SAAL1
 From Wikipedia - Reading time: 9 min
From Wikipedia - Reading time: 9 min
| SAAL1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | SAAL1, SPACIA1, serum amyloid A like 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | MGI: 1926185; HomoloGene: 34706; GeneCards: SAAL1; OMA:SAAL1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Serum amyloid A-like 1 (also known as SAAL1, Synoviocyte proliferation-associated in collagen-induced arthritis 1, and SPACIA1) is a protein in humans encoded by the SAAL1 gene.[5][6]
Gene
[edit]Locus
[edit]The human SAAL1 gene is located at position 11p15.1 on the minus strand spanning from base pairs 18080292-18106082 (25,790 bases).[5] It has 12 exons and 11 introns and encodes a single isoform.[5][7]
Members of the serum amyloid-A family such as SAA1 reside in the same loci as SAAL1.[7]

Promoter
[edit]The promoter region (GXP_169676) is predicted to span from basepairs 18105980-18107207 and extends into the first exon of SAAL1.[9] Predicted transcription factors include TATA binding factors, NF-κB, and KLF4, KLF5, and KLF6.[10]
Expression
[edit]SAAL1 is ubiquitously expressed at moderate levels across all human tissues with highest expression in testes as determined by RNA-sequencing and microarray expression profiling.[11][12]
Transcript
[edit]Predicted 5' UTR binding proteins of the human SAAL1 transcript include SRSF3 and FXR2.[13] Predicted 3' UTR binding proteins include SRSF5 and U2AF2.[13] All predicted proteins are involved in mRNA splicing, export, and translation.[14][15][16][17]

Protein structure
[edit]
General properties
[edit]The SAAL1 protein has a single known isoform consisting of 474 amino acids with a molecular weight of 53.5 kDa.[5] The unmodified SAAL1 protein is acidic with an isoelectric point of 4.4.[21]
Composition
[edit]SAAL1 is abundant in aspartic acid (7.8% by composition) and deficient in glycine (3.4% by composition)compared to other human proteins.[22] It also has 44 more aspartic acid and glutamic acid residues compared to lysine and arginine, indicating an overall negative charge.[23] Two negatively charged and glutamic acid abundant segments were identified and labeled in the SAAL1 conceptual translation.[22]
Domains and motifs
[edit]SAAL1 contains an armadillo-like fold with an enveloped fungal symportin-1 like region.[24][25] Other motifs were predicted by ELM[26] and MyHits Motif Scan.[27]
| Predicted Motifs | Amino Acids | Tools |
|---|---|---|
| Casein kinase 2 phosphorylation site | 152-155, 165-168 | MyHits,[27] ELM[26] |
| Nuclear Export Signal | 72-84 | ELM[26] |
| MAPK docking site | 106-115, 344-352 | ELM[26] |
Sub-cellular localization
[edit]Immunofluorescent staining has identified SAAL1 localization in the nucleus of Caco-2 cells.[28] However, western blotting of hepatocellular carcinoma cell lines identified SAAL1 localization in the cytoplasm with minor amounts in the cell membrane and nucleus.[29]
Post-translational modifications
[edit]SAAL1 undergoes phosphorylation at two experimentally verified sites: Ser6 and Thr387.[25] Predicted post-translational modifications are detailed in the following table.
