform external digestion by releasing enzymes into the environment and decomposing organic matter[2] into simpler molecules that can be absorbed. This external digestion has a distinct advantage: it allows greater control over the substances that are allowed to enter the cell, thus minimizing the intake of harmful substances or infection.[142]
Probably all eukaryotes are capable of osmotrophy, but some have no alternative of acquiring nutrients. Obligate osmotrophs and saprotrophs include some euglenids, some green algae, the human parasite Blastocystis, some metamonads,[2] the parasitic trypanosomatids,[143] and the fungus-like oomycetes and hyphochytrids.[142]

Phagotrophy
[edit]
Phagotrophic feeding consists of two phases: the concentration of food particles in the environment, and the phagocytosis, which encloses the food particle in a vacuole (the phagosome)[138] where digestion takes place. In ciliates and most phagotrophic flagellates, digestion occurs at the oral region or cytostome, which is covered by a single membrane from which vacuoles are formed; the phagosomes then may be shuttled to the interior of the cell along the cytopharynx.[145] In amoebae, phagocytosis takes place anywhere on the cell surface. The average food particle size is around one tenth the size of the protist cell.[146]
Phagotrophic protists can be further classified according to how they approach the nutrients. The filter feeders acquire small, suspended food particles or prokaryotic cells and accumulate them by filtration into the cytostome (e.g., choanoflagellates, some chrysomonads, most ciliates);[2] filter-feeding flagellates accumulate particles by propelling them with a flagellum through a collar of rigid tentacles or pseudopodia that act as a filter, while filter-feeding ciliates generate water currents through cilia and membranelle zones surrounding the cytostome. The raptorial feeders (e.g., bicosoecids, chrysomonads, kinetoplastids, some euglenids, many dinoflagellates and ciliates), instead of retaining all particles in bulk, capture each particle individually.[146] Among raptorial protists, the grazers search and ingest prey from surfaces covered with potential food items such as bacterial lawns, while the predators actively pursue scarce prey.[2] Predators that feed on filamentous algae or fungal hyphae either swallow the filaments entirely or penetrate the cell wall and ingest the cytoplasm (e.g., Viridiraptoridae).[2] Predators may have adaptations to hunt prey, such as 'toxicysts' that immobilize prey cells. Certain ciliates have developed a specialized kind of raptorial feeding called histophagy, where they attack damaged but live animals (e.g., annelids and small crustaceans), enter the wounds, and ingest animal tissue. Large raptorial amoebae enclose their prey in a "food cup" of pseudopodia, prior to the formation of the food vacuole.[146] Lastly, diffusion feeders (e.g., heliozoa, foraminifera and many other amoebae, suctorian ciliates) engulf prey that happen to collide with their pseudopods or, in the case of ciliates, tentacles that carry toxicysts or extrusomes to immobilize the prey.[146]
Consumers of prokaryotes are popularly called bacterivores (e.g., most amoebae), while consumers (including osmotrophic parasites) of eukaryotes are known as eukaryovores. In particular, eukaryovores that feed on unicellular protists are cytotrophs (e.g., colponemids, colpodellids, many amoebae, some ciliates); those that feed on fungi are mycophages or mycotrophs (e.g., the ciliate family Grossglockneriidae of obligate mycophages);[147] those that prey on nematodes are nematophages;[148] and those that feed on algae are phycotrophs (e.g., vampyrellids).[2]
Mixotrophy
[edit]Most autotrophic protists are mixotrophs[150] and combine photosynthesis with phagocytosis.[e] They are classified into various functional groups or 'mixotypes'.[152][153] Constitutive mixotrophs have the innate ability to photosynthesize through already present chloroplasts, and have diverse feeding behaviors, as some require phototrophy, others phagotrophy, and others are obligate mixotrophs (e.g., nanoflagellates such as some haptophytes and dinoflagellates). Non-constitutive mixotrophs acquire the ability to photosynthesize by stealing chloroplasts from their prey, a process known as kleptoplasty. Non-constitutives can be divided into two: generalists, which can steal chloroplasts from a variety of prey (e.g., oligotrich ciliates), or specialists, which can only acquire chloroplasts from a few specific prey (e.g., Rapaza viridis can only steal from Tetraselmis cells).[149] The specialists are further divided into two types: plastidic, which contain differentiated plastids (e.g., Mesodinium, Dinophysis), and endosymbiotic, which contain whole endosymbionts (e.g., mixotrophic Rhizaria such as Foraminifera and Radiolaria, dinoflagellates like Noctiluca).[152]
Among exclusively heterotrophic protists, variation of nutritional modes is also observed. The diplonemids, which inhabit deep waters where photosynthesis is absent, can flexibly switch between osmotrophy and bacterivory depending on the environmental conditions.[154]
Osmoregulation
[edit]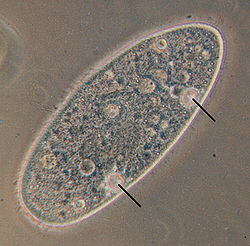
Many freshwater protists need to osmoregulate (i.e., remove excess water volume to adjust the ion concentrations) because non-saline water enters in excess by osmosis from the environment[155] and by endocytosis when feeding.[145] Osmoregulation is done through active ion transporters of the cell membrane and through contractile vacuoles, specialized organelles that periodically excrete fluid high in potassium and sodium through a cycle of diastole and systole. The cycle stops when the cells are placed in a medium with different salinity, until the cell adapts.[137]
The contractile vacuoles are surrounded by the spongiome, an array of cytoplasmic vesicles or tubes that slowly collect fluid from the cytoplasm into the vacuole. The vacuoles then contract and discharge the fluid outside of the cell through a pore. The contractile mechanism varies depending on the protist: in ciliates, the spongiome is composed of irregular tubules and actin filaments wind around the pore and over the vacuole surface, together with microtubules; in most flagellates and amoebae, the spongiome is composed of both vesicles and tubules; in dinoflagellates, a flagellar rootlet branches to form a contractile sheath around the vacuole (known as pusule).[145] The location and amount also varies: unicellular flagellated algae (cryptomonads, euglenids, prasinophytes, golden algae, haptophytes, etc.) typically have a single contractile vacuole in a fixed position; naked amoebae have numerous small vesicles that fuse into one vacuole and then split again after excretion. Marine or parasitic protists (e.g., metamonads), as well as those with rigid cell walls, lack these vacuoles.[155]
Respiration
[edit]The last eukaryotic common ancestor was aerobic, bearing mitochondria for oxidative metabolism. Many lineages of free-living and parasitic protists have independently evolved and adapted to inhabit anaerobic or microaerophilic habitats, by modifying the early mitochondria into hydrogenosomes, organelles that generate ATP anaerobically through fermentation of pyruvate. In a parallel manner, in the microaerophilic trypanosomatid protists, the fermentative glycosome evolved from the peroxisome.[137]
Sensory perception
[edit]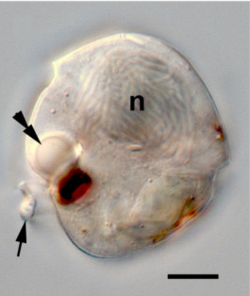
Many flagellates and probably all motile algae exhibit a positive phototaxis (i.e. they swim or glide toward a source of light). For this purpose, they exhibit three kinds of photoreceptors or "eyespots": (1) receptors with light antennae, found in many green algae, dinoflagellates and cryptophytes; (2) receptors with opaque screens; and (3) complex ocelloids with intracellular lenses, found in one group of predatory dinoflagellates, the Warnowiaceae. Additionally, some ciliates orient themselves in relation to the Earth's gravitational field while moving (geotaxis), and others swim in relation to the concentration of dissolved oxygen in the water.[137]
Endosymbionts
[edit]Protists have an accentuated tendency to include endosymbionts in their cells, and these have produced new physiological opportunities. Some associations are more permanent, such as Paramecium bursaria and its endosymbiont Chlorella; others more transient. Many protists contain captured chloroplasts, chloroplast-mitochondrial complexes, and even eyespots from algae. The xenosomes are bacterial endosymbionts found in ciliates, sometimes with a methanogenic role inside anaerobic ciliates.[137]
Life cycle and reproduction
[edit]
Protists exhibit a large range of life cycles and strategies involving multiple stages of different morphologies which have allowed them to thrive in most environments. Nevertheless, most of the knowledge concerning protist life cycles concerns model organisms and important parasites. Free-living uncultivated protists represent the majority, but knowledge on their life cycles remains fragmentary.[157]
Asexual reproduction
[edit]Protists typically reproduce asexually under favorable environmental conditions,[158] allowing for rapid exponential population growth with minimal genetic diversification. This asexual reproduction, occurs through mitosis and has historically been regarded as the primary reproductive mode in protists.[157] This process is also known as vegetative reproduction, as it is only performed by the 'vegetative stage' or individual.[159]
Unicellular protists often multiply via binary fission, similarly to bacteria.[157] They can also divide through budding, similarly to yeasts, or through multiple fissions, a process known as schizogony.[160] In multicellular protists, vegetative reproduction can take the form of fragmentation of body parts, or specialized propagules composed of numerous cells (e.g., in red algae).[159]
Sexual reproduction
[edit]While asexual reproduction remains the most common strategy among protists, sexual reproduction is also a fundamental characteristic of eukaryotes.[161][162] Sexual reproduction involves meiosis (a specialized nuclear division enabling genetic recombination) and syngamy (the fusion of nuclei from two parents).[157] These processes are thought to have been present in the last eukaryotic common ancestor,[163] which likely had the ability to reproduce sexually on a facultative (non-obligate) basis.[164] Even protists that no longer reproduce sexually still retain a core set of meiosis-related genes, reflecting their descent from sexual ancestors.[165][166] For example, although amoebae are traditionally considered asexual organisms, most asexual amoebae likely arose recently and independently from sexually reproducing amoeboid ancestors.[167] Even in the early 20th century, some researchers interpreted phenomena related to chromidia (chromatin granules free in the cytoplasm) in amoebae as sexual reproduction.[168]
Basic sexual cycles
[edit]Every sexual cycle involves the events of syngamy and meiosis, which increase or decrease the ploidy (i.e., number of chromosome sets, represented by the letter n), respectively. Syngamy implies the fusion of two haploid (1n) reproductive cells, known as gametes, which generates a diploid (2n) cell called zygote. The diploid cell then undergoes meiosis to generate haploid cells. Depending on which cells compose the individual or vegetative stage (i.e., the stage that grows by mitosis), there are three distinguishable sexual cycles observed in free-living protists:[157]
- In the haploid cycle, the individual is haploid and differentiates into haploid gametes through mitosis. The gametes fuse into a zygote which immediately undergoes meiosis to generate new haploid individuals.[157] This is the case for some green algae (namely Volvocales), many dinoflagellates, some metamonads, and apicomplexans.[145]: 26
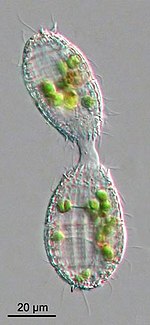
- In the diploid cycle, the individual is diploid and undergoes meiosis to generate haploid gametes, which in turn fuse with others to form a zygote that develops into a new individual.[157] This is the case for some metamonads, heliozoans, many green algae, diatoms, and ciliates, as well as animals.[145]: 26 Instead of generating gametes, ciliates divide their diploid micronucleus into two haploid nuclei, exchange one of them by conjugation with another ciliate, and fuse the two nuclei into a new diploid nucleus.[67]
- In the haplo-diploid cycle, there are two alternating generations of individuals. One generation is the diploid 'agamont', which undergoes meiosis to generate haploid cells (spores) that develop into the other generation, the haploid 'gamont'. The gamont then generates gametes by mitosis, which in turn fuse to form the zygote that develops into the agamont.[157] This is the case for many foraminifera and many algae, as well as land plants.[145]: 26 There are three modes of this cycle depending on the relative growth and lifespan of one generation compared to the other: haploid-dominant, diploid-dominant, or equally dominant generations. Brown algae exhibit the full range of these modes.[169]
Free-living protists tend to reproduce sexually under stressful conditions, such as starvation or heat shock. Oxidative stress, which leads to DNA damage, also appears to be an important factor in the induction of sex in protists.[158]
Sexual cycles in pathogenic protists
[edit]Pathogenic protists tend to have extremely complex life cycles that involve multiple forms of the organism, some of which reproduce sexually and others asexually.[170] The stages that feed and multiply inside the host are generally known as trophozoites (from Greek trophos 'nutrition' and zoia 'animals'), but the names of each stage vary depending on the protist group.[160] For example:
- In apicomplexans, a haploid sporozoite is released into the host, penetrates a host cell, begins the infection and transforms into a meront that grows and asexually divides into numerous merozoites (a schizogony called merogony); each merozoite continues the infection by multiplying. Eventually, the merozoites differentiate (gamogony) into female (macrogametocytes) and male (microgametocytes) that generate gametes, which in turn fuse (sporogony) into a diploid zygote that grows into a sporocyst. The sporocyst then undergoes meiosis to form sporozoites that transmit the infection.[70][162]
- In phytomyxeans, the diploid primary zoospores enter the host, encyst, and penetrate cells as a uninucleate protoplast or plasmodium. Inside the cells, the protoplast grows into a multinucleate zoosporangium, which then divides into secondary zoospores that infect more cells. These multiply into thick-walled resting spores that begin meiosis and divide into binucleate resting spores; one nucleus is lost, and the spores hatch as primary zoospores.[171]
Some protist pathogens undergo asexual reproduction in a wide variety of organisms – which act as secondary or intermediate hosts – but can undergo sexual reproduction only in the primary or definitive host (e.g., Toxoplasma gondii in felids such as domestic cats).[172] Others, such as Leishmania, are capable of performing syngamy in the secondary vector.[173] In apicomplexans, sexual reproduction is obligatory for parasite transmission.[174]
Despite undergoing sexual reproduction, it is unclear how frequently there is genetic exchange between different strains of pathogenic protists, as most populations may be clonal lines that rarely exchange genes with other members of their species.[175]
Ecology
[edit]Protists are indispensable to modern ecosystems worldwide. They also have been the only eukaryotic component of all ecosystems for much of Earth's history, which allowed them to evolve a vast functional diversity that explains their critical ecological significance. They are essential as primary producers, as intermediates in multiple trophic levels, as key regulating parasites or parasitoids, and as partners in diverse symbioses.[37]
Habitat diversity
[edit]Protists are abundant and diverse in nearly all habitats. They contribute 4 gigatons (Gt) to Earth's biomass—double that of animals (2 Gt), but less than 1% of the total. Combined, protists, animals, archaea (7 Gt), and fungi (12 Gt) make up less than 10% of global biomass, with plants (450 Gt) and bacteria (70 Gt) dominating.[176] Protist diversity, as detected through environmental DNA surveys, is vast in every sampled environment, but it is mostly undescribed.[177] The richest protist communities appear in soils, followed by oceanic and lastly freshwater habitats, mostly as part of the plankton.[178] Freshwater protist communities are characterized by a higher "beta diversity" (i.e. highly heterogeneous between samples) than soil and marine plankton. The high diversity can be a result of the hydrological dynamic of recruiting organisms from different habitats through extreme floods.[179] Soil-dwelling protist communities are ecologically the richest, possibly be due to the complex and highly dynamic distribution of water in the sediment, which creates extremely heterogenous environmental conditions. The constantly changing environment promotes the activity of only one part of the community at a time, while the rest remains inactive; this phenomenon promotes high microbial diversity in prokaryotes as well as protists.[178]
Primary producers
[edit]Microscopic phototrophic protists (or microalgae) are the main contributors to the biomass and primary production in nearly all aquatic environments, where they are collectively known as phytoplankton (together with cyanobacteria). In marine phytoplankton, the smallest fractions, the picoplankton (<2 μm) and nanoplankton (2–20 μm), are dominated by several different algae (prymnesiophytes, pelagophytes, prasinophytes); fractions larger than 5 μm are instead dominated by diatoms and dinoflagellates.[177] In freshwater phytoplankton, golden algae, cryptophytes and dinoflagellates are the most abundant groups.[178] Altogether, they are responsible for almost half of the global primary production.[180] They are the main providers of much of the energy and organic matter used by bacteria, archaea, and higher trophic levels (zooplankton and fish), including essential nutrients such as fatty acids.[181] Their abundance in the oceans depends mostly on the availability of inorganic nutrients, rather than temperature or sunlight; they are most abundant in coastal waters that receive nutrient-rich run-off from land, and areas where nutrient-rich deep ocean water reaches the surface, namely the upwelling zones in the Arctic Ocean and along continental margins.[180] In freshwater habitats, most phototrophic protists are mixotrophic, meaning they also behave as consumers, while strict consumers (heterotrophs) are less abundant.[178]
Macroalgae (namely red algae, green algae and brown algae), unlike phytoplankton, generally require a fixation point, which limits their marine distribution to coastal waters, and particularly to rocky substrates. They support numerous herbivorous animals, especially benthic ones, as both food and refuge from predators. Some communities of seaweeds exist adrift on the ocean surface, serving as a refuge and means of dispersal for associated organisms.[182][183]
Phototrophic protists are as abundant in soils as their aquatic counterparts. Given the importance of aquatic algae, soil algae may provide a larger contribution to the global carbon cycle than previously thought, but the magnitude of their carbon fixation has yet to be quantified.[178] Most soil algae are stramenopiles (diatoms, xanthophytes and eustigmatophytes) and archaeplastids (green algae). There is also presence of environmental DNA from dinoflagellates and haptophytes in soil, but no living forms have been seen.[184]
Consumers
[edit]Phagotrophic protists are the most diverse functional group in all ecosystems, primarily represented by cercozoans (dominant in freshwater and soils), radiolarians (dominant in oceans), non-photosynthetic stramenopiles (with higher abundance in soils than in oceans), and ciliates.[178]
Contrary to the common division between phytoplankton and zooplankton, much of the marine plankton is composed of mixotrophic protists, which pose a largely underestimated importance and abundance (around 12% of all marine environmental DNA sequences). Mixotrophs have varied presence due to seasonal abundance[185] and depending on their specific type of mixotrophy. Constitutive mixotrophs are present in almost the entire range of oceanic conditions, from eutrophic shallow habitats to oligotrophic subtropical waters but mostly dominating the photic zone, and they account for most of the predation of bacteria. They are also responsible for harmful algal blooms. Plastidic and generalist non-constitutive mixotrophs have similar biogeographies and low abundance, mostly found in eutrophic coastal waters, with generalist ciliates dominating up to half of ciliate communities in the photic zone. Lastly, endosymbiotic mixotrophs are by far the most widespread and abundant non-constitutive type, representing over 90% of all mixotroph sequences (mostly radiolarians).[153][152]

In the trophic webs of soils, protists are the main consumers of both bacteria and fungi, the two main pathways of nutrient flow towards higher trophic levels.[186] Amoeboflagellates like the glissomonads and cercomonads are among the most abundant soil protists: they possess both flagella and pseudopodia, a morphological variability well suited for foraging between soil particles. Testate amoebae are also acclimated to the soil environment, as their shells protect against desiccation.[184] As bacterial grazers, they have a significant role in the foodweb: they excrete nitrogen in the form of NH3, making it available to plants and other microbes.[186] Traditionally, protists were considered primarily bacterivorous due to biases in cultivation techniques, but many (e.g., vampyrellids, cercomonads, gymnamoebae, testate amoebae, small flagellates) are omnivores that feed on a wide range of soil eukaryotes, including fungi and even some animals such as nematodes. Bacterivorous and mycophagous protists amount to similar biomasses.[147]
Decomposers
[edit]Necrophagy (the degradation of dead biomass) among microbes is mainly attributed to bacteria and fungi, but protists have a still poorly recognized role as decomposers with specialized lytic enzymes.[187] In soils, fungus-like protists and slime molds (e.g., oomycetes, myxomycetes, acrasids) are present abundantly as osmotrophs and saprotrophs.[184] In marine and estuarine environments, the well-studied thraustochytrids (part of labyrinthulomycetes) are relevant saprotrophs that decompose various substrates, including dead plant and animal tissue. Various ciliates and testate amoebae scavenge on dead animals. Some nucleariid amoebae specifically consume the contents of dead or damaged cells, but not healthy cells. However, all these examples are only facultative necrophages that also feed on live prey. In contrast, the algivorous cercozoan family Viridiraptoridae, present in shallow bog waters, are broad-range but sophisticated necrophages that feed on a variety of exclusively dead algae, potentially fulfilling an important role in cleaning up the environment and releasing nutrients for live microbes.[187]
Parasites and pathogens
[edit]Parasitic protists occupy around 15–20% of all environmental DNA in marine and soil systems, but only around 5% in freshwater systems, where chytrid fungi likely fill that ecological niche. In oceanic systems, parasitoids (i.e. those which kill their hosts, e.g. Syndiniales) are more abundant. In freshwater ecosystems, parasitoids are mainly Perkinsea and Syndiniales (Alveolata), while true parasites (i.e. those which do not kill their hosts) in freshwater are mostly oomycetes, Apicomplexa and Ichthyosporea.[178] In soil ecosystems, true parasites are primarily animal-hosted apicomplexans and plant-hosted oomycetes and plasmodiophorids.[184] In Neotropical forest soils, apicomplexans dominate eukaryotic diversity and have an important role as parasites of small invertebrates, while oomycetes are very scarce in contrast.[188]
Some protists are significant parasites of animals (e.g.; five species of the parasitic genus Plasmodium cause malaria in humans and many others cause similar diseases in other vertebrates), land plants[189][190] (the oomycete Phytophthora infestans causes late blight in potatoes)[191] or even of other protists.[192][193] Around 100 protist species can infect humans.[184]
Biogeochemical cycles
[edit]Marine protists have a fundamental impact on biogeochemical cycles, particularly the carbon cycle.[194] As phytoplankton, they fix as much carbon as all terrestrial plants combined.[178] Soil protists, particularly testate amoebae, contribute to the silica cycle as much as forest trees through the biomineralization of their shells.[184]
History of classification
[edit]Early classification
[edit]
From the start of the 18th century, the popular term "infusion animals" (later infusoria) was used for protists, bacteria and small invertebrates. In the mid-18th century, while Swedish biologist Carl Linnaeus largely ignored the protists,[f] his Danish contemporary Otto Friedrich Müller was the first to introduce protists to the binomial nomenclature system.[195][196]
In 1820, German naturalist Georg August Goldfuss coined the term "Protozoa" (meaning 'early animals') as a class within Kingdom Animalia[197] that consisted of four groups: Infusoria (ciliates), Lithozoa (corals), Phytozoa, and Medusinae (jellyfish). Later, in 1845, Carl Theodor von Siebold used the term "Protozoa" as a phylum of exclusively unicellular animals consisting of two classes: Infusoria (ciliates) and Rhizopoda (amoebae, foraminifera).[198] Other scientists did not consider all protozoans part of the animal kingdom, and by the middle of the century most biologists grouped microorganisms into Protozoa, Protophyta (primitive plants), Phytozoa (animal-like plants), and Bacteria (mostly considered plants). In 1860, palaeontolgist Richard Owen was the first to define Protozoa as its own kingdom of eukaryotes, although he also included sponges within his group.[28]

In 1860, British naturalist John Hogg proposed "Protoctista" as the name for a fourth kingdom, (the other kingdoms being plant, animal and mineral) which he described as containing "all the lower creatures, or the primary organic beings", which included Protophyta, Protozoa and sponges.[199][28]

In 1866, the 'father of protistology', German scientist Ernst Haeckel, addressed the problem of classifying all these organisms as a mixture of animal and vegetable characters, and proposed Protistenreich[200] (Kingdom Protista) as the third kingdom of life, comprising primitive forms that were "neither animals nor plants". He grouped both bacteria[201] and eukaryotes, both unicellular and multicellular organisms, as Protista. He retained the Infusoria in the animal kingdom, until German zoologist Otto Bütschli demonstrated that they were unicellular.[202][203] At first, he included sponges and fungi, but in later publications he explicitly restricted Protista to predominantly unicellular organisms or colonies incapable of forming tissues. He clearly separated Protista from true animals on the basis that the defining character of protists was the absence of sexual reproduction, while the defining character of animals was the blastula stage of animal development. He also returned the terms Protozoa and Protophyta as subkingdoms of Protista.[28]
End of the animal-plant dichotomy
[edit]Bütschli considered the kingdom to be too polyphyletic and rejected the inclusion of bacteria. He fragmented the kingdom into protozoa (only nucleated, unicellular animal-like organisms), while bacteria and the protophyta were a separate grouping. This strengthened the old dichotomy of protozoa/protophyta from German scientist Carl Theodor von Siebold, and the German naturalists asserted this view over the worldwide scientific community by the turn of the century. However, British biologist C. Clifford Dobell in 1911 brought attention to the fact that protists functioned very differently compared to the animal and vegetable cellular organization, and gave importance to Protista as a group with a different organization that he called "acellularity", shifting away from the dogma of German cell theory. He coined the term protistology and solidified it as a branch of study independent from zoology and botany.[28]
In 1938, American biologist Herbert Copeland resurrected Hogg's label, arguing that Haeckel's term Protista included anucleated microbes such as bacteria, which the term Protoctista (meaning "first established beings") did not. Under his four-kingdom classification (Monera, Protoctista, Plantae, Animalia), the protists and bacteria were finally split apart, recognizing the difference between anucleate (prokaryotic) and nucleate (eukaryotic) organisms. To firmly separate protists from plants, he followed Haeckel's blastular definition of true animals, and proposed defining true plants as those with chlorophyll a and b, carotene, xanthophyll and production of starch. He also was the first to recognize that the unicellular/multicellular dichotomy was invalid. Still, he kept fungi within Protoctista, together with red algae, brown algae and protozoans.[28][204] This classification was the basis for Whittaker's later definition of Fungi, Animalia, Plantae and Protista as the four kingdoms of life.[205]
In the popular five-kingdom scheme published by American plant ecologist Robert Whittaker in 1969, Protista was defined as eukaryotic "organisms which are unicellular or unicellular-colonial and which form no tissues". Just as the prokaryotic/eukaryotic division was becoming mainstream, Whittaker, after a decade from Copeland's system,[205] recognized the fundamental division of life between the prokaryotic Monera and the eukaryotic kingdoms: Animalia (ingestion), Plantae (photosynthesis), Fungi (absorption) and the remaining Protista.[206][207][28]
In the five-kingdom system of American evolutionary biologist Lynn Margulis, the term "protist" was reserved for microscopic organisms, while the more inclusive kingdom Protoctista (or protoctists) included certain large multicellular eukaryotes, such as kelp, red algae, and slime molds.[208] Some use the term protist interchangeably with Margulis' protoctist, to encompass both single-celled and multicellular eukaryotes, including those that form specialized tissues but do not fit into any of the other traditional kingdoms.[209]
Advances in electron microscopy and molecular phylogenetics
[edit]
The five-kingdom model remained the accepted classification until the development of molecular phylogenetics in the late 20th century, when it became apparent that protists are a paraphyletic group from which animals, fungi and land plants evolved, and the three-domain system (Bacteria, Archaea, Eukarya) became prevalent.[210] Today, protists are not treated as a formal taxon, but the term is commonly used for convenience in two ways:[13]
- Phylogenetic definition: protists are a paraphyletic group.[211] A protist is any eukaryote that is not an animal, land plant or fungus,[212] thus excluding many unicellular groups like the fungal Microsporidia, Chytridiomycetes and yeasts, and the non-unicellular Myxozoan animals included in Protista in the past.[213]
- Functional definition: protists are essentially those eukaryotes that are never multicellular,[13] that either exist as independent cells, or if they occur in colonies, do not show differentiation into tissues.[214] While in popular usage, this definition excludes the variety of non-colonial multicellularity types that protists exhibit, such as aggregative (e.g., choanoflagellates) or complex multicellularity (e.g., brown algae).[215]
There is, however, one classification of protists based on traditional ranks that lasted until the 21st century. The British protozoologist Thomas Cavalier-Smith, since 1998, developed a six-kingdom model:[g] Bacteria, Animalia, Plantae, Fungi, Protozoa and Chromista.[10][216] In his context, paraphyletic groups take preference over clades:[10] both protist kingdoms Protozoa and Chromista contain paraphyletic phyla such as Apusozoa, Eolouka or Opisthosporidia. Additionally, red and green algae are considered true plants, while the fungal groups Microsporidia, Rozellida and Aphelida are considered protozoans under the phylum Opisthosporidia. This scheme endured until 2021, the year of his last publication.[9]
Fossil record
[edit]Before the existence of land plants, animals and fungi, all eukaryotes were protists. As a result, the early fossil record of protists is equivalent to the early record of eukaryotic life.[174] The protist fossil record is mainly represented by protists with fossilizable coverings, such as foraminifera, radiolaria, testate amoebae and diatoms, as well as multicellular algae.[217]
accepted fossil record (including name of earliest fossil), putative fossil record, biochemical signatures, molecular clock estimate, †major extinctions.
Paleo- and Mesoproterozoic
[edit]Modern or crown-group eukaryotes originated from the last eukaryotic common ancestor (LECA) and emerged between 1600 and 2400 million years ago (Ma), during the Paleoproterozoic and Mesoproterozoic eras.[1] However, the fossil record through this time is scarce and dominated by stem-group eukaryotes, extinct lineages preceding LECA. These lineages displayed early eukaryotic traits like flexible cell membranes and complex cell wall ornamentations, which require a flexible endomembrane system, but they lacked crown-group eukaryotes' advanced sterols (e.g., cholesterol), and instead produced simpler protosterols that require less oxygen during biosynthesis.[218] Examples of these are: Trachyhystrichosphaera and Leiosphaeridia dated at 1100 Ma,[219] Satka dated at 1300 Ma,[220] Tappania and Shuiyousphaeridium dated at 1600 Ma,[221] Grypania dated at 1800–1900 Ma, and Valeria which ranges from 1650 to 700 Ma.[222]
Crown-group eukaryotes achieved significant morphological and ecological diversity before 1000 Ma, with multicellular algae capable of sexual reproduction and unicellular protists exhibiting modern phagocytosis and locomotion. Their advanced but metabolically expensive sterols likely provided numerous evolutionary advantages due to the increased membrane flexibility, including resilience to osmotic shock during desiccation and rehydration cycles, extreme temperatures, UV light exposure, and protection against changing oxygen levels. These adaptations allowed crown-group eukaryotes to colonize diverse and harsh environments (e.g., mudflats, rivers, agitated shorelines and land). In contrast, stem-group eukaryotes occupied the low-oxygen marine waters as anaerobes.[218] The oldest definitive crown-group eukaryotic fossils include Rafatazmia and Ramathallus, both putative red algae, dated at 1600 Ma.[1]
Neoproterozoic
[edit]As oxygen levels rose during the Tonian period, crown-group eukaryotes outcompeted stem-group eukaryotes, expanding into oxygen-rich marine environments that supported an aerobic metabolism enabled by their mitochondria. Stem-group eukaryotes may have gone extinct due to competition and the extreme climatic changes of the Cryogenian glaciations and subsequent global warming, cementing the dominance of crown-group eukaryotes.[218] Crown-group eukaryotes began to appear abundantly in this era, fueled by the proliferation of red algae. The oldest fossils firmly assigned to existing protist groups include three multicellular algae: the rhodophyte Bangiomorpha (1047 Ma),[223] the chlorophyte Proterocladus (1000 Ma),[218] and the xanthophyte Paleovaucheria (1000 Ma).[224][225] Also included are the oldest fossils of Opisthokonta: Ourasphaira giraldae (1010–890 Ma), interpreted as the earliest fungus,[218] and Bicellum brasieri (1000 Ma), the earliest holozoan, showing traits associated with complex multicellularity.[226]
Abundant fossils of heterotrophic protists appear significantly later, parallel to the emergence of fungi.[218] Vase-shaped microfossils (VSMs), widespread rocks dated at 780–720 Ma (Tonian to Cryogenian), have been described as a variety of organisms across the decades (e.g., algae, chitinozoans, tintinnids), but current scientific consensus relates most VSMs to marine testate amoebae.[227] As such, VSMs comprise the oldest known fossils of both filose (Cercozoa) and lobose (Amoebozoa) testate amoebae.[228][229]