| Tool | Predicted Modification | Amino Acids |
|---|---|---|
| NetPhos[30][31] | Casein kinase 2 phosphorylation | Thr152, Ser165 |
| YinOYang[32][33] | O-linked glycosylation | Ser6 |
| SMART[34] | Ubiquitination | Lys209, Val302 |
Clinical significance
[edit]SAAL1 overexpression has been correlated with the proliferation of rheumatoid and osteoarthritic synovial fibroblasts as well as disease progression.[24][35] RNAi knockouts of SAAL1 reduced arrested fibroblasts in G0/G1 phase and reduced proliferation by 20% with a 50% reduction when fibroblasts were stimulated by TNF-α.[24] Stability assays reveal that SAAL1 promotes G1/S transition via CDK6 mRNA stabilization.[24][35] This finding was corroborated by SAAL1 knockdowns in hepatocellular carcinomas which also demonstrated impaired HGF-induced migration and increased sensitivity to sorafenib and foretinib treatment.[29] Additionally, SAAL1 is overexpressed in hepatocellular carcinoma cells and in chondrocytes stimulated by interleukin-1 beta, but this effect is diminished in the presence of glucosamine.[29][36]
Studies of the rock bream SAAL1 ortholog noted an increase in gene expression in response to bacterial and viral pathogens.[37] Human SAAL1 has been reported to interact with the M protein of SARS-Cov-2,[38] Orf4 of Kaposi's sarcoma-associated herpesvirus,[39] and the M and M2 proteins of influenza A.[40] It has also been reported as an interferon stimulator and TRIM25 interactor.[41][42] Other interacting proteins include PNKD (which plays a role in cardiac hypertrophy via NF-κB signaling),[43][44] TMIGD3(which inhibits NF-κB activity),[45][46] and MARK3.[47]
Evolution
[edit]Homology
[edit]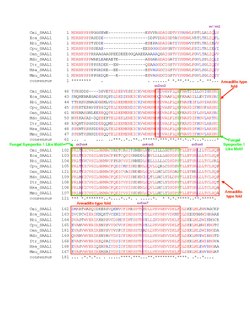
BLAST searches have found homologs for SAAL1 in organisms as distant as plants, though few orthologs were found for fungi.[49] The following table provides a sample of the ortholog space. Vertebrate orthologs share >50% identity with human protein SAAL1 while displayed invertebrates and non-metazoan orthologs have 30% or less identity.
| Species | Organism Common Name | Multiple Sequence Alignment Abbreviation | Date of Divergence from Humans
(Millions of Years Ago)[50] |
Length (AAs) | Identity | NCBI Accession |
|---|---|---|---|---|---|---|
| Homo sapiens | Humans | Hsa_SAAL1 | 0 | 474 | 100 | NP_612430.2 |
| Macaca mulatta | Rhesus Monkey | Mmu_SAAL1 | 29 | 473 | 98 | XP_001087433.2 |
| Ictidomys tridecemlineatus | Thirteen-Lined Ground Squirrel | Itr_SAAL1 | 90 | 474 | 90 | XP_005326805.1 |
| Monodelphis domestica | Gray Short-Tailed Opossum | Mdo_SAAL1 | 159 | 475 | 73 | XP_007497074.1 |
| Ornithorhynchus anatinus | Platypus | Oan_SAAL1 | 177 | 486 | 71 | XP_028915648.1 |
| Calidris pugnax | Ruff | Cpu_SAAL1 | 312 | 472 | 70 | XP_014815565.1 |
| Rhinatrema bivittatum | Two-Lined Caecilian | Rbi_SAAL1 | 352 | 472 | 61 | XP_029438391.1 |
| Erpetoichthys calabaricus | Reedfish | Eca_SAAL1 | 435 | 484 | 50 | XP_028650019.1 |
| Callorhinchus milii | Australian Ghost Shark | Cmi_SAAL1 | 473 | 474 | 54 | XP_007885592.1 |
| Saccoglossus kowalevskii | Acorn Worm | Ski_SAAL1 | 684 | 508 | 28 | XP_002732678.2 |
| Pomacea canaliculata | Golden Apple Snail | Pca_SAAL1 | 797 | 563 | 30 | XP_025086883.1 |
| Orbicella faveolata | Mountainous Star Coral | Ofa_SAAL1 | 824 | 561 | 25 | XP_020625180.1 |
| Rhizopus microsporus | (a fungal plant pathogen) | Rmi_SAAL1 | 1105 | 323 | 14 | XP_023467779.1 |
| Phycomyces blakesleeanus | (a type of fungus) | Pbl_SAAL1 | 1105 | 346 | 14 | XP_018285622.1 |
| Manihot esculenta | Cassava | Mes_SAAL1 | 1496 | 536 | 20 | XP_021611223.1 |
| Lactuca sativa | Lettuce | Lsa_SAAL1 | 1496 | 534 | 19 | XP_023753062.1 |
| Lupinus angustifolius | Narrowleaf Lupin | Lan_SAAL1 | 1496 | 488 | 18 | XP_019436310.1 |
| Elaeis guineensis | Oil Palm | Egu_SAAL1 | 1496 | 568 | 18 | XP_010933466.1 |
| Phalaenopsis equestris | (a type of orchid) | Peq_SAAL1 | 1496 | 551 | 17 | XP_020591929.1 |
| Phoenix dactylifera | Date Palm | Pda_SAAL1 | 1496 | 508 | 17 | XP_026661658.1 |
SAAL1 exists in up to four isoforms in other vertebrates. Across these orthologs, it is the only member of its gene family.