After the Gaskiers glaciation of the Late Ediacaran (~579 Ma), fossils of heterotrophic protists undergo diversification. Some fossils similar to VSMs are interpreted as the oldest fossils of Foraminifera dated at 548 Ma (e.g., Protolagena),[227] but their foraminiferal affinity is doubtful. Other microfossils that are possibly foraminifera include some poorly preserved tubular shells from 716–635 Ma rocks.[230]
Paleozoic
[edit]Radiolarian shells appear abundantly in the fossil record since the Cambrian, with the first definitive radiolarian fossils found at the very start of this period (~540 Ma) together with the first small shelly fauna.[231] Radiolarian records from older Precambrian rocks have been disregarded due to the lack of reliable fossils.[232][233][234] Around this time, between 540 and 510 Ma, the oldest Foraminifera shells appear, first multi-chambered and later tubular.[235][217][230]
Following the Cambrian explosion and rapid diversification of animals, the Precambrian microbe-dominated ecosystems were replaced by primarily benthic and nekto-benthic communities, with most marine organisms (animals, foraminifers, radiolarians) limited to the depths of shallow water environments.[236] Mirroring the animal radiation, there was a radiation of phytoplanktonic protists (i.e., acritarchs)[237] around 520–510 Ma, followed by a decrease in diversity around 500 Ma.[238] Later, the surviving acritarchs expanded in diversity and morphological innovation[237] due to a decrease in predation from benthic animals (particularly trilobites and brachiopods), which suffered extinction due to various proposed environmental factors such as anoxia.[239] Both phytoplankton and zooplankton (e.g., radiolarians) flourished, as signaled by an increase of organic carbon buried in the sediment known as the SPICE event (~497 Ma).[236][239] This abundant biomass supported a second animal radiation known as the Great Ordovician Biodiversification Event (GOBE), where many animals switched to a planktonic lifestyle and pelagic predators first appeared (e.g., cephalopods, swimming arthropods). This event is also known as the 'Ordovician Plankton Revolution' due to the significant diversification of planktonic protists, and it spanned from the late Cambrian well into the Ordovician.[236]
The Ordovician also includes the oldest euglenid fossil, known as Moyeria, which is found in rocks spanning from the middle Ordovician (~471 Ma) to the Silurian.[240] There are putative records of calcareous foraminifera from the Early Ordovician to the Silurian, but these are not widely accepted; the oldest trusted and well-known calcaerous foraminifera appear in the Middle Devonian, the next geological period.[217][241]
In Early Devonian terrestrial ecosystems the first fossils of freshwater arcellinid testate amoebae are found (e.g., Palaeoleptochlamys, Cangweulla),[242] as well as various types of freshwater green algae, including charophytes, volvocaceans and desmids,[243] and some putative algal fossils that might represent glaucophytes.[244] During the Devonian some benthic foraminifera acquired the ability of calcifying, and particularly the giant fusulinids became the dominant fossilizable protists. This time interval is also considered the molecular origin of haptophytes (~310 Ma) and silicoflagellates (397–382 Ma), which did not leave fossil traces until later in the Mesozoic. After the Late Devonian extinction (372 Ma), nassellarian-like radiolarians appeared for the first time, with a unique body plan among marine protists.[217]
During the Carboniferous period, no new fossilizable protists originated despite the major environmental changes. However, starting in the Late Carboniferous, radiolarian diversity and productivity increased, causing a large amount of biosiliceous sediment (chert) to be accumulated worldwide; this is known as the Radiolarian Optimum Event, which lasted primarily from the Middle Permian until the Early Cretaceous.[245][246][247] Around the Capitanian mass extinction event (262–259 Ma) of the Permian period, coccolithophores genetically diverged from the rest of haptophytes, possibly as a response to a reduction in atmospheric oxygen, and there was a faunal turnover from larger to smaller fusulinids.[217] Spumellarian radiolarians appear in the latest Permian.[245]
Mesozoic
[edit]The Permian-Triassic extinction event (~251.9 Ma) caused the extinction of many radiolarians, which manifests as a gap in the chert record.[245] The extinction is hypothesized as resulting in the molecular origin of diatoms and modern coccolithophores.[217] The Middle to Late Triassic period saw the acceleration of radiolarian diversity[245] and the appearance of several groups of calcaerous nannofossils. First, various nannofossils, some of which belonged to dinocysts, appeared early at around 235 Ma. Later originated the oldest identifiable coccolithophore, Crucirhabdus minutus (205–201 Ma), as well as the oldest fossils of Phaeodaria.[217] There's a variety of protozoa, including soft-bodied ciliates, and filamentous algae found in amber from the Late Triassic (220–230 Ma).[248]
Around the Early–Middle Jurassic, after the global Toarcian Oceanic Anoxic Event there was a diversification of dinoflagellates and coccolithophores, in both species and abundance. This interval also saw the completion of a symbiosis between Acantharia radiolarians and lineages of Phaeocystis haptophytes, as well as the appearance of planktonic foraminifera.[217] The period of low atmospheric oxygen ends in the Aptian-Albian boundary during the Early Cretaceous, and the first fossils of diatoms and silicoflagellates appear.[217] Samples of amber from around 100 Ma contain the oldest fossil records of apicomplexans (particularly malarian agents and gregarines), trypanosomes,[249] and metamonads—particularly mutualistic parabasalids of cockroaches, representing the earliest record of mutualism between protists and animals.[250][251]
The diversification of coccolithophores, mixotrophic dinoflagellates, and later diatoms across the Mesozoic era caused an accelerated transfer of primary production into higher trophic levels. This evolutionary radiation of phytoplankton was, in turn, responsible for the animal "Mesozoic marine revolution", characterized by the appearance of widespread predation among most invertebrate phyla. Coccolithophores, dinoflagellates and especially diatoms became the dominating eukaryotic producers in oceans until today, as opposed to cyanobacteria and green algae which dominated earlier.[252]
Cenozoic
[edit]The Cretaceous-Paleogene extinction event (~66 Ma) caused the extinction of many marine dinoflagellates, foraminifers, coccolithophores, and silicoflagellates; mesozoic types of these groups were substituted with types that dominate marine habitats today. Right after this event, putative ebridians begin appearing in the fossil record (e.g., Ammodochium), but the oldest reliable ebridian fossils belong to the upper middle Eocene (42–33.7 Ma).[217] Around this time, the oldest fossils of Synurophyceae appear (~49–40 Ma).[253] Following the Middle Eocene Climatic Optimum (~40 Ma), diatoms became the dominant agents of marine silicon precipitation as opposed to radiolarians, and the fossil record shows the first raphid diatoms and collodarians.[217]
See also
[edit]Footnotes
[edit]- ^ a b The distinction between protists and the other three eukaryotic kingdoms has been difficult to settle. Historically, the heterotrophic protists, known as protozoa, were considered part of the animal kingdom, while the phototrophic ones, called algae, were studied as part of the plant kingdom. Even after the creation of a separate protist kingdom, some minuscule animals (the myxozoans)[6] and 'lower' fungi (namely the aphelids, rozellids and microsporidians, collectively known as Opisthosporidia) were studied as protists,[7][8][9] and some algae (particularly red and green algae) remained classified as plants.[10] According to the current consensus, the label 'protist' specifically excludes animals, embryophytes (land plants) —meaning that all algae fall under this label— and all fungi. Opisthosporidians are considered part of a larger fungal kingdom, although they are often studied by protistologists and mycologists alike.[2][11][12]
- ^ The terms 'cilium' and 'eukaryotic flagellum' are interchangeable from a biological perspective. However, their usage depends on the author: some prefer to reserve cilia for shorter appendages and flagella for longer ones, while others prefer cilia for eukaryotes and flagella for prokaryotes. The term 'undulipodium' was proposed to unify the two concepts, as it refers specifically to the homologous microtubular structure found in both, but not found in prokaryotic flagella.[24][25][26]
- ^ A 2007 report on protist diversity included a table listing the described number of species for protist and fungal groups. The total sum of the listed species, excluding fungi, is 76,144.[36]
- ^ a b The phylum Percolozoa is usually better known as Heterolobosea.[2][46] However, in the strictest sense, Heterolobosea refers only to a class within this phylum, containing the orders Acrasida and Schizopyrenida. The name Percolozoa encompasses these and other related single-celled protists, not just the 'true' heteroloboseans.[9]
- ^ The terms "mixotroph" and "mixoplankton" almost exclusively refer to protists that perform photosynthesis and phagocytosis (photo-phagotrophs). Osmotrophy is always present, but not taken into account. As such, "pure" phototrophs (incapable of phagocytosis) and "pure" phagotrophs (incapable of photosynthesis) are technically mixotrophic due to their innate ability for osmotrophy, but are not usually reported in this sense.[151]
- ^ Carl von Linnaeus did not mention a single protist genus until the tenth edition of Systema Naturae of 1758, where Volvox was recorded.[195]
- ^ In 2015, Cavalier-Smith's initial six-kingdom model was revised into a seven-kingdom model after the inclusion of Archaea.[216]
References
[edit]- ^ a b c Strassert, Jürgen F. H.; Irisarri, Iker; Williams, Tom A.; Burki, Fabien (25 March 2021). "A molecular timescale for eukaryote evolution with implications for the origin of red algal-derived plastids". Nature Communications. 12 (1): 1879. Bibcode:2021NatCo..12.1879S. doi:10.1038/s41467-021-22044-z. PMC 7994803. PMID 33767194.
- ^ a b c d e f g h i j k l m n o p q r s t u v w Adl, Sina M.; Bass, David; Lane, Christopher E.; Lukeš, Julius; Schoch, Conrad L.; Smirnov, Alexey; et al. (2019). "Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes". Journal of Eukaryotic Microbiology. 66 (1): 4–119. doi:10.1111/JEU.12691. PMC 6492006. PMID 30257078.
- ^ a b c Tikhonenkov DV, Mikhailov KV, Gawryluk RMR, et al. (2022). "Microbial predators form a new supergroup of eukaryotes". Nature. 612 (7941): 714–719. Bibcode:2022Natur.612..714T. doi:10.1038/s41586-022-05511-5. PMID 36477531. S2CID 254436650.
- ^ Simonite T (November 2005). "Protists push animals aside in rule revamp". Nature. 438 (7064): 8–9. Bibcode:2005Natur.438....8S. doi:10.1038/438008b. PMID 16267517.
- ^ Harper D, Benton, Michael (2009). Introduction to Paleobiology and the Fossil Record. Wiley-Blackwell. p. 207. ISBN 978-1-4051-4157-4.
- ^ a b Levine ND, Corliss JO, Cox FEG, Deroux G, Grain J, Honigberg BM, et al. (1980). "A newly revised classification of the Protozoa". Journal of Protozoology. 27 (1): 37–58. doi:10.1111/j.1550-7408.1980.tb04228.x. PMID 6989987.
- ^ Weiss, Louis M. (2001). "Microsporidia: emerging pathogenic protists". Acta Tropica. 78 (2): 89–102. doi:10.1016/S0001-706X(00)00178-9. PMID 11230819."
- ^ Karpov, Sergey A.; Mamkaeva, Maria A.; Aleoshin, Vladimir V.; Nassonova, Elena; Lilje, Osu; Gleason, Frank H. (2014). "Morphology, phylogeny, and ecology of the aphelids (Aphelidea, Opisthokonta) and proposal for the new superphylum Opisthosporidia". Frontiers in Microbiology. 5: 112. doi:10.3389/fmicb.2014.00112. PMC 3975115. PMID 24734027.
- ^ a b c d Cavalier-Smith T (May 2022). "Ciliary transition zone evolution and the root of the eukaryote tree: implications for opisthokont origin and classification of kingdoms Protozoa, Plantae, and Fungi". Protoplasma. 259 (3): 487–593. Bibcode:2022Prpls.259..487C. doi:10.1007/s00709-021-01665-7. PMC 9010356. PMID 34940909.
- ^ a b c Cavalier-Smith T (August 1998). "A revised six-kingdom system of life". Biological Reviews of the Cambridge Philosophical Society. 73 (3): 203–266. doi:10.1111/j.1469-185X.1998.tb00030.x. PMID 9809012.
- ^ Tedersoo, Leho; Sánchez-Ramírez, Santiago; Kõljalg, Urmas; Bahram, Mohammad; Döring, Markus; Schigel, Dmitry; May, Tom; Ryberg, Martin; Abarenkov, Kessy (2018), "High-level classification of the Fungi and a tool for evolutionary ecological analyses", Fungal Diversity, 90: 135–159, doi:10.1007/s13225-018-0401-0, hdl:10138/238983, S2CID 21714270
- ^ a b Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; et al. (2022). "Outline of Fungi and fungus-like taxa – 2021". Mycosphere. 13 (1): 53–453. doi:10.5943/mycosphere/13/1/2. hdl:10481/76378. S2CID 249054641.
- ^ a b c O'Malley MA, Simpson AG, Roger AJ (2012). "The other eukaryotes in light of evolutionary protistology". Biology & Philosophy. 28 (2): 299–330. doi:10.1007/s10539-012-9354-y. S2CID 85406712.
- ^ Sebé-Pedrós, Arnau; Degnan, Bernard M.; Ruiz-Trillo, Iñaki (8 May 2017). "The origin of Metazoa: a unicellular perspective". Nature Reviews Genetics. 18 (8): 498–512. doi:10.1038/nrg.2017.21. PMID 28479598.
- ^ Corliss, J.O. (1995). "The ambiregnal protists and the codes of nomenclature: a brief review of the problem and of proposed solutions". Bulletin of Zoological Nomenclature. 52: 11–17. doi:10.5962/bhl.part.6717.
- ^ Richard Barnes; Stephen Kent (2001). The Invertebrates: A Synthesis. Wiley-Blackwell. p. 41. ISBN 978-0-632-04761-1.
- ^ a b c d e f g h i j k l m n o p q r s t Simpson, Alastair G. B.; Slamovits, Claudio H.; Archibald, John M. (2017). "Protist Diversity and Eukaryote Phylogeny". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 1–22. doi:10.1007/978-3-319-28149-0_45. ISBN 978-3-319-28147-6.
- ^ De Bruyn, P. P. H. (March 1947). "Theories of amoeboid movement". The Quarterly Review of Biology. 22 (1). The University of Chicago Press: 1–24. doi:10.1086/395577. JSTOR 2813332. PMID 20287832.
- ^ Brown MW, Kolisko M, Silberman JD, Roger AJ (2012). "Aggregative Multicellularity Evolved Independently in the Eukaryotic Supergroup Rhizaria". Current Biology. 22 (12): 1123–1127. Bibcode:2012CBio...22.1123B. doi:10.1016/j.cub.2012.04.021. PMID 22608512. S2CID 17510471.
- ^ Pawlowski, Jan (2008). "The twilight of Sarcodina: a molecular perspective on the polyphyletic origin of amoeboid protists" (PDF). Protistology. 5 (4): 281–302.
- ^ Pawlowski, Jan; Burki, Fabien (2009). "Untangling the Phylogeny of Amoeboid Protists". The Journal of Eukaryotic Microbiology. 56 (1): 16–25. doi:10.1111/j.1550-7408.2008.00379.x. PMID 19335771.
- ^ Matz, Mikhail V.; Frank, Tamara M.; Marshall, N. Justin; Widder, Edith A.; Johnsen, Sönke (2008). "Giant deep-sea protist produces bilaterian-like traces". Current Biology. 18 (23): 1849–1854. Bibcode:2008CBio...18.1849M. doi:10.1016/j.cub.2008.10.028. PMID 19026540.
- ^ Levin, Lisa A.; Rouse, Greg W. (2019). "Giant protists (xenophyophores) function as fish nurseries". Ecology. 101 (4): e02933. doi:10.1002/ecy.2933. PMC 7341444. PMID 31742677.
- ^ "Lynn Margulis replies". BioScience. 36 (5): 293–294. 1986. doi:10.1093/bioscience/36.5.293-a.
- ^ Margulis, Lynn (1980). "Undulipodia, flagella and cilia". Biosystems. 12 (1–2): 105–108. Bibcode:1980BiSys..12..105M. doi:10.1016/0303-2647(80)90041-6. PMID 7378551.
- ^ Andersen, R. A.; Barr, D. J. S.; Lynn, D. H.; Melkonian, M.; Moestrup, Ø.; Sleigh, M. A. (1991). "Terminology and nomenclature of the cytoskeletal elements associated with the flagellar/ciliary apparatus in protists". Protoplasma. 164 (1–3): 1–8. Bibcode:1991Prpls.164....1A. doi:10.1007/BF01320809.
- ^ Thibaut Brunet; Marvin Albert; William Roman; Maxwell C Coyle; Danielle C Spitzer; Nicole King (15 January 2021). "A flagellate-to-amoeboid switch in the closest living relatives of animals". eLife. 10. doi:10.7554/ELIFE.61037. ISSN 2050-084X. PMC 7895527. PMID 33448265. Wikidata Q105870433.
- ^ a b c d e f g Scamardella JM (1999). "Not plants or animals: A brief history of the origin of Kingdoms Protozoa, Protista, and Protoctista". International Microbiology. 2 (4): 207–221. PMID 10943416.
- ^ Eliáš, Marek (2021). "Protist diversity: Novel groups enrich the algal tree of life". Current Biology. 31 (11): R714 – R740. Bibcode:2021CBio...31.R733E. doi:10.1016/j.cub.2021.04.025. PMID 34102125.
- ^ Gleason, Frank H.; Lilje, Osu; Lange, Lene (2018). "What has happened to the "aquatic phycomycetes" (sensu Sparrow)? Part II: Shared properties of zoosporic true fungi and fungus-like microorganisms". Fungal Biology Reviews. 32 (2): 52–61. Bibcode:2018FunBR..32...52G. doi:10.1016/j.fbr.2017.09.003.
- ^ Neuhauser, Sigrid; Glockling, Sally L.; Leaño, Eduardo M.; Lilje, Osu; Marano, Agostina V.; Gleason, Frank H. (2012). "An introduction to fungus-like microorganisms". In Jones, E. B. Gareth; Pang, Ka-Lai (eds.). Marine fungi. Marine and Freshwater Botany. De Gruyter. pp. 137–152. doi:10.1515/9783110264067.137. ISBN 9783110264067.
- ^ Cavalier-Smith, Thomas (1993). "Kingdom Protozoa and its 18 phyla". Microbiological Reviews. 57 (4): 953–994. doi:10.1128/mr.57.4.953-994.1993. PMC 372943. PMID 8302218.
- ^ "Facts about malaria". www.ecdc.europa.eu. June 9, 2017.
- ^ a b c Pawlowski J, Audic S, Adl S, Bass D, Belbahri L, Berney C, Bowser SS, Cepicka I, Decelle J, Dunthorn M, Fiore-Donno AM, Gile GH, Holzmann M, Jahn R, Jirků M, Keeling PJ, Kostka M, Kudryavtsev A, Lara E, Lukeš J, Mann DG, Mitchell EAD, Nitsche F, Romeralo M, Saunders GW, Simpson AGB, Smirnov AV, Spouge JL, Stern JF, Stoeck T, Zimmermann J, Schindel D, de Vargas C (2012). "CBOL Protist Working Group: Barcoding Eukaryotic Richness beyond the Animal, Plant, and Fungal Kingdoms". PLOS Biology. 10 (11): e1001419. doi:10.1371/journal.pbio.1001419. PMC 3491025. PMID 23139639. S2CID 6330045.
- ^ Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B (2011). "How Many Species Are There on Earth and in the Ocean?". PLOS Biology. 9 (8): e1001127. doi:10.1371/journal.pbio.1001127. PMC 3160336. PMID 21886479.