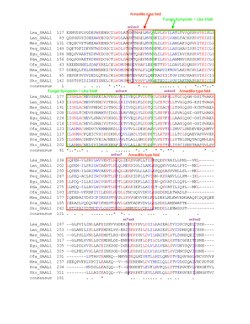
A multiple sequence alignment of the vertebrate homologs demonstrated high conservation of the protein, especially in the armadillo-type fold and fungal symportin-1 like motif. An alignment of invertebrate and non-metazoan orthologs indicates drastic changes in the protein's primary structure, but some conservation in the labeled motifs. Highly similar amino acids were colored red and less similar amino acids were colored blue; "*" denotes conservation and "." denotes similarity.
Phylogeny
[edit]The date of divergence from the human ortholog was compared to the corrected % divergence for SAAL1 orthologs. Compared against data for cytochrome c and fibrinogen alpha proteins in similar orthologs, SAAL1 evolved at a moderate rate.

References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000166788 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000006763 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c d "SAAL1 Gene - GeneCards | SIM23 Protein | SIM23 Antibody". GeneCards.
- ^ "SAAL1 - Protein SAAL1 - Homo sapiens (Human) - SAAL1 gene & protein". www.uniprot.org. Uniprot. Retrieved 2020-08-01.
- ^ a b "SAAL1 serum amyloid A like 1 [Homo Sapiens (human)] - Gene - NCBI". National Center for Biotechnology Information (NCBI).
- ^ "Human hg38 chr11:18080292-18106082 UCSC Genome Browser v401".
- ^ Search Query SAAL1. "Genomatix Annotation and Analysis". Genomatix.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^ GXP_169676 Predicted Transcription Factor Binding Sites. "Genomatix Matinspector Result". Genomatix.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^ Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. (February 2014). "Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics". Molecular & Cellular Proteomics. 13 (2): 397–406. doi:10.1074/mcp.M113.035600. PMC 3916642. PMID 24309898.
- ^ Duff MO, Olson S, Wei X, Garrett SC, Osman A, Bolisetty M, et al. (May 2015). "Genome-wide identification of zero nucleotide recursive splicing in Drosophila". Nature. 521 (7552): 376–9. Bibcode:2015Natur.521..376D. doi:10.1038/nature14475. PMC 4529404. PMID 25970244.
- ^ a b "RBPmap - Motif Analysis and Prediction of mRNA Binding Sites". RBPmap.
- ^ "SRSF3 Serine/arginine rich splicing factor 3 - Homo sapiens (Human) - SRSF3 gene & protein". UniProt.
- ^ "SRSF5 - Serine/arginine-rich splicing factor 5 - Homo sapiens (Human) - SRSF5 gene & protein". UniProt.
- ^ "U2AF2- Splicing factor U2AF2 65kDa subunit - Homo sapiens (Human) - U2AF2 gene & protein". UniProt.
- ^ "FXR2 - Fragile X mental retardation syndrome-related protein 2 - Homo sapiens (Human) - FXR2 gene & protein". UniProt.
- ^ Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y (January 2015). "The I-TASSER Suite: protein structure and function prediction". Nature Methods. 12 (1): 7–8. doi:10.1038/nmeth.3213. PMC 4428668. PMID 25549265.
- ^ Roy A, Kucukural A, Zhang Y (April 2010). "I-TASSER: a unified platform for automated protein structure and function prediction". Nature Protocols. 5 (4): 725–38. doi:10.1038/nprot.2010.5. PMC 2849174. PMID 20360767.