- ^ a b c Adl, Sina M.; Leander, Brian S.; Simpson, Alastair G. B.; Archibald, John M.; Anderson, O. Roger; Bass, David; Bowser, Samuel S.; Brugerolle, Guy; Farmer, Mark A.; Karpov, Sergey; Kolisko, Martin; Lane, Christopher E.; Lodge, Deborah J.; Mann, David G.; Meisterfeld, Ralf; Mendoza, Leonel; Moestrup, Øjvind; Mozley-Standridge, Sharon E.; Smirnov, Alexey V.; Spiegel, Frederick (2007). "Diversity, Nomenclature, and Taxonomy of Protists". Systematic Biology. 56 (4): 684–689. doi:10.1080/10635150701494127. PMID 17661235.
- ^ a b c Burki, Fabien; Sandin, Miguel M.; Jamy, Mahwash (2021). "Diversity and ecology of protists revealed by metabarcoding". Current Biology. 31 (19): R1267 – R1280. Bibcode:2021CBio...31R1267B. doi:10.1016/j.cub.2021.07.066. PMID 34637739. S2CID 238588753.
- ^ a b c Brown MW, et al. (2018), "Phylogenomics Places Orphan Protistan Lineages in a Novel Eukaryotic Super-Group", Genome Biology and Evolution, 10 (2): 427–433, doi:10.1093/gbe/evy014, PMC 5793813, PMID 29360967
- ^ a b c d Yazaki, Euki; Yabuki, Akinori; Imaizumi, Ayaka; Kume, Keitaro; Hashimoto, Tetsuo; Inagaki, Yuji (2022). "The closest lineage of Archaeplastida is revealed by phylogenomics analyses that include Microheliella maris". Open Biology. 12 (4): 210376. doi:10.1098/rsob.210376. PMC 9006020. PMID 35414259.
- ^ a b c d e Eglit, Yana; Shiratori, Takashi; Jerlström-Hultqvist, Jon; Williamson, Kelsey; Roger, Andrew J.; Ishida, Ken-Ichiro; Simpson, Alastair G.B. (22 January 2024). "Meteora sporadica, a protist with incredible cell architecture, is related to Hemimastigophora". Current Biology. 34 (2): 451–459. Bibcode:2024CBio...34E.451E. doi:10.1016/j.cub.2023.12.032. PMID 38262350.
- ^ Burki F, Shalchian-Tabrizi K, Minge M, Skjaeveland A, Nikolaev SI, Jakobsen KS, Pawlowski J (August 2007). Butler G (ed.). "Phylogenomics reshuffles the eukaryotic supergroups". PLOS ONE. 2 (8): e790. Bibcode:2007PLoSO...2..790B. doi:10.1371/journal.pone.0000790. PMC 1949142. PMID 17726520.
- ^ a b c d e Madigan, Michael T.; Bender, Kelly S.; Buckley, Daniel H.; Sattley, W. Matthew; Stahl, David A. (2019). "Diversity of Microbial Eukarya". Brock Biology of Microorganisms (15th, Global ed.). Pearson. pp. 593–618. ISBN 9781292235103.
- ^ Simpson, Alastair G. B. (2003). "Cytoskeletal organization, phylogenetic affinities and systematics in the contentious taxon Excavata (Eukaryota)". International Journal of Systematic and Evolutionary Microbiology. 53 (6): 1759–1777. doi:10.1099/ijs.0.02578-0. PMID 14657103.
- ^ Suzuki-Tellier, Sei; Kiørboe, Thomas; Simpson, Alastair G. B. (2023). "The function of the feeding groove of 'typical excavate' flagellates". Journal of Eukaryotic Microbiology. 71 (2): e13016. doi:10.1111/jeu.13016. PMID 38108228.
- ^ Al Jewari, Caesar; Baldauf, Sandra L. (2023). "An excavate root for the eukaryote tree of life". Science Advances. 9 (17): eade4973. Bibcode:2023SciA....9E4973A. doi:10.1126/sciadv.ade4973. PMC 10146883. PMID 37115919.
- ^ a b Pánek, Tomáš; Simpson, Alastair G. B.; Brown, Matthew W.; Dyer, Betsey Dexter (2017). "Heterolobosea". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 2 (2nd ed.). Springer. pp. 1005–1046. doi:10.1007/978-3-319-28149-0_10. ISBN 978-3-319-28147-6.
- ^ a b Simpson, Alastair G. B. (2017). "Jakobida". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 2 (2nd ed.). Springer. pp. 973–1004. doi:10.1007/978-3-319-28149-0_6. ISBN 978-3-319-28147-6.
- ^ a b c d e f g h Guiry, Michael D. (2024). "How many species of algae are there? A reprise. Four kingdoms, 14 phyla, 63 classes and still growing". Journal of Phycology. 60 (2): 214–228. Bibcode:2024JPcgy..60..214G. doi:10.1111/jpy.13431. PMID 38245909.
- ^ Kostygov, Alexei Y.; Karnkowska, Anna; Votýpka, Jan; Tashyreva, Daria; Maciszewski, Kacper; Yurchenko, Vyacheslav; Lukeš, Julius (2021). "Euglenozoa: taxonomy, diversity and ecology, symbioses and viruses". Open Biology. 11 (3): 200407. doi:10.1098/rsob.200407. PMC 8061765. PMID 33715388.
- ^ Karnkowska A, Vacek V, Zubáčová Z, Treitli SC, Petrželková R, Eme L, Novák L, Žárský V, Barlow LD, Herman EK, Soukal P, Hroudová M, Doležal P, Stairs CW, Roger AJ, Eliáš M, Dacks JB, Vlček Č, Hampl V (23 May 2016). "A Eukaryote without a Mitochondrial Organelle". Current Biology. 26 (10): 1274–1284. Bibcode:2016CBio...26.1274K. doi:10.1016/j.cub.2016.03.053. PMID 27185558.
- ^ a b Novák, Lukáš V. F.; Treitli, Sebastian C.; Pyrih, Jan; Hałakuc, Paweł; Pipaliya, Shweta V.; Vacek, Vojtěch; Brzoň, Ondřej; Soukal, Petr; Eme, Laura; Dacks, Joel B.; Karnkowska, Anna; Eliáš, Marek; Hampl, Vladimír (2023). "Genomics of Preaxostyla Flagellates Illuminates the Path Towards the Loss of Mitochondria". PLOS Genetics. 19 (12): e1011050. doi:10.1371/journal.pgen.1011050. PMC 10703272. PMID 38060519.
- ^ Zhang, Qianqian; Táborský, Petr; Silberman, Jeffrey D.; Pánek, Tomáš; Čepička, Ivan; Simpson, Alastair G.B. (September 2015). "Marine Isolates of Trimastix marina Form a Plesiomorphic Deep-branching Lineage within Preaxostyla, Separate from Other Known Trimastigids (Paratrimastix n. gen.)". Protist. 166 (4): 468–491. doi:10.1016/j.protis.2015.07.003. PMID 26312987.
- ^ Eglit, Yana; Williams, Shelby K.; Roger, Andrew J.; Simpson, Alastair G.B. (2024). "Characterization of Skoliomonas gen. nov., a haloalkaliphilic anaerobe related to barthelonids (Metamonada)". Journal of Eukaryotic Microbiology. 71 (6): e13048. doi:10.1111/jeu.13048. PMC 11603281. PMID 39225178.
- ^ Heiss AA, Warring SD, Lukacs K, Favate J, Yang A, Gyaltshen Y, Filardi C, Simpson AGB, Kim E (December 2020). "Description of Imasa heleensis, gen. nov., sp. nov. (Imasidae, fam. nov.), a Deep-Branching Marine Malawimonad and Possible Key Taxon in Understanding Early Eukaryotic Evolution". Journal of Eukaryotic Microbiology. 68 (2): e12837. doi:10.1111/jeu.12837. PMID 33274482.
- ^ a b Tikhonenkov, Denis V.; Jamy, Mahwash; Borodina, Anastasia S.; Belyaev, Artem O.; Zagumyonnyi, Dmitry G.; Prokina, Kristina I.; Mylnikov, Alexander P.; Burki, Fabien; Karpov, Sergey A. (2022). "On the origin of TSAR: morphology, diversity and phylogeny of Telonemia". Open Biology. 12 (3). The Royal Society. doi:10.1098/rsob.210325. ISSN 2046-2441. PMC 8924772. PMID 35291881.
- ^ Strassert JF, Jamy M, Mylnikov AP, Tikhonenkov DV, Burki F (April 2019). Shapiro B (ed.). "New Phylogenomic Analysis of the Enigmatic Phylum Telonemia Further Resolves the Eukaryote Tree of Life". Molecular Biology and Evolution. 36 (4): 757–765. doi:10.1093/molbev/msz012. PMC 6844682. PMID 30668767.
- ^ Yazaki, Euki; Yabuki, Akinori; Imaizumi, Ayaka; Kume, Keitaro; Hashimoto, Tetsuo; Inagaki, Yuji (2022). "The closest lineage of Archaeplastida is revealed by phylogenomics analyses that include Microheliella maris". Open Biol. 12 (4): 210376. doi:10.1098/rsob.210376. PMC 9006020. PMID 35414259.
- ^ Torruella, Guifré; Galindo, Luis Javier; Moreira, David; López-García, Purificación (27 August 2024). "Phylogenomics of neglected flagellated protists supports a revised eukaryotic tree of life". bioRxiv.org. doi:10.1101/2024.05.15.594285. Retrieved 12 November 2024.
- ^ a b c d e Jirsová, Dagmar; Wideman, Jeremy G. (2024). "Integrated overview of stramenopile ecology, taxonomy, and heterotrophic origin". The ISME Journal. 18 (1): wrae150. doi:10.1093/ismejo/wrae150. PMC 11412368. PMID 39077993.
- ^ Weston EJ, Eglit Y, Simpson AG (2023). "Kaonashia insperata gen. et sp. nov., a eukaryotrophic flagellate, represents a novel major lineage of heterotrophic stramenopiles". Journal of Eukaryotic Microbiology. 71 (1): e13003. doi:10.1111/jeu.13003. PMID 37803921.
- ^ Cho, Anna; Tikhonenkov, Denis V.; Lax, Gordon; Prokina, Kristina I.; Keeling, Patrick J. (2025). "Phylogenomic position of genetically diverse phagotrophic stramenopile flagellates in the sediment-associated MAST-6 lineage and a potentially halotolerant placididean". Molecular Phylogenetics and Evolution. 190 107964. doi:10.1016/j.ympev.2023.107964. PMID 37951557.
- ^ Mann, David G.; Crawford, Richard M.; Round, Frank E. (2017). "Bacillariophyta". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 205–266. doi:10.1007/978-3-319-28149-0_29. ISBN 978-3-319-28147-6.
- ^ Kawai, Hiroshi; Henry, Eric C. (2017). "Phaeophyta". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 267–304. doi:10.1007/978-3-319-28149-0_31. ISBN 978-3-319-28147-6.
- ^ Kristiansen, Jørgen; Škaloud, Pavel (2017). "Chrysophyta". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 331–366. doi:10.1007/978-3-319-28149-0_43. ISBN 978-3-319-28147-6.
- ^ Thines M (2018). "Oomycetes". Current Biology. 28 (15): R812 – R813. Bibcode:2018CBio...28.R812T. doi:10.1016/j.cub.2018.05.062. PMID 30086308.
- ^ Azuma, Tomonori; Pánek, Tomáš; Tice, Alexander K.; Kayama, Motoki; Kobayashi, Mayumi; Miyashita, Hideaki; Suzaki, Toshinobu; Yabuki, Akinori; Brown, Matthew W.; Kamikawa, Ryoma (10 April 2022). "An Enigmatic Stramenopile Sheds Light on Early Evolution in Ochrophyta Plastid Organellogenesis". Molecular Biology and Evolution. 39 (4). doi:10.1093/molbev/msac065. PMC 9004409. PMID 35348760.
- ^ a b Lynn, Denis H. (2017). "Ciliophora". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 679–730. doi:10.1007/978-3-319-28149-0_23. ISBN 978-3-319-28147-6.
- ^ Janouškovec, Jan; Tikhonenkov, Denis V.; Mikhailov, Kirill V.; Simdyanov, Timur G.; Aleoshin, Vladimir V.; Mylnikov, Alexander P.; Keeling, Patrick J. (2013). "Colponemids Represent Multiple Ancient Alveolate Lineages". Current Biology. 23 (24): 2546–2552. Bibcode:2013CBio...23.2546J. doi:10.1016/j.cub.2013.10.062. PMID 24316202.
- ^ a b c Janouškovec, Jan; Tikhonenkov, Denis V.; Burki, Fabien; Howe, Alexis T.; Kolísko, Martin; Mylnikov, Alexander P.; Keeling, Patrick J. (2015). "Factors mediating plastid dependency and the origins of parasitism in apicomplexans and their close relatives". PNAS. 112 (33): 10200–10207. Bibcode:2015PNAS..11210200J. doi:10.1073/pnas.1423790112. PMC 4547307. PMID 25717057.
- ^ a b Votýpka, Jan; Modrý, David; Oborník, Miroslav; Šlapeta, Jan; Lukeš, Julius (2017). "Apicomplexa". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 567–624. doi:10.1007/978-3-319-28149-0_20. ISBN 978-3-319-28147-6.
- ^ Itoïz, Sarah; Metz, Sebastian; Derelle, Evelyne; Reñé, Albert; Garcés, Esther; Bass, David; Soudant, Philippe; Chambouvet, Aurélie (2021). "Emerging Parasitic Protists: The Case of Perkinsea". Frontiers in Microbiology. 12: 735815. doi:10.3389/FMICB.2021.735815. PMC 8792838. PMID 35095782.
- ^ a b Saldarriaga, Juan F.; Taylor, F. J. R. 'Max' (2017). "Dinoflagellata". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 625–678. doi:10.1007/978-3-319-28149-0_22. ISBN 978-3-319-28147-6.
- ^ Waller, Ross F.; Kořený, Ludĕk (2017). "Plastid Complexity in Dinoflagellates: A Picture of Gains, Losses, Replacements and Revisions". Advances in Botanical Research. 84: 105–143. doi:10.1016/bs.abr.2017.06.004. ISBN 978-0-12-802651-9.
- ^ Van Houten, Judith (2023). "A Review for the Special Issue on Paramecium as a Modern Model Organism". Microorganisms. 11 (4): 937. doi:10.3390/microorganisms11040937. PMC 10143506. PMID 37110360.
- ^ Cavalier-Smith, Thomas; Chao, Ema E.; Lewis, Rhodri (2018). "Multigene phylogeny and cell evolution of chromist infrakingdom Rhizaria: contrasting cell organisation of sister phyla Cercozoa and Retaria". Protoplasma. 255 (5): 1517–1574. Bibcode:2018Prpls.255.1517C. doi:10.1007/s00709-018-1241-1. PMC 6133090. PMID 29666938.
- ^ Burki, Fabien; Keeling, Patrick J. (2014). "Rhizaria". Current Biology. 24 (3): R103 – R107. Bibcode:2014CBio...24.R103B. doi:10.1016/j.cub.2013.12.025. PMID 2450277.
- ^ Biard, Tristan (2022). "Diversity and ecology of Radiolaria in modern oceans". Environmental Microbiology. 24 (5): 2179–2200. Bibcode:2022EnvMi..24.2179B. doi:10.1111/1462-2920.16004. PMC 9322464. PMID 35412019.
- ^ a b Pawlowski, J.; Lejzerowicz, F.; Esling, P. (2014-10-01). "Next-Generation Environmental Diversity Surveys of Foraminifera: Preparing the Future". The Biological Bulletin. 227 (2): 93–106. doi:10.1086/BBLv227n2p93. ISSN 0006-3185. PMID 25411369. S2CID 24388876.
- ^ a b Boltovskoy, Demetrio; Anderson, O. Roger; Correa, Nancy M. (2017). "Radiolaria and Phaeodaria". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 731–764. doi:10.1007/978-3-319-28149-0_19. ISBN 978-3-319-28147-6.
- ^ a b Dumack, Kenneth; Mylnikov, Alexander P.; Bonkowski, Michael (July 2017). "Evolutionary Relationship of the Scale-Bearing Kraken (incertae sedis, Monadofilosa, Cercozoa, Rhizaria): Combining Ultrastructure Data and a Two-Gene Phylogeny". Protist. 168 (3): 362–373. doi:10.1016/j.protis.2017.04.004. PMID 28582680.
- ^ Brown, Matthew W.; Kolisko, Martin; Silberman, Jeffrey D.; Rogers, Andrew J. (2012). "Aggregative multicellularity evolved independently in the eukaryotic supergroup Rhizaria". Current Biology. 22 (12): 1123–1127. Bibcode:2012CBio...22.1123B. doi:10.1016/j.cub.2012.04.021. PMID 22608512.
- ^ a b Howe, Alexis T.; Bass, David; Scoble, Josephine M.; Lewis, Rhodri; Vickerman, Keith; Arndt, Hartmut; Cavalier-Smith, Thomas (2011). "Novel Cultured Protists Identify Deep-branching Environmental DNA Clades of Cercozoa: New Genera Tremula, Micrometopion, Minimassisteria, Nudifila, Peregrinia". Protist. 162 (2): 332–372. doi:10.1016/j.protis.2010.10.002. ISSN 1434-4610. PMID 21295519.
- ^ Bass, David; Ward, Georgia M.; Burki, Fabien (2019). "Ascetosporea". Current Biology. 29 (1): R7 – R8. Bibcode:2019CBio...29...R7B. doi:10.1016/j.cub.2018.11.025. PMID 30620917.
- ^ Hittorf, Michaela; Letsch-Praxmarer, Susanne; Windegger, Alexandra; Bass, David; Kirchmair, Martin; Neuhauser, Sigrid (2020). "Revised Taxonomy and Expanded Biodiversity of the Phytomyxea (Rhizaria, Endomyxa)". Journal of Eukaryotic Microbiology. 67 (6): 648–659. doi:10.1111/jeu.12817. PMC 7756720. PMID 32654223.
- ^ Hess S, Suthaus A (2022). "The Vampyrellid Amoebae (Vampyrellida, Rhizaria)". Protist. 173 (1) 125854. doi:10.1016/j.protis.2021.125854. PMID 35091168.
- ^ Gast, Rebecca J. (2017). "Centrohelida and Other Heliozoan-Like Protists". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 2 (2nd ed.). Springer. pp. 955–1004. doi:10.1007/978-3-319-28149-0_28. ISBN 978-3-319-28147-6.
- ^ Bass, David; Tikhonenkov, Denis Victorovich; Foster, Rachel; Dyal, Patricia; Janouškovec, Jan; Keeling, Patrick J.; et al. (2018). "Rhizarian 'Novel Clade 10' revealed as abundant and diverse planktonic and terrestrial flagellates, including Aquavolon n. gen". Journal of Eukaryotic Microbiology. 65 (6): 828–842. doi:10.1111/jeu.12524. PMC 6282753. PMID 29658156.
- ^ a b Cavalier-Smith; Chao; Lewis (2015), "Multiple origins of Heliozoa from flagellate ancestors: New cryptist subphylum Corbihelia, superclass Corbistoma, and monophyly of Haptista, Cryptista, Hacrobia and Chromista", Molecular Phylogenetics and Evolution, 93: 331–362, Bibcode:2015MolPE..93..331C, doi:10.1016/j.ympev.2015.07.004, PMID 26234272
- ^ Burki, Fabien; Okamoto, Noriko; Pombert, Jean-François; Keeling, Patrick J. (2012). "The evolutionary history of haptophytes and cryptophytes: phylogenomic evidence for separate origins". Proceedings of the Royal Society B: Biological Sciences. 279 (1736): 2246–2254. doi:10.1098/rspb.2011.2301. PMC 3321700. PMID 22298847.
- ^ Burki, Fabien; Roger, Andrew J.; Brown, Matthew W.; Simpson, Alastair G.B. (2020). "The New Tree of Eukaryotes". Trends in Ecology & Evolution. 35 (1): 43–55. Bibcode:2020TEcoE..35...43B. doi:10.1016/j.tree.2019.08.008. PMID 31606140.
- ^ Torruella, Guifré; Galindo, Luis Javier; Moreira, David; López-García, Purificación (6 January 2025). "Phylogenomics of neglected flagellated protists supports a revised eukaryotic tree of life". Current Biology. 35 (1): 198–207.e4. Bibcode:2025CBio...35..198T. doi:10.1016/j.cub.2024.10.075. ISSN 1879-0445. PMID 39642877.
- ^ Williamson, Kelsey; Eme, Laura; Baños, Hector; McCarthy, Charley G. P.; Susko, Edward; Kamikawa, Ryoma; Orr, Russell J. S.; Muñoz-Gómez, Sergio A.; Minh, Bui Quang; Simpson, Alastair G. B.; Roger, Andrew J. (24 April 2025). "A robustly rooted tree of eukaryotes reveals their excavate ancestry". Nature. 640 (8060): 974–981. Bibcode:2025Natur.640..974W. doi:10.1038/s41586-025-08709-5. PMID 40074902.
- ^ Eikrem, Wenche; Medlin, Linda K.; Henderiks, Jorijntje; Rokitta, Sebastian; Rost, Björn; Probert, Ian; Throndsen, Jahn; Edvardsen, Bente (2017). "Haptophyta". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 2 (2nd ed.). Springer. pp. 893–954. doi:10.1007/978-3-319-28149-0_38. ISBN 978-3-319-28147-6.
- ^ Cavalier-Smith, Thomas; von der Heyden, Sophie (2007). "Molecular phylogeny, scale evolution and taxonomy of centrohelid heliozoa". Molecular Phylogenetics and Evolution. 44 (3): 1186–1203. Bibcode:2007MolPE..44.1186C. doi:10.1016/j.ympev.2007.04.019. PMID 17588778.
- ^ Zagumyonnyi, Dmitry G.; Radaykina, Liudmila V.; Keeling, Patrick J.; Tikhonenkov, Denis V. (2022). "Centrohelid heliozoans of Ukraine with a description of a new genus and species (Haptista: Centroplasthelida)". European Journal of Protistology. 86 125916. doi:10.1016/j.ejop.2022.125916. PMID 36137331.
- ^ Hoef-Emden, Kerstin; Archibald, John M. (2017). "Cryptophyta (Cryptomonads)". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 2 (2nd ed.). Springer. pp. 851–892. doi:10.1007/978-3-319-28149-0_35. ISBN 978-3-319-28147-6.
- ^ Yabuki, Akinori; Kamikawa, Ryoma; Ishikawa, Sohta A.; Kolisko, Martin; Kim, Eunsoo; Tanabe, Akifumi S.; Kume, Keitaro; Ishida, Ken-ichiro; Inagki, Yuji (2014-04-10). "Palpitomonas bilix represents a basal cryptist lineage: insight into the character evolution in Cryptista". Scientific Reports. 4 (1): 4641. Bibcode:2014NatSR...4.4641Y. doi:10.1038/srep04641. ISSN 2045-2322. PMC 3982174. PMID 24717814.
- ^ a b Olivier De Clerck; Kenny A. Bogaert; Frederik Leliaert (2012). "Chapter Two – Diversity and Evolution of Algae: Primary Endosymbiosis". In Gwenaël Piganeau (ed.). Advances in Botanical Research. Vol. 64. Academic Press. pp. 55–86. doi:10.1016/B978-0-12-391499-6.00002-5. ISBN 9780123914996. ISSN 0065-2296.
- ^ Yoon, Hwan Su; Nelson, Wendy; Lindstrom, Sandra C.; Boo, Sung Min; Pueschel, Curt; Qiu, Huan; Bhattacharya, Debashish (2017). "Rhodophyta". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 89–135. doi:10.1007/978-3-319-28149-0_33. ISBN 978-3-319-28147-6.
- ^ Prokina, Kristina I.; Tikhonenkov, Denis V.; López-García, Purificación; Moreira, David (2023). "Morphological and molecular characterization of a new member of the phylum Rhodelphidia". Journal of Eukaryotic Microbiology. 71 (2): e12995. doi:10.1111/jeu.12995. PMID 37548159.
- ^ Gawryluk, Ryan M. R.; Tikhonenkov, Denis V.; Hehenberger, Elisabeth; Husnik, Filip; Mylnikov, Alexander P.; Keeling, Patrick J. (August 2019). "Non-photosynthetic predators are sister to red algae". Nature. 572 (7768): 240–243. doi:10.1038/s41586-019-1398-6. ISSN 1476-4687. PMID 31316212. S2CID 197542583.
- ^ Schön, Max E.; Zlatogursky, Vasily V.; Singh, Rohan P.; Poirier, Camille; Wilken, Susanne; Mathur, Varsha; Strassert, Jürgen F. H.; Pinhassi, Jarone; Z. Worden, Alexandra; Keeling, Patrick J.; Ettema, Thijs J. G.; Wideman, Jeremy G.; Burki, Fabien (2021). "Single cell genomics reveals plastid-lacking Picozoa are close relatives of red algae". Nature Communications. 12 (1): 6651. Bibcode:2021NatCo..12.6651S. doi:10.1038/s41467-021-26918-0. PMC 8599508. PMID 34789758.
- ^ Linzhou Li; Sibo Wang; Hongli Wang; Sunil Kumar Sahu; Birger Marin; Haoyuan Li; Yan Xu; Hongping Liang; Zhen Li; Shifeng Chen; Tanja Reder; Zehra Çebi; Sebastian Wittek; Morten Petersen; Barbara Melkonian; Hongli Du; Huanming Yang; Jian Wang; Gane Ka-Shu Wong; Xun Xu; Xin Liu; Yves Van de Peer; Michael Melkonian; Huan Liu (22 June 2020). "The genome of Prasinoderma coloniale unveils the existence of a third phylum within green plants". Nature Ecology & Evolution. 4 (9): 1220–1231. Bibcode:2020NatEE...4.1220L. doi:10.1038/s41559-020-1221-7. PMC 7455551. PMID 32572216.
- ^ Hall, John D.; MCCourt, Richard M. (2017). "Zygnematophyta". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 135–163. doi:10.1007/978-3-319-28149-0_41. ISBN 978-3-319-28147-6.
- ^ McCourt, Richard M.; Karol, Kenneth G.; Hall, John D.; Casanova, Michelle T.; Grant, Michael C. (2017). "Charophyceae (Charales)". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 165–183. doi:10.1007/978-3-319-28149-0_40. ISBN 978-3-319-28147-6.
- ^ Cook, Martha E.; Graham, Linda E. (2017). "Chlorokybophyceae, Klebsormidiophyceae, Coleochaetophyceae". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 185–204. doi:10.1007/978-3-319-28149-0_36. ISBN 978-3-319-28147-6.
- ^ a b Heiss, Aaron A.; Kolisko, Martin; Ekelund, Fleming; Brown, Matthew W.; Roger, Andrew J.; Simpson, Alastair G. B. (4 April 2018). "Combined morphological and phylogenomic re-examination of malawimonads, a critical taxon for inferring the evolutionary history of eukaryotes". Royal Society Open Science. 5 (4). Bibcode:2018RSOS....571707H. doi:10.1098/rsos.171707. PMC 5936906. PMID 29765641.
- ^ Lamża, Łukasz (December 2023). "Diversity of 'simple' multicellular eukaryotes: 45 independent cases and six types of multicellularity". Biological Reviews. 98 (6): 2188–2209. doi:10.1111/brv.13001. PMID 37475165.
- ^ a b Brown, Matthew W.; Sharpe, Susan C.; Silberman, Jeffrey D.; Heiss, Aaron A.; Lang, B. Franz; Simpson, Alastair G. B.; Roger, Andrew J. (28 August 2013). "Phylogenomics demonstrates that breviate flagellates are related to opisthokonts and apusomonads". Proceedings of the Royal Society B: Biological Sciences. 280 (1769): 20131755. doi:10.1098/rspb.2013.1755. PMC 3768317. PMID 23986111.
- ^ Torruella G, Galindo LJ, Moreira D, Ciobanu M, Heiss AA, Yubuki N, et al. (2022). "Expanding the molecular and morphological diversity of Apusomonadida, a deep-branching group of gliding bacterivorous protists". Journal of Eukaryotic Microbiology. 70 (2): e12956. doi:10.1111/jeu.12956. hdl:2117/404026. PMID 36453005. S2CID 253460648.
- ^ Smirnov, Alexey; Nassonova, Elena; Berney, Cédric; Fahrni, José; Bolivar, Ignacio; Pawlowski, Jan (2005). "Molecular Phylogeny and Classification of the Lobose Amoebae". Protist. 156 (2): 129–142. doi:10.1016/j.protis.2005.06.002. PMID 16171181.
- ^ Page, Frederick C. (1987). "The Classification of 'Naked' Amoebae (Phylum Rhizopoda)". Archiv für Protistenkunde. 133 (3–4): 199–217. doi:10.1016/S0003-9365(87)80053-2.
- ^ a b Tekle, Yonas I.; Wang, Fang; Wood, Fiona C.; Anderson, O. Roger; Smirnov, Alexey (1 July 2022). "New insights on the evolutionary relationships between the major lineages of Amoebozoa". Scientific Reports. 12 (1): 11173. Bibcode:2022NatSR..1211173T. doi:10.1038/s41598-022-15372-7. PMC 9249873. PMID 35778543.
- ^ Brown, Matthew W.; Silberman, Jeffrey D.; Spiegel, Frederick W. (26 November 2010). ""Slime Molds" among the Tubulinea (Amoebozoa): Molecular Systematics and Taxonomy of Copromyxa". Protist. 162 (2): 277–287. doi:10.1016/j.protis.2010.09.003. PMID 21112814.
- ^ Kang, Seungho; Tice, Alexander K; Spiegel, Frederick W; Silberman, Jeffrey D; Pánek, Tomáš; Čepička, Ivan; Kostka, Martin; Kosakyan, Anush; Alcântara, Daniel M C; Roger, Andrew J; Shadwick, Lora L; Smirnov, Alexey; Kudryavtsev, Alexander; Lahr, Daniel J G; Brown, Matthew W (September 2017). "Between a Pod and a Hard Test: The Deep Evolution of Amoebae". Molecular Biology and Evolution. 34 (9): 2258–2270. doi:10.1093/molbev/msx162. PMC 5850466. PMID 28505375.
- ^ González-Miguéns, Rubén; Todorov, Milcho; Blandenier, Quentin; Duckert, Clément; Porfirio-Sousa, Alfredo L.; Ribeiro, Giulia M.; Ramos, Diana; Lahr, Daniel J.G.; Buckley, David; Lara, Enrique (2022). "Deconstructing Difflugia: The tangled evolution of lobose testate amoebae shells (Amoebozoa: Arcellinida) illustrates the importance of convergent evolution in protist phylogeny". Molecular Phylogenetics and Evolution. 175 107557. Bibcode:2022MolPE.17507557G. doi:10.1016/j.ympev.2022.107557. hdl:10261/281619. PMID 35777650.
- ^ Berney, Cédric; Geisen, Stefan; Van Wichelen, Jeroen; Nitsche, Frank; Vanormelingen, Pieter; Bonkowski, Michael; Bass, David (20 April 2015). "Expansion of the 'Reticulosphere': Diversity of Novel Branching and Network-forming Amoebae Helps to Define Variosea (Amoebozoa)". Protist. 166 (2): 271–295. doi:10.1016/j.protis.2015.04.001. PMID 25965302.
- ^ Galindo, Luis Javier; López-García, Purificación; Torruella, Guifré; Karpov, Sergey; Moreira, David (17 August 2021). "Phylogenomics of a new fungal phylum reveals multiple waves of reductive evolution across Holomycota". Nature Communications. 12 (1): 4973. Bibcode:2021NatCo..12.4973G. doi:10.1038/s41467-021-25308-w. PMC 8371127. PMID 34404788.
- ^ Gabaldón, Toni; Völcker, Eckhard; Torruella, Guifré (2022). "On the Biology, Diversity and Evolution of Nucleariid Amoebae (Amorphea, Obazoa, Opisthokonta)". Protist. 173 (4): 125895. doi:10.1016/j.protis.2022.125895. hdl:2117/369912. PMID 35841659.
- ^ Letcher, Peter M.; Powell, Martha J. (1 December 2018). "A taxonomic summary and revision of Rozella (Cryptomycota)". IMA Fungus. 9 (2): 383–399. doi:10.5598/imafungus.2018.09.02.09. PMC 6317583. PMID 30622888.
- ^ Cali, Ann; Becnel, James J.; Takvorian, Peter M. (2017). "Microsporidia". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 2 (2nd ed.). Springer. pp. 1559–1618. doi:10.1007/978-3-319-28149-0_27. ISBN 978-3-319-28147-6.
- ^ Galindo, Luis Javier; Torruella, Guifré; López-García, Purificación; Ciobanu, Maria; Gutiérrez-Preciado, Ana; Karpov, Sergey A.; Moreira, David (28 July 2022). "Phylogenomics Supports the Monophyly of Aphelids and Fungi and Identifies New Molecular Synapomorphies". Systematic Biology. 72 (3): 505–515. doi:10.1093/sysbio/syac054. PMID 35900180.
- ^ Richter, Daniel J.; Nitsche, Frank (2017). "Choanoflagellatea". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 2 (2nd ed.). Springer. pp. 1479–1496. doi:10.1007/978-3-319-28149-0_5. ISBN 978-3-319-28147-6.
- ^ Glockling, Sally L.; Marshall, Wyth L.; Gleason, Frank H. (25 April 2013). "Phylogenetic interpretations and ecological potentials of the Mesomycetozoea (Ichthyosporea)". Fungal Ecology. 6 (4): 237–247. Bibcode:2013FunE....6..237G. doi:10.1016/j.funeco.2013.03.005.
- ^ Urrutia, Ander; Mitsi, Konstantina; Foster, Rachel; Ross, Stuart; Carr, Martin; Ward, Georgia M.; van Aerle, Ronny; Marigomez, Ionan; Leger, Michelle M.; Ruiz-Trillo, Iñaqui; Feist, Stephen W.; Bass, David (2022). "Txikispora philomaios n. sp., n. g., a micro-eukaryotic pathogen of amphipods, reveals parasitism and hidden diversity in Class Filasterea". Journal of Eukaryotic Microbiology. 69 (2): e12875. doi:10.1111/jeu.12875. PMID 34726818.