- ^ Zhang Y (January 2008). "I-TASSER server for protein 3D structure prediction". BMC Bioinformatics. 9: 40. doi:10.1186/1471-2105-9-40. PMC 2245901. PMID 18215316.
- ^ "ExPasy - Compute pI/Mw tool". ExPasy.
- ^ a b c d Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, et al. (July 2019). "The EMBL-EBI search and sequence analysis tools APIs in 2019". Nucleic Acids Research. 47 (W1): W636 – W641. doi:10.1093/nar/gkz268. PMC 6602479. PMID 30976793.
- ^ "SAPS < Sequence Statistics < EMBL-EBI". EMBL-EBI.
- ^ a b c d Sato T, Fujii R, Konomi K, Yagishita N, Aratani S, Araya N, Aono H, Yudoh K, Suzuki N, Beppu M, Yamano Y, Nishioka K, Nakajima T (December 2011). "Overexpression of SPACIA1/SAAL1, a newly identified gene that is involved in synoviocyte proliferation, accelerates the progression of synovitis in mice and humans". Arthritis and Rheumatism. 63 (12): 3833–42. doi:10.1002/art.30617. PMID 22127701.
- ^ a b "protein SAAL1 [Homo sapiens] - Protein - NCBI". National Center for Biotechnology Information (NCBI).
- ^ a b c d Kumar M, Gouw M, Michael S, Sámano-Sánchez H, Pancsa R, Glavina J, et al. (January 2020). "ELM-the eukaryotic linear motif resource in 2020". Nucleic Acids Research. 48 (D1): D296 – D306. doi:10.1093/nar/gkz1030. PMC 7145657. PMID 31680160.
- ^ a b Pagni M, Ioannidis V, Cerutti L, Zahn-Zabal M, Jongeneel CV, Hau J, et al. (July 2007). "MyHits: improvements to an interactive resource for analyzing protein sequences". Nucleic Acids Research. 35 (Web Server issue): W433–7. doi:10.1093/nar/gkm352. PMC 1933190. PMID 17545200.
- ^ "Cell atlas - SAAL1 - The Human Protein Atlas". The Human Protein Atlas.
- ^ a b c Chu PY, Tung SL, Tsai KW, Shen FP, Chan SH (July 2020). "Identification of the Novel Oncogenic Role of SAAL1 and Its Therapeutic Potential in Hepatocellular Carcinoma". Cancers. 12 (7): 1843. doi:10.3390/cancers12071843. PMC 7408781. PMID 32650537.
- ^ Blom N, Gammeltoft S, Brunak S (December 1999). "Sequence and structure-based prediction of eukaryotic protein phosphorylation sites". Journal of Molecular Biology. 294 (5): 1351–62. doi:10.1006/jmbi.1999.3310. PMID 10600390.
- ^ Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S (June 2004). "Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence". Proteomics. 4 (6): 1633–49. doi:10.1002/pmic.200300771. PMID 15174133. S2CID 18810164.
- ^ Gupta, R (2001). "Prediction of glycosylation sites in proteomes: from post-translational modifications to protein function". Ph.D Thesis at the Center for Biological Sequence Analysis as the Bioinformatic Unit at Technical University of Denmark.
- ^ Gupta R, Brunak S (2002). "Prediction of glycosylation across the human proteome and the correlation to protein function". Pacific Symposium on Biocomputing. Pacific Symposium on Biocomputing: 310–22. PMID 11928486.
- ^ Letunic I, Bork P (January 2018). "20 years of the SMART protein domain annotation resource". Nucleic Acids Research. 46 (D1): D493 – D496. doi:10.1093/nar/gkx922. PMC 5753352. PMID 29040681.
- ^ a b Fujii R, Komatsu R, Sato T, Seki I, Konomi K, Aono H, Niki H, Yudoh K, Nishioka K, Nakajima T (November 2018). "SPACIA1/SAAL1 Deletion Results in a Moderate Delay in Collagen-Induced Arthritis Activity, along with mRNA Decay of Cyclin-dependent Kinase 6 Gene". International Journal of Molecular Sciences. 19 (12): 3828. doi:10.3390/ijms19123828. PMC 6320788. PMID 30513680.