- ^ Hehenberger, Elisabeth; Tikhonenkov, Denis V.; Kolisko, Martin; del Campo, Javier; Esaulov, Anton S.; Mylnikov, Alexander P.; Keeling, Patrick J. (10 July 2017). "Novel Predators Reshape Holozoan Phylogeny and Reveal the Presence of a Two-Component Signaling System in the Ancestor of Animals". Current Biology. 27 (13): 2043–2050. Bibcode:2017CBio...27E2043H. doi:10.1016/j.cub.2017.06.006. PMID 28648822.
- ^ Tikhonenkov DV, Mikhailov KV, Hehenberger E, Mylnikov AP, Aleoshin VV, Keeling PJ, et al. (2020). "New Lineage of Microbial Predators Adds Complexity to Reconstructing the Evolutionary Origin of Animals". Current Biology. 30 (22): 4500–4509. Bibcode:2020CBio...30E4500T. doi:10.1016/j.cub.2020.08.061. PMID 32976804.
- ^ Blaz, Jazmin; Galindo, Luis Javier; Heiss, Aaron A.; Kaur, Harpreet; Torruella, Guifré; Yang, Ashley; Thompson, L. Alexa; Filbert, Alexander; Warring, Sally; Narechania, Apurva; Shiratori, Takashi; Ishida, Ken-ichiro; Dacks, Joel B.; López-García, Purificación; Moreira, David; Kim, Eunsoo; Eme, Laura (January 2021). "High quality genome and transcriptome data for two new species of Mantamonas, a deep-branching eukaryote clade". bioRxiv 10.1101/2023.01.20.524885.
- ^ Yazaki, Euki; Harada, Ryo; Isogai, Ryu; Bamba, Kohei; Ishida, Ken-ichiro; Inagaki, Yuji; Shiratori, Takashi (June 2025). "Glissandra oviformis n. sp.: a novel predatory flagellate illuminates the character evolution within the eukaryotic clade CRuMs". Open Biology. 15 (6). doi:10.1098/rsob.250057. PMC 12133344. PMID 40460873.
- ^ Patterson, David J.; Simpson, A.G.B. (December 1996). "Heterotrophic flagellates from coastal marine and hypersaline sediments in Western Australia". European Journal of Protistology. 32 (4): 423–448. doi:10.1016/S0932-4739(96)80003-4.
- ^ Yubuki, Naoji; Torruella, Guifré; Galindo, Luis Javier; Heiss, Aaron A.; Ciobanu, Maria Cristina; Shiratori, Takashi; Ishida, Ken-ichiro; Blaz, Jazmin; Kim, Eunsoo; Moreira, David; López-García, Purificación; Eme, Laura (November 2023). "Molecular and morphological characterization of four new ancyromonad genera and proposal for an updated taxonomy of the Ancyromonadida". Journal of Eukaryotic Microbiology. 70 (6: e12997): e12997. doi:10.1111/jeu.12997. hdl:2117/404022. ISSN 1550-7408. PMID 37606230.
- ^ Lax, Gordon; Eglit, Yana; Eme, Laura; Bertrand, Erin M.; Roger, Andrew J.; Simpson, Alastair G. B. (14 November 2018). "Hemimastigophora is a novel supra-kingdom-level lineage of eukaryotes". Nature. 564 (7736): 410–414. Bibcode:2018Natur.564..410L. doi:10.1038/s41586-018-0708-8. ISSN 0028-0836. PMID 30429611. S2CID 205570993.
- ^ Belyaev, Artem O.; Karpov, Sergey A.; Keeling, Patrick J.; Tikhonenkov, Denis V. (18 December 2024). "The nature of 'jaws': a new predatory representative of Provora and the ultrastructure of nibbling protists". Open Biology. 14 (12): 240158. doi:10.1098/rsob.240158. PMC 11651884. PMID 39689855.
- ^ Shishkin, Yegor; Drachko, Daria; Zlatogursky, Vasily V. (22 April 2021). "The smallest known heliozoans are the Erebor lineage (nom. clad. n.) inside Microheliella maris (Eukaryota, Diaphoretickes), with the amendation of M. maris diagnosis and description of Berkeleyaesol magnus gen. nov., comb. nov. (Eukaryota, incertae sedis)". International Journal of Systematic and Evolutionary Microbiology. 71 (4). doi:10.1099/ijsem.0.004776. PMID 33886450. S2CID 233370018.
- ^ Yamaguchi M, Mori Y, Kozuka Y, Okada H, Uematsu K, Tame A, Furukawa H, Maruyama T, Worman CO, Yokoyama K (2012). "Prokaryote or eukaryote? A unique microorganism from the deep sea". J Electron Microsc (Tokyo). 61 (6): 423–431. doi:10.1093/jmicro/dfs062. PMID 23024290.
- ^ Plattner H (2018). "Evolutionary cell biology of proteins from protists to humans and plants". J. Eukaryot. Microbiol. 65 (2): 255–289. doi:10.1111/jeu.12449. PMID 28719054. S2CID 206055044.
- ^ a b c d e Levandowsky, Michael (2012). "Physiological Adaptations of Protists". In Sperelakis, Nicholas (ed.). Cell Physiology Sourcebook: Essentials of Membrane Biophysics (Fourth ed.). Amsterdam; Boston: Elsevier/AP. pp. 874–890. ISBN 978-0-12-387738-3.
- ^ a b c d e f g Wiser, Mark F. (1 July 2024). "Feeding Mechanisms of Pathogenic Protozoa with a Focus on Endocytosis and the Digestive Vacuole". Parasitologia. 4 (3): 222–237. doi:10.3390/parasitologia4030019.
- ^ a b c Hickman, Cleveland P. Jr.; Keen, Susan L.; Eisenhour, David J.; Larson, Allan; l'Anson, Helen (2017). "Unicellular Eukaryotes: Protozoan Groups". Integrated Principles of Zoology (7th ed.). New York: McGraw Hill. pp. 216–245. ISBN 9781259562310. LCCN 2016026850.
- ^ Lwoff A, Van Niel CB, Ryan TF, Tatum EL (1946). "Nomenclature of nutritional types of microorganisms" (PDF). Cold Spring Harbor Symposia on Quantitative Biology. 11 (5th ed.): 302–3.
- ^ Swanson, Joel A.; Watts, Colin (November 1995). "Macropinocytosis". Trends in Cell Biology. 5 (11): 424–428. doi:10.1016/S0962-8924(00)89101-1. PMID 14732047.
- ^ a b Richards, Thomas A.; Talbot, Nicholas J. (10 September 2013). "Horizontal gene transfer in osmotrophs: playing with public goods". Nature Reviews Microbiology. 11 (10): 720–727. doi:10.1038/nrmicro3108. hdl:10871/15898. PMID 24018383.
- ^ Harmer, Jane; Yurchenko, Vyacheslav; Nenarokova, Anna; Lukeš, Julius; Ginger, Michael L. (13 June 2018). "Farming, slaving and enslavement: histories of endosymbioses during kinetoplastid evolution" (PDF). Parasitology. 145 (10): 1311–1323. doi:10.1017/S0031182018000781. PMID 29895336.
- ^ Etheridge, Ronald Drew (2022). "Protozoan phagotrophy from predators to parasites: An overview of the enigmatic cytostome-cytopharynx complex of Trypanosoma cruzi". Journal of Eukaryotic Microbiology. 69 (6): e12896. doi:10.1111/jeu.12896. PMC 11110969. PMID 35175673.
- ^ a b c d e f Ruppert, Edward E.; Fox, Richard S.; Barnes, Robert D. (2004). "Protozoa". Invertebrate Zoology: A Functional Evolutionary Approach (7th ed.). Thomson Brooks/Cole. pp. 22–57. ISBN 0-03-025982-7.
- ^ a b c d Esteban, Genoveva F.; Fenchel, Tom M. (2020). "Feeding". Ecology of Protozoa: The Biology of Free-living Phagotrophic Protists (2nd ed.). Cham: Springer Nature Switzerland AG. pp. 33–54. doi:10.1007/978-3-030-59979-9_4. ISBN 978-3-030-59979-9.
- ^ a b c Geisen, Stefan; Koller, Robert; Hünninghaus, Maike; Dumack, Kenneth; Urich, Tim; Bonkowski, Michael (2016). "The soil food web revisited: Diverse and widespread mycophagous soil protists". Soil Biology and Biochemistry. 94: 10–18. Bibcode:2016SBiBi..94...10G. doi:10.1016/j.soilbio.2015.11.010.
- ^ Geisen, Stefan; Rosengarten, Jamila; Koller, Robert; Mulder, Christian; Urich, Tim; Bonkowski, Michael (16 June 2015). "Pack-hunting protists attacking nematodes". Environmental Microbiology. 17 (11): 4538–4546. doi:10.1111/1462-2920.12949. PMID 26079718.
- ^ a b Karnkowska A, Yubuki N, Maruyama M, Yamaguchi A, Kashiyama Y, Suzaki T, Keeling PJ, Hampl V, Leander BS (21 March 2023). "Euglenozoan kleptoplasty illuminates the early evolution of photoendosymbiosis". Proceedings of the National Academy of Sciences of the United States of America. 120 (12): e2220100120. Bibcode:2023PNAS..12020100K. doi:10.1073/pnas.2220100120. PMC 10041101. PMID 36927158.
- ^ Sanders RW (2011). "Alternative Nutritional Strategies in Protists: Symposium Introduction and a Review of Freshwater Protists that Combine Photosynthesis and Heterotrophy". Journal of Eukaryotic Microbiology. 58 (3): 181–184. doi:10.1111/j.1550-7408.2011.00543.x. PMID 21477096.
- ^ Flynn, Kevin J.; Mitra, Aditee; Anestis, Konstantinos; Anschütz, Anna A.; Calbet, Albert; Ferreira, Guilherme Duarte; Gypens, Nathalie; Hansen, Per J.; John, Uwe; Martin, Jon Lapeyra; Mansour, Joost S.; Maselli, Maira; Medić, Nikola; Norlin, Andreas; Not, Fabrice; Pitta, Paraskevi; Romano, Filomena; Saiz, Enric; Schneider, Lisa K.; Stolte, Willem; Traboni, Claudia (15 July 2019). "Mixotrophic protists and a new paradigm for marine ecology: where does plankton research go now?". Journal of Plankton Research. 41 (4): 375–391. doi:10.1093/plankt/fbz026. hdl:10261/192145.
- ^ a b c Mitra, Aditee; Flynn, Kevin J.; Tillmann, Urban; Raven, John A.; Caron, David; Stoecker, Diane K.; Not, Fabrice; Hansen, Per J.; Hallegraeff, Gustaaf; Sanders, Robert; Wilken, Susanne; McManus, George; Johnson, Mathew; Pitta, Paraskevi; Våge, Selina; Berge, Terje; Calbet, Albert; Thingstad, Frede; Jeong, Hae Jin; Burkholder, JoAnn; Glibert, Patricia M.; Granéli, Edna; Lundgren, Veronica (2016). "Defining Planktonic Protist Functional Groups on Mechanisms for Energy and Nutrient Acquisition: Incorporation of Diverse Mixotrophic Strategies". Protist. 167 (2): 106–120. doi:10.1016/j.protis.2016.01.003. hdl:10261/131722. PMID 26927496.
- ^ a b Faure, Emile; Not, Fabrice; Benoiston, Anne-Sophie; Labadie, Karine; Bittner, Lucie; Ayata, Sakina-Dorothée (April 2019). "Mixotrophic protists display contrasted biogeographies in the global ocean". The ISME Journal. 13 (4): 1072–1083. Bibcode:2019ISMEJ..13.1072F. doi:10.1038/s41396-018-0340-5. PMC 6461780. PMID 30643201.
- ^ Prokopchuk, Galina; Korytář, Tomáš; Juricová, Valéria; Majstorović, Jovana; Horák, Aleš; Šimek, Karel; Lukeš, Julius (18 January 2022). "Trophic flexibility of marine diplonemids - switching from osmotrophy to bacterivory". The ISME Journal. 16 (5): 1409–1419. Bibcode:2022ISMEJ..16.1409P. doi:10.1038/s41396-022-01192-0. PMC 9039065. PMID 35042972.
- ^ a b Patterson, D. J. (February 1980). "Contractile vacuoles and associated structures: their organization and function". Biological Reviews. 55 (1): 1–46. doi:10.1111/j.1469-185x.1980.tb00686.x.
- ^ Hoppenrath, M; Bachvaroff, TR; Handy, SM; Delwiche, CF; Leander, BS (25 May 2009). "Molecular phylogeny of ocelloid-bearing dinoflagellates (Warnowiaceae) as inferred from SSU and LSU rDNA sequences". BMC Evolutionary Biology. 9 (1): 116. Bibcode:2009BMCEE...9..116H. doi:10.1186/1471-2148-9-116. PMC 2694157. PMID 19467154.
- ^ a b c d e f g h i Rizos, Iris; Frada, Miguel J.; Bittner, Lucie; Not, Fabrice (31 July 2024). "Life cycle strategies in free- living unicellular eukaryotes: Diversity, evolution, and current molecular tools to unravel the private life of microorganisms". Journal of Eukaryotic Microbiology. 71 (6): e13052. doi:10.1111/jeu.13052. PMC 11603280. PMID 39085163.
- ^ a b Bernstein H, Bernstein C, Michod RE (2012). "Chapter 1. DNA repair as the primary adaptive function of sex in bacteria and eukaryotes". In Kimura S, Shimizu S (eds.). DNA Repair: New Research. Hauppauge, N.Y.: Nova Sci. Publ. pp. 1–49. ISBN 9781621007562.
- ^ a b Cecere, Ester; Petrocelli, Antonella; Verlaque, Marc (2011). "Vegetative reproduction by multicellular propagules in Rhodophyta: an overview". Marine Ecology. 32 (4): 419–437. Bibcode:2011MarEc..32..419C. doi:10.1111/j.1439-0485.2011.00448.x.
- ^ a b Milgroom, Michael G. (26 November 2023). "Protozoa". Biology of Infectious Disease. Cham: Springer. pp. 71–87. doi:10.1007/978-3-031-38941-2_6. ISBN 978-3-031-38940-5.
- ^ Hörandl, Elvira; Bast, Jens; Brandt, Alexander; Scheu, Stefan; Bleidorn, Christoph; Cordellier, Mathilde; Nowrousian, Minou; Begerow, Dominik; Sturm, Anja; Verhoeven, Koen; Boenigk, Jens; Friedl, Thomas; Dunthorn, Micah (2020). "Chapter 7. Genome Evolution of Asexual Organisms and the Paradox of Sex in Eukaryotes". In Pontarotti, Pierre (ed.). Evolutionary Biology—A Transdisciplinary Approach. Cham: Springer Nature Switzerland AG. doi:10.1007/978-3-030-57246-4_7. ISBN 978-3-030-57246-4.
- ^ a b Gibson, Wendy (16 April 2021). "The sexual side of parasitic protists". Molecular and Biochemical Parasitology. 243 111371. doi:10.1016/j.molbiopara.2021.111371. PMID 33872659.
- ^ Malik SB, Pightling AW, Stefaniak LM, Schurko AM, Logsdon JM (August 2007). Hahn MW (ed.). "An expanded inventory of conserved meiotic genes provides evidence for sex in Trichomonas vaginalis". PLOS ONE. 3 (8): e2879. Bibcode:2008PLoSO...3.2879M. doi:10.1371/journal.pone.0002879. PMC 2488364. PMID 18663385.
- ^ Dacks J, Roger AJ (June 1999). "The first sexual lineage and the relevance of facultative sex". Journal of Molecular Evolution. 48 (6): 779–783. Bibcode:1999JMolE..48..779D. doi:10.1007/PL00013156. PMID 10229582. S2CID 9441768.
- ^ Ramesh MA, Malik SB, Logsdon JM (January 2005). "A phylogenomic inventory of meiotic genes; evidence for sex in Giardia and an early eukaryotic origin of meiosis". Current Biology. 15 (2): 185–191. Bibcode:2005CBio...15..185R. doi:10.1016/j.cub.2005.01.003. PMID 15668177. S2CID 17013247.
- ^ Cooper MA, Adam RD, Worobey M, Sterling CR (November 2007). "Population genetics provides evidence for recombination in Giardia". Current Biology. 17 (22): 1984–1988. Bibcode:2007CBio...17.1984C. doi:10.1016/j.cub.2007.10.020. PMID 17980591. S2CID 15991722.
- ^ Lahr DJ, Parfrey LW, Mitchell EA, Katz LA, Lara E (July 2011). "The chastity of amoebae: re-evaluating evidence for sex in amoeboid organisms". Proceedings of the Royal Society B: Biological Sciences. 278 (1715): 2081–2090. doi:10.1098/rspb.2011.0289. PMC 3107637. PMID 21429931.
- ^ Dobell, C. (1909). "Chromidia and the binuclearity hypotheses: A review and a criticism" (PDF). Quarterly Journal of Microscopical Science. 53: 279–326.
- ^ Heesch, Svenja; Serrano-Serrano, Martha; Barrera-Redondo, Josué; Luthringer, Rémy; Peters, Akira F.; Destombe, Christophe; Cock, J. Mark; Valero, Myriam; Roze, Denis; Salamin, Nicolas; Coelho, Susana M. (1 July 2021). "Evolution of life cycles and reproductive traits: Insights from the brown algae". Journal of Evolutionary Biology. 34 (7): 992–1009. doi:10.1111/jeb.13880. PMID 34096650.
- ^ Talman AM, Domarle O, McKenzie FE, Ariey F, Robert V (July 2004). "Gametocytogenesis: the puberty of Plasmodium falciparum". Malaria Journal. 3: 24. doi:10.1186/1475-2875-3-24. PMC 497046. PMID 15253774.
- ^ Bulman, Simon; Neuhauser, Sigrid (2017). "Phytomyxea". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 783–803. doi:10.1007/978-3-319-28149-0_24. ISBN 978-3-319-28147-6.
- ^ Dubey, Jitender P. (2014). "The History and Life Cycle of Toxoplasma gondii". In Weiss, Louis M.; Kim, Kami (eds.). Toxoplasma gondii: The Model Apicomplexan - Perspectives and Methods (2nd ed.). Academic Press. pp. 1–17. doi:10.1016/B978-0-12-396481-6.00001-5. ISBN 978-0-12-396481-6.
- ^ Akopyants NS, et al. (April 2009). "Demonstration of genetic exchange during cyclical development of Leishmania in the sand fly vector". Science. 324 (5924): 265–268. Bibcode:2009Sci...324..265A. doi:10.1126/science.1169464. PMC 2729066. PMID 19359589.
- ^ a b da Silva, Verônica Santana; Machado, Carlos Renato (2022). "Sex in protists: A new perspective on the reproduction mechanisms of trypanosomatids". Genetics and Molecular Biology. 45 (3): e20220065. doi:10.1590/1678-4685-GMB-2022-0065. PMC 9552303. PMID 36218381.
- ^ Tibayrenc M, et al. (June 1991). "Are eukaryotic microorganisms clonal or sexual? A population genetics vantage". Proceedings of the National Academy of Sciences of the United States of America. 88 (12): 5129–33. Bibcode:1991PNAS...88.5129T. doi:10.1073/pnas.88.12.5129. PMC 51825. PMID 1675793.
- ^ Bar-On, Yinon M.; Phillips, Rob; Milo, Ron (17 May 2018). "The biomass distribution on Earth". Proceedings of the National Academy of Sciences. 115 (25): 6506–6511. Bibcode:2018PNAS..115.6506B. doi:10.1073/pnas.1711842115. ISSN 0027-8424. PMC 6016768. PMID 29784790.
- ^ a b Epstein, Slava; López-García, Purificación (2007). ""Missing" protists: a molecular perspective". Biodiversity and Conservation. 17 (2): 261–276. doi:10.1007/s10531-007-9250-y. S2CID 3960288.
- ^ a b c d e f g h Singer, David; Seppey, Christophe V.W.; Lentendu, Guillaume; Dunthorn, Micah; Bass, David; Belbahri, Lassâad; Blandenier, Quentin; Debroas, Didier; de Groot, G. Arjen; de Vargas, Colomban; Domaizon, Isabelle; Duckert, Clément; Izaguirre, Irina; Koenig, Isabelle; Mataloni, Gabriela; Schiaffino, M. Romina; Mitchell, Edward A.D.; Geisen, Stefan; Lara, Enrique (January 2021). "Protist taxonomic and functional diversity in soil, freshwater and marine ecosystems". Environment International. 146 (106262): 106262. Bibcode:2021EnInt.14606262S. doi:10.1016/j.envint.2020.106262. hdl:10261/265020. PMID 33221595.
- ^ Metz S, Huber P, Accattatis V, Lopes dos Santos A, Bigeard E, Unrein F, Chambouvet A, Not F, Lara E, Devercelli M (2022). "Freshwater protists: unveiling the unexplored in a large floodplain system". Environmental Microbiology. 24 (4): 1731–1745. Bibcode:2022EnvMi..24.1731M. doi:10.1111/1462-2920.15838. PMID 34783136. S2CID 244133100.
- ^ a b Thoré, Eli S.J.; Muylaert, Koenraad; Bertram, Michael G.; Brodin, Thomas (6 February 2023). "Microalgae". Current Biology. 33 (3): R91 – R95. Bibcode:2023CBio...33R..91T. doi:10.1016/j.cub.2022.12.032. PMID 36750029.
- ^ Caron, David A.; Worden, Alexandra Z.; Countway, Peter D.; Demir, Elif; Heidelberg, Karla B. (January 2009). "Protists are microbes too: a perspective". The ISME Journal. 3 (1): 4–12. Bibcode:2009ISMEJ...3....4C. doi:10.1038/ismej.2008.101. PMID 19005497.
- ^ Pereira, Leonel (7 February 2021). "Macroalgae". Encyclopedia. 1 (1): 177–188. doi:10.3390/encyclopedia1010017.
- ^ Rothäusler, Eva; Gutow, Lars; Thiel, Martin (1 January 2012). "Floating Seaweeds and Their Communities". In Wiencke, Christian; Bischof, Kai (eds.). Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization. Ecological Studies. Vol. 219. Springer. pp. 359–380. doi:10.1007/978-3-642-28451-9_17. ISBN 978-3-642-28451-9.
- ^ a b c d e f Geisen, Stefan; Mitchell, Edward A. D.; Adl, Sina; Bonkowski, Michael; Dunthorn, Micah; Ekelund, Flemming; Fernández, Leonardo D.; Jousset, Alexandre; Krashevska, Valentyna; Singer, David; Spiegel, Frederick W.; Walochnik, Julia; Lara, Enrique (May 2018). "Soil protists: a fertile frontier in soil biology research". FEMS Microbiology Reviews. 42 (3): 293–323. doi:10.1093/femsre/fuy006. PMID 29447350.
- ^ Leles SG, Mitra A, Flynn KJ, Stoecker DK, Hansen PJ, Calbet A, McManus GB, Sanders RW, Caron DA, Not F, Hallegraeff GM, Pitta P, Raven JA, Johnson MD, Glibert PM, Våge S (August 2017). "Oceanic protists with different forms of acquired phototrophy display contrasting biogeographies and abundance". Proceedings of the Royal Society B: Biological Sciences. 284 (1860): 20170664. doi:10.1098/rspb.2017.0664. PMC 5563798. PMID 28768886.
- ^ a b Harder C, Rønn R, Brejnrod A, et al. (8 March 2016). "Local diversity of heathland Cercozoa explored by in-depth sequencing". The ISME Journal. 10 (10): 2488–2497. Bibcode:2016ISMEJ..10.2488H. doi:10.1038/ismej.2016.31. PMC 5030685. PMID 26953604.
- ^ a b Moye, Jannika; Hess, Sebastian (3 November 2024). "Broad- range necrophytophagy in the flagellate Orciraptor agilis (Viridiraptoridae, Cercozoa) and the underappreciated role of scavenging among protists". Journal of Eukaryotic Microbiology. 72 (2): e13065. doi:10.1111/jeu.13065. PMC 11822879. PMID 39489698.
- ^ Mahé, Frédéric; de Vargas, Colomban; Bass, David; Czech, Lucas; Stamatakis, Alexandros; Lara, Enrique; Singer, David; Mayor, Jordan; Bunge, John; Sernaker, Sarah; Siemensmeyer, Tobias; Trautmann, Isabelle; Romac, Sarah; Berney, Cédric; Kozlov, Alexey; Mitchell, Edward A. D.; Seppey, Christophe V. W.; Egge, Elianne; Lentendu, Guillaume; Wirth, Rainer; Trueba, Gabriel; Dunthorn, Micah (2017). "Parasites dominate hyperdiverse soil protist communities in Neotropical rainforests". Nature Ecology and Evolution. 1 (4): 0091. doi:10.1038/s41559-017-0091. PMID 28812652.
- ^ Schwelm A, Badstöber J, Bulman S, Desoignies N, Etemadi M, Falloon RE, Gachon CM, Legreve A, Lukeš J, Merz U, Nenarokova A, Strittmatter M, Sullivan BK, Neuhauser S (April 2018). "Not in your usual Top 10: protists that infect plants and algae". Molecular Plant Pathology. 19 (4): 1029–1044. Bibcode:2018MolPP..19.1029S. doi:10.1111/mpp.12580. PMC 5772912. PMID 29024322.
- ^ Kamoun S, Furzer O, Jones JD, Judelson HS, Ali GS, Dalio RJ, Roy SG, Schena L, Zambounis A, Panabières F, Cahill D, Ruocco M, Figueiredo A, Chen XR, Hulvey J, Stam R, Lamour K, Gijzen M, Tyler BM, Grünwald NJ, Mukhtar MS, Tomé DF, Tör M, Van Den Ackerveken G, McDowell J, Daayf F, Fry WE, Lindqvist-Kreuze H, Meijer HJ, Petre B, Ristaino J, Yoshida K, Birch PR, Govers F (May 2015). "The Top 10 oomycete pathogens in molecular plant pathology". Molecular Plant Pathology. 16 (4): 413–34. Bibcode:2015MolPP..16..413K. doi:10.1111/mpp.12190. PMC 6638381. PMID 25178392.
- ^ Campbell, N. and Reese, J. (2008) Biology. Pearson Benjamin Cummings; 8 ed. ISBN 0805368442. pp. 583, 588
- ^ Lauckner, G. (1980). "Diseases of protozoa". In: Diseases of Marine Animals. Kinne, O. (ed.). Vol. 1, p. 84, John Wiley & Sons, Chichester, UK.
- ^ Cox, F.E.G. (1991). "Systematics of parasitic protozoa". In: Kreier, J.P. & J. R. Baker (ed.). Parasitic Protozoa, 2nd ed., vol. 1. San Diego: Academic Press.
- ^ Keeling PJ, Campo JD (June 2017). "Marine Protists Are Not Just Big Bacteria". Current Biology. 27 (11): R541 – R549. Bibcode:2017CBio...27.R541K. doi:10.1016/j.cub.2017.03.075. PMID 28586691.
- ^ a b Barry S. C. Leadbeater; Sharon M. M. McReady (2000). "Chapter 1. The flagellates: historical perspectives". In Barry S. C. Leadbeater; J. C. Green (eds.). The Flagellates. Unity, diversity and evolution. London: Taylor & Francis. pp. 1–26. doi:10.1201/9781482268225. ISBN 9780429182136.
- ^ Marc J. Ratcliff (2009). "The Emergence of the Systematics of Infusoria". The Quest for the Invisible: Microscopy in the Enlightenment. Ashgate. pp. 177–216. ISBN 9781409480266.
- ^ Goldfuß (1818). "Ueber die Classification der Zoophyten" [On the classification of zoophytes]. Isis, Oder, Encyclopädische Zeitung von Oken (in German). 2 (6): 1008–1019. From p. 1008: "Erste Klasse. Urthiere. Protozoa." (First class. Primordial animals. Protozoa.) [Note: each column of each page of this journal is numbered; there are two columns per page.]
- ^ Carl Theodor Ernst von Siebold; Hermann Stannius (1846–1848). Lehrbuch der vergleichenden Anatomie Vol. 1: Wirbellose Thiere [Textbook of Comparative Anatomy Vol. 1: Invertebrate Animals] (in German). Vol. 1. Berlin, Germany: Veit. p. 3. p. 3:
Erste Hauptgruppe. Protozoa. Thiere, in welchen die verschiedenen Systeme der Organe nicht scharf ausgeschieden sind, und deren unregelmässige Form und einfache Organisation sich auf eine Zelle reduziren lassen.
[First principal group. Protozoa. Animals, in which the different systems of organs are not sharply separated, and whose irregular form and simple organization can be reduced to one cell.] - ^ John Hogg (1860). "On the distinctions of a Plant and an Animal, and on a Fourth Kingdom of Nature". Edinburgh New Philosophical Journal. 2nd series. 12: 216–225. p. 223:
... I here suggest a fourth or an additional kingdom, under the title of the Primigenal kingdom, ... This Primigenal kingdom would comprise all the lower creatures, or the primary organic beings, – 'Protoctista,' – from πρώτος, first, and χτιστά, created beings; ...
- ^ Haeckel, Ernst (1878). Das protistenreich. Eine populäre uebersicht über das formengebiet der niedersten lebewesen [The protistan kingdom. A popular survey of the forms of the lowest living beings] (in German). Leipzig: E. Günther. doi:10.5962/bhl.title.58542.
- ^ Taylor, F. J. R. 'Max' (11 January 2003). "The collapse of the two-kingdom system, the rise of protistology and the founding of the International Society for Evolutionary Protistology (ISEP)". International Journal of Systematic and Evolutionary Microbiology. 53 (6): 1707–1714. doi:10.1099/ijs.0.02587-0. PMID 14657097.
- ^ Haeckel, Ernst (1866). Generelle Morphologie der Organismen [The General Morphology of Organisms] (in German). Vol. 1. Berlin, (Germany): G. Reimer. pp. 215ff. From p. 215: "VII. Character des Protistenreiches." (VII. Character of the kingdom of Protists.)
- ^ Rothschild, Lynn J. (1989). "Protozoa, Protista, Protoctista: what's in a name?". Journal of the History of Biology. 22 (2): 277–305. doi:10.1007/BF00139515. PMID 11542176. S2CID 32462158.
- ^ Copeland HF (1938). "The Kingdoms of Organisms". Quarterly Review of Biology. 13 (4): 383–420. doi:10.1086/394568. JSTOR 2808554. S2CID 84634277.
- ^ a b Whittaker RH (1959). "On the Broad Classification of Organisms". Quarterly Review of Biology. 34 (3): 210–226. doi:10.1086/402733. JSTOR 2816520. PMID 13844483. S2CID 28836075.
- ^ Whittaker RH (January 1969). "New concepts of kingdoms or organisms. Evolutionary relations are better represented by new classifications than by the traditional two kingdoms". Science. 163 (3863): 150–160. Bibcode:1969Sci...163..150W. CiteSeerX 10.1.1.403.5430. doi:10.1126/science.163.3863.150. PMID 5762760.
- ^ Hagen JB (2012). "depiction of Whittaker's early four-kingdom system, based on three modes of nutrition and the distinction between unicellular and multicellular body plans". BioScience. 62: 67–74. doi:10.1525/bio.2012.62.1.11.
- ^ Margulis L, Chapman MJ (2009-03-19). Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth. Academic Press. ISBN 9780080920146.
- ^ Archibald, John M.; Simpson, Alastair G. B.; Slamovits, Claudio H., eds. (2017). Handbook of the Protists (2 ed.). Springer International Publishing. pp. ix. ISBN 978-3-319-28147-6.
- ^ Stechmann A, Cavalier-Smith T (September 2003). "The root of the eukaryote tree pinpointed" (PDF). Current Biology. 13 (17): R665–667. Bibcode:2003CBio...13.R665S. doi:10.1016/S0960-9822(03)00602-X. PMID 12956967. S2CID 6702524.
- ^ Schlegel, M.; Hulsmann, N. (2007). "Protists – A textbook example for a paraphyletic taxon☆". Organisms Diversity & Evolution. 7 (2): 166–172. Bibcode:2007ODivE...7..166S. doi:10.1016/j.ode.2006.11.001.
- ^ "Protista". microbeworld.org. Archived from the original on 13 June 2016. Retrieved 11 June 2016.
- ^ Štolc A (1899). "Actinomyxidies, nouveau groupe de Mesozoaires parent des Myxosporidies". Bull. Int. l'Acad. Sci. Bohème. 12: 1–12.
- ^ Adl SM, Simpson AG, Farmer MA, Andersen RA, Anderson OR, Barta JR, Bowser SS, Brugerolle G, Fensome RA, Fredericq S, James TY, Karpov S, Kugrens P, Krug J, Lane CE, Lewis LA, Lodge J, Lynn DH, Mann DG, McCourt RM, Mendoza L, Moestrup O, Mozley-Standridge SE, Nerad TA, Shearer CA, Smirnov AV, Spiegel FW, Taylor MF (2005). "The new higher level classification of eukaryotes with emphasis on the taxonomy of protists". The Journal of Eukaryotic Microbiology. 52 (5): 399–451. doi:10.1111/j.1550-7408.2005.00053.x. PMID 16248873. S2CID 8060916.
- ^ O'Malley, Maureen A. (2022). "Getting at the Basics of Multicellularity". In Herron, Matthew D.; Conlin, Peter L.; Ratcliff, William C. (eds.). The Evolution of Multicellularity. Evolutionary Cell Biology (1st ed.). CRC Press. pp. 9–24. doi:10.1201/9780429351907. ISBN 9780429351907. S2CID 248578172.
- ^ a b Ruggiero, Michael A.; Gordon, Dennis P.; Orrell, Thomas M.; Bailly, Nicolas; Bourgoin, Thierry; Brusca, Richard C.; Cavalier-Smith, Thomas; Guiry, Michael D.; Kirk, Paul M.; Thuesen, Erik V. (2015). "A higher level classification of all living organisms". PLOS ONE. 10 (4): e0119248. Bibcode:2015PLoSO..1019248R. doi:10.1371/journal.pone.0119248. PMC 4418965. PMID 25923521.
- ^ a b c d e f g h i j k Suzuki, Noritoshi; Oba, Masahiro (2015). "Oldest Fossil Records of Marine Protists and the Geologic History Toward the Establishment of the Modern-Type Marine Protist World". In Ohtsuka, Susumu; Suzaki, Toshinobu; Horiguchi, Takeo; Suzuki, Noritoshi; Not, Fabrice (eds.). Marine Protists: Diversity and Dynamics. Springer Japan. pp. 359–394. doi:10.1007/978-4-431-55130-0_15. ISBN 978-4-431-55130-0.