- ^ Gouze JN, Gouze E, Popp MP, Bush ML, Dacanay EA, Kay JD, et al. (2006). "Exogenous glucosamine globally protects chondrocytes from the arthritogenic effects of IL-1beta". Arthritis Research & Therapy. 8 (6): R173. doi:10.1186/ar2082. PMC 1794517. PMID 17109745.
- ^ Saranya Revathy K, Umasuthan N, Whang I, Lee Y, Lee S, Oh MJ, Jung SJ, Choi CY, Park CJ, Park HC, Lee J (June 2012). "A novel acute phase reactant, serum amyloid A-like 1, from Oplegnathus fasciatus: genomic and molecular characterization and transcriptional expression analysis". Developmental and Comparative Immunology. 37 (2): 294–305. doi:10.1016/j.dci.2012.03.014. PMID 22504166.
- ^ Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. (July 2020). "A SARS-CoV-2 protein interaction map reveals targets for drug repurposing". Nature. 583 (7816): 459–468. Bibcode:2020Natur.583..459G. doi:10.1038/s41586-020-2286-9. PMC 7431030. PMID 32353859.
- ^ Davis ZH, Verschueren E, Jang GM, Kleffman K, Johnson JR, Park J, et al. (January 2015). "Global mapping of herpesvirus-host protein complexes reveals a transcription strategy for late genes". Molecular Cell. 57 (2): 349–60. doi:10.1016/j.molcel.2014.11.026. PMC 4305015. PMID 25544563.
- ^ Wang L, Fu B, Li W, Patil G, Liu L, Dorf ME, Li S (February 2017). "Comparative influenza protein interactomes identify the role of plakophilin 2 in virus restriction". Nature Communications. 8: 13876. Bibcode:2017NatCo...813876W. doi:10.1038/ncomms13876. PMC 5309701. PMID 28169297.
- ^ Hubel P, Urban C, Bergant V, Schneider WM, Knauer B, Stukalov A, et al. (April 2019). "A protein-interaction network of interferon-stimulated genes extends the innate immune system landscape". Nature Immunology. 20 (4): 493–502. doi:10.1038/s41590-019-0323-3. PMID 30833792. S2CID 71144955.
- ^ Choudhury NR, Heikel G, Trubitsyna M, Kubik P, Nowak JS, Webb S, et al. (November 2017). "RNA-binding activity of TRIM25 is mediated by its PRY/SPRY domain and is required for ubiquitination". BMC Biology. 15 (1): 105. doi:10.1186/s12915-017-0444-9. PMC 5678581. PMID 29117863.
- ^ Huttlin EL, Bruckner RJ, Paulo JA, Cannon JR, Ting L, Baltier K, et al. (May 2017). "Architecture of the human interactome defines protein communities and disease networks". Nature. 545 (7655): 505–509. Bibcode:2017Natur.545..505H. doi:10.1038/nature22366. PMC 5531611. PMID 28514442.
- ^ "PNKD - Probably hydrolase PNKD - Homo sapiens (Human) - PNKD gene & protein". UniProt.
- ^ Huttlin EL, Ting L, Bruckner RJ, Gebreab F, Gygi MP, Szpyt J, et al. (July 2015). "The BioPlex Network: A Systematic Exploration of the Human Interactome". Cell. 162 (2): 425–440. doi:10.1016/j.cell.2015.06.043. PMC 4617211. PMID 26186194.
- ^ "TMIGD3 - Transmembrane domain-containing protein TMIGD3 - Homo sapiens (Humans) - TMIGD3 gene & protein". UniProt.
- ^ Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, et al. (September 2005). "A human protein-protein interaction network: a resource for annotating the proteome". Cell. 122 (6): 957–68. doi:10.1016/j.cell.2005.08.029. hdl:11858/00-001M-0000-0010-8592-0. PMID 16169070. S2CID 8235923.
- ^ a b "BoxShade Server". ExPASy.
- ^ "Protein BLAST: search protein databases using protein query". National Center for Biotechnology Information (NCBI).
- ^ "Time Tree :: Timescale of Life". TimeTree.
 KSF
KSF