- ^ a b c d e f Brocks, Jochen J.; Nettersheim, Benjamin J.; Adam, Pierre; Schaeffer, Philippe; Jarrett, Amber J. M.; Güneli, Nur; Liyanage, Tharika; van Maldegem, Lennart M.; Hallmann, Christian; Hope, Janet M. (2023). "Lost world of complex life and the late rise of the eukaryotic crown" (PDF). Nature. 618 (7966): 767–773. Bibcode:2023Natur.618..767B. doi:10.1038/s41586-023-06170-w. PMID 37286610. S2CID 259111647.
- ^ Beghin, Jérémie; Storme, Jean-Yves; Blanpied, Christian; Gueneli, Nur; Brocks, Jochen J.; Poulton, Simon W.; Javaux, Emmanuelle J. (April 2017). "Microfossils from the late Mesoproterozoic–early Neoproterozoic Atar/El Mreïti Group, Taoudeni Basin, Mauritania, northwestern Africa". Precambrian Research. 291: 63–82. Bibcode:2017PreR..291...63B. doi:10.1016/j.precamres.2017.01.009. hdl:1885/234547.
- ^ Javaux, Emmanuelle J.; Knoll, Andrew H. (22 December 2016). "Micropaleontology of the lower Mesoproterozoic Roper Group, Australia, and implications for early eukaryotic evolution". Journal of Paleontology. 91 (2): 199–229. doi:10.1017/jpa.2016.124.
- ^ Butterfield, Nicholas J. (2015). "Early evolution of the Eukaryota". Palaeontology. 58 (1): 5–17. Bibcode:2015Palgy..58....5B. doi:10.1111/pala.12139.
- ^ Xiao, Shuhai (2013). "Written in Stone: The Fossil Record of Early Eukaryotes". In Trueba, Gabriel; Montúfar, Carlos (eds.). Evolution from the Galapagos. Social and Ecological Interactions in the Galapagos Islands. Vol. 2. New York: Springer. pp. 107–124. doi:10.1007/978-1-4614-6732-8_8. ISBN 978-1-4614-6731-1.
- ^ Gibson, Timothy M.; Shih, Patrick M.; Cumming, Vivien M.; Fischer, Woodward W.; Crockford, Peter W.; Hodgskiss, Malcolm S.W.; Wörndle, Sarah; Creaser, Robert A.; Rainbird, Robert H.; Skulski, Thomas M.; Halverson, Galen P. (2017). "Precise age of Bangiomorpha pubescens dates the origin of eukaryotic photosynthesis". Geology. 46 (2): 135–138. Bibcode:2018Geo....46..135G. doi:10.1130/G39829.1.
- ^ Butterfield, Nicholas J. (2004). "A vaucheriacean alga from the middle Neoproterozoic of Spitsbergen: implications for the evolution of Proterozoic eukaryotes and the Cambrian explosion". Paleobiology. 30 (2): 231–252. Bibcode:2004Pbio...30..231B. doi:10.1666/0094-8373(2004)030<0231:avaftm>2.0.co;2.
- ^ Javaux, Emmanuelle J. (2007). "The Early Eukaryotic Fossil Record". In Jékely, Gáspár (ed.). Eukaryotic Membranes and Cytoskeleton. Advances in Experimental Medicine and Biology. Vol. 607. New York: Springer. pp. 1–19. doi:10.1007/978-0-387-74021-8_1. ISBN 978-0-387-74020-1. PMID 17977455.
- ^ Strother, Paul K.; Brasier, Martin D.; Wacey, David; Timpe, Leslie; Saunders, Martin; Wellman, Charles H. (April 2021). "A possible billion-year-old holozoan with differentiated multicellularity". Current Biology. 31 (12): 2658–2665.e2. Bibcode:2021CBio...31E2658S. doi:10.1016/j.cub.2021.03.051. PMID 33852871.
- ^ a b Chai, Shu; Hua, Hong; Ren, Jinjie; Dai, Qiaokun; Cui, Zaihang (January 2021). "Vase-shaped microfossils from the late Ediacaran Dengying Formation of Ningqiang, South China: taxonomy, preservation and biological affinity". Precambrian Research. 352 105968. Bibcode:2021PreR..35205968C. doi:10.1016/j.precamres.2020.105968.
- ^ Porter SM, Meisterfeld R, Knoll AH (May 2003). "Vase-shaped microfossils from the Neoproterozoic Chuar Group, Grand Canyon: a classification guided by modern testate amoebae" (PDF). Journal of Paleontology. 77 (3): 409–429. Bibcode:2003JPal...77..409P. doi:10.1666/0022-3360(2003)077<0409:VMFTNC>2.0.CO;2. Archived from the original (PDF) on 23 January 2022.
- ^ Parfrey LW, Lahr DJ, Knoll AH, Katz LA (August 2011). "Estimating the timing of early eukaryotic diversification with multigene molecular clocks". Proceedings of the National Academy of Sciences of the United States of America. 108 (33): 13624–9. Bibcode:2011PNAS..10813624P. doi:10.1073/pnas.1110633108. PMC 3158185. PMID 21810989.
- ^ a b Min, Xiao; Hua, Hong; Sun, Bo; Dai, Qiaokun; Luo, Jinzhou; Pan, Xiaoqiang; Liu, Ziwei (August 2021). "Diversification of heterotrophic protists at the eve of Cambrian explosion". Global and Planetary Change. 203 103545. Bibcode:2021GPC...20303545M. doi:10.1016/j.gloplacha.2021.103545.
- ^ A. Braun; J. Chen; D. Waloszek; A. Maas (2007), "First Early Cambrian Radiolaria", in Vickers-Rich, Patricia; Komarower, Patricia (eds.), The Rise and Fall of the Ediacaran Biota, Special publications, vol. 286, London: Geological Society, pp. 143–149, doi:10.1144/SP286.10, ISBN 978-1-86239-233-5, OCLC 156823511
- ^ Chang, Shan; Feng, Qinglai; Zhang, Lei (14 August 2018). "New Siliceous Microfossils from the Terreneuvian Yanjiahe Formation, South China: The Possible Earliest Radiolarian Fossil Record". Journal of Earth Science. 29 (4): 912–919. Bibcode:2018JEaSc..29..912C. doi:10.1007/s12583-017-0960-0. S2CID 134890245.
- ^ Zhang, Ke; Feng, Qing-Lai (September 2019). "Early Cambrian radiolarians and sponge spicules from the Niujiaohe Formation in South China". Palaeoworld. 28 (3): 234–242. doi:10.1016/j.palwor.2019.04.001. S2CID 146452469.
- ^ Maletz, Jörg (June 2017). "The identification of putative Lower Cambrian Radiolaria". Revue de Micropaléontologie. 60 (2): 233–240. Bibcode:2017RvMic..60..233M. doi:10.1016/j.revmic.2017.04.001.
- ^ Pawlowski, Jan; Holzmann, Maria; Berney, Cédric; Fahrni, José; Gooday, Andrew J.; Cedhagen, Thomas; Habura, Andrea; Bowser, Samuel S. (2003). "The evolution of early Foraminifera". Proceedings of the National Academy of Sciences. 100 (20): 11494–11498. Bibcode:2003PNAS..10011494P. doi:10.1073/pnas.2035132100. PMC 208786. PMID 14504394.
- ^ a b c Servais, Thomas; Perrier, Vincent; Danelian, Taniel; Klug, Christian; Martin, Ronald; Munnecke, Axel; Nowak, Hendrik; Nützel, Alexander; Vandenbroucke, Thijs R.A.; Williams, Mark; Rasmussen, Christian M.Ø. (15 September 2016). "The onset of the 'Ordovician Plankton Revolution' in the late Cambrian". Palaeogeography, Palaeoclimatology, Palaeoecology. 458: 12–28. Bibcode:2016PPP...458...12S. doi:10.1016/j.palaeo.2015.11.003.
- ^ a b Vecoli, Marco; Le Hérissé, Alain (October 2004). "Biostratigraphy, taxonomic diversity and patterns of morphological evolution of Ordovician acritarchs (organic-walled microphytoplankton) from the northern Gondwana margin in relation to palaeoclimatic and palaeogeographic changes". Earth-Science Reviews. 67 (3–4): 267–311. Bibcode:2004ESRv...67..267V. doi:10.1016/j.earscirev.2004.03.002.
- ^ Nowak, Hendrik; Servais, Thomas; Monnet, Claude; Molyneux, Stewart G.; Vandenbroucke, Thijs R.A. (December 2015). "Phytoplankton dynamics from the Cambrian Explosion to the onset of the Great Ordovician Biodiversification Event: A review of Cambrian acritarch diversity" (PDF). Earth-Science Reviews. 151: 117–131. Bibcode:2015ESRv..151..117N. doi:10.1016/j.earscirev.2015.09.005. hdl:20.500.12210/34278.
- ^ a b Zhang, Lei; Algeo, Thomas J.; Zhao, Laishi; Dahl, Tais W.; Chen, Zhong-Qiang; Zhang, Zihu; Poulton, Simon W.; Hughes, Nigel C.; Gou, Xueqing; Li, Chao (2023-05-12). "Environmental and trilobite diversity changes during the middle-late Cambrian SPICE event". Geological Society of America Bulletin. doi:10.1130/b36421.1. ISSN 0016-7606.
- ^ Strother, Paul K.; Taylor, Wilson A.; van de Schootbrugge, Bas; Leander, Brian S.; Wellman, Charles H. (2020). "Pellicle ultrastructure demonstrates that Moyeria is a fossil euglenid". Palynology. 44 (3): 461–471. Bibcode:2020Paly...44..461S. doi:10.1080/01916122.2019.1625457.
- ^ Nestell, Galina; Heredia, Susana; Mestre, Ana; Beresi, Matilde; González, Mercedes (November–December 2011). "The oldest Ordovician foraminifers (Oepikodus evae conodont Zone, Floian) from South America" (PDF). Geobios. 44 (6): 601–608. Bibcode:2011Geobi..44..601N. doi:10.1016/j.geobios.2011.02.007. hdl:11336/139154. Archived from the original (PDF) on 29 March 2024.
- ^ Wang, Kai; Xu, Hong-He; Liu, Bing-Cai; Bai, Jiao; Wang, Yao; Tang, Peng; Lu, Jian-Feng; Wang, Yi (19 May 2023). "Shallow-marine testate amoebae with internal structures from the Lower Devonian of China". iScience. 26 (5): 106678. Bibcode:2023iSci...26j6678W. doi:10.1016/j.isci.2023.106678. PMC 10173733. PMID 37182111.
- ^ Krings, Michael (September 2022). "Algae from the Lower Devonian Rhynie chert: Populations of a probable saccoderm desmid (Mesotaeniaceae, Zygnematales) preserved in a microbial mat". Review of Palaeobotany and Palynology. 304 104697. Bibcode:2022RPaPa.30404697K. doi:10.1016/j.revpalbo.2022.104697.
- ^ Krings, Michael; Kerp, Hans (December 2019). "A tiny parasite of unicellular microorganisms from the Lower Devonian Rhynie and Windyfield cherts, Scotland". Review of Palaeobotany and Palynology. 271 104106. Bibcode:2019RPaPa.27104106K. doi:10.1016/j.revpalbo.2019.104106.
- ^ a b c d Wever, Patrick; O’Dogherty, Luis; Gorican, Spela (2007). "The plankton turnover at the Permo-Triassic boundary, emphasis on radiolarians". In Baumgartner, Peter O.; Aitchison, Jonathan C.; De Wever, Patrick; Jackett, Sarah-Jane (eds.). Radiolaria: Siliceous Plankton through Time. 10th International Meeting of Radiolarian Palaeontologists. Swiss Journal of Geosciences Supplement. Vol. 2. Basel: Birkhäuser Verlag. pp. 49–62. doi:10.1007/978-3-7643-8344-2_4.
- ^ Suzuki, Noritoshi; Aita, Yoshiaki; Yamakita, Satoshi; Kamata, Yoshihito; Takemura, Atsushi; Fujiki, Toru; Ogane, Kaoru; Sakai, Toyosaburo; Hori, Rie S. (2007). "The depositional environment of the Induan (Early Triassic) biosiliceous sequence (units 2B and 3 of the Oruatemanu Formation), Arrow Rocks, New Zealand". In Spörli, K. B.; Takemura, A.; Hori, R. S. (eds.). The oceanic Permian/Triassic boundary sequence at Arrow Rocks (Oruatemanu), Northland, New Zealand. GNS Science Monograph. Vol. 24. Lower Hutt, New Zealand: GNS Science. pp. 45–67. ISBN 9780478099195.
- ^ Saesaengseerung, Doungrutai; Agematsu, Sachiko; Sashida, Katsuo; Sardsud, Apsorn (30 June 2009). "Discovery of Lower Permian radiolarian and conodont faunas from the bedded chert of the Chanthaburi area along the Sra Kaeo suture zone, eastern Thailand". Paleontological Research. 13 (2): 119–138. Bibcode:2009PalRe..13..119S. doi:10.2517/1342-8144-13.2.119.
- ^ Poinar, George O.; Waggoner, Benjamin M.; Bauer, Ulf-Christian (1993). "Terrestrial Soft-Bodied Protists and Other Microorganisms in Triassic Amber". Science. 259 (5092): 222–224. Bibcode:1993Sci...259..222P. doi:10.1126/science.259.5092.222. PMID 17790989.
- ^ Leung, Tommy L. F. (2017). "Fossils of parasites: what can the fossil record tell us about the evolution of parasitism?". Biological Reviews. 92 (1): 410–430. doi:10.1111/brv.12238. PMID 26538112.
- ^ Poinar, George O. (18 February 2009). "Description of an early Cretaceous termite (Isoptera: Kalotermitidae) and its associated intestinal protozoa, with comments on their co-evolution". Parasites & Vectors. 2 (12) 12. doi:10.1186/1756-3305-2-12. PMC 2669471. PMID 19226475.
- ^ Poinar, George (October 2009). "Early Cretaceous protist flagellates (Parabasalia: Hypermastigia: Oxymonada) of cockroaches (Insecta: Blattaria) in Burmese amber". Cretaceous Research. 30 (5): 1066–1072. Bibcode:2009CrRes..30.1066P. doi:10.1016/j.cretres.2009.03.008.
- ^ Knoll, Andrew H.; Follows, Michael J. (26 October 2016). "A bottom-up perspective on ecosystem change in Mesozoic oceans". Proceedings of the Royal Society B: Biological Sciences. 283 (1841). doi:10.1098/rspb.2016.1755. PMC 5095382. PMID 27798303.
- ^ Brown JW, Sorhannus U (2010). "A Molecular Genetic Timescale for the Diversification of Autotrophic Stramenopiles (Ochrophyta): Substantive Underestimation of Putative Fossil Ages". PLOS ONE. 5 (9): e12759. Bibcode:2010PLoSO...512759B. doi:10.1371/journal.pone.0012759. PMC 2940848. PMID 20862282.
Bibliography
[edit]General
[edit]- Hausmann, K., N. Hulsmann, R. Radek. Protistology. Schweizerbart'sche Verlagsbuchshandlung, Stuttgart, 2003.
- Margulis, L., J.O. Corliss, M. Melkonian, D.J. Chapman. Handbook of Protoctista. Jones and Bartlett Publishers, Boston, 1990.
- Margulis, L., K.V. Schwartz. Five Kingdoms: An Illustrated Guide to the Phyla of Life on Earth, 3rd ed. New York: W.H. Freeman, 1998.
- Margulis, L., L. Olendzenski, H.I. McKhann. Illustrated Glossary of the Protoctista, 1993.
- Margulis, L., M.J. Chapman. Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth. Amsterdam: Academic Press/Elsevier, 2009.
- Schaechter, M. Eukaryotic microbes. Amsterdam, Academic Press, 2012.
Physiology, ecology and paleontology
[edit]- Fontaneto, D. Biogeography of Microscopic Organisms. Is Everything Small Everywhere? Cambridge University Press, Cambridge, 2011.
- Moore, R. C., and other editors. Treatise on Invertebrate Paleontology. Protista, part B (vol. 1[permanent dead link], Charophyta, vol. 2, Chrysomonadida, Coccolithophorida, Charophyta, Diatomacea & Pyrrhophyta), part C (SARcodina, Chiefly "Thecamoebians" and Foraminiferida) and part D[permanent dead link] (Chiefly Radiolaria and Tintinnina). Boulder, Colorado: Geological Society of America; & Lawrence, Kansas: University of Kansas Press.
External links
[edit]- UniEuk Taxonomy App
- Tree of Life: Eukaryotes
- Tsukii, Y. (1996). Protist Information Server (database of protist images). Laboratory of Biology, Hosei University. Protist Information Server. Updated: March 22, 2016.
 KSF
KSF








![Paramecium, a well-studied genus of ciliates[74]](https://upload.wikimedia.org/wikipedia/commons/thumb/0/07/%D0%98%D0%BD%D1%84%D1%83%D0%B7%D0%BE%D1%80%D0%B8%D1%8F_%D1%82%D1%83%D1%84%D0%B5%D0%BB%D1%8C%D0%BA%D0%B0_2.tif/lossy-page1-110px-%D0%98%D0%BD%D1%84%D1%83%D0%B7%D0%BE%D1%80%D0%B8%D1%8F_%D1%82%D1%83%D1%84%D0%B5%D0%BB%D1%8C%D0%BA%D0%B0_2.tif.jpg)