Transition metal dichalcogenide monolayers
 From Wikipedia - Reading time: 34 min
From Wikipedia - Reading time: 34 min

Transition-metal dichalcogenide (TMD or TMDC) monolayers are atomically thin semiconductors of the type MX2, with M a transition-metal atom (Mo, W, etc.) and X a chalcogen atom (S, Se, or Te). One layer of M atoms is sandwiched between two layers of X atoms. They are part of the large family of so-called 2D materials, named so to emphasize their extraordinary thinness. For example, a MoS2 monolayer is only 6.5 Å thick. The key feature of these materials is the interaction of large atoms in the 2D structure as compared with first-row transition-metal dichalcogenides, e.g., WTe2 exhibits anomalous giant magnetoresistance and superconductivity.[1]
The discovery of graphene shows how new physical properties emerge when a bulk crystal of macroscopic dimensions is thinned down to one atomic layer. Like graphite, TMD bulk crystals are formed of monolayers bound to each other by van-der-Waals attraction. TMD monolayers have properties that are distinctly different from those of the semimetal graphene:
- TMD monolayers MoS2, WS2, MoSe2, WSe2, MoTe2 have a direct band gap, and can be used in electronics as transistors and in optics as emitters and detectors.[2][3][4][5]
- The TMD monolayer crystal structure has no inversion center, which allows to access a new degree of freedom of charge carriers, namely the k-valley index, and to open up a new field of physics: valleytronics[6][7][8][9]
- The strong spin–orbit coupling in TMD monolayers leads to a spin–orbit splitting[10] of hundreds meV in the valence band and a few meV in the conduction band, which allows control of the electron spin by tuning the excitation laser photon energy and handedness.[11]
- 2D nature and high spin–orbit coupling in TMD layers can be used as promising materials for spintronic applications.[12][13]
The work on TMD monolayers is an emerging research and development field since the discovery of the direct bandgap[2] and the potential applications in electronics [14][3] and valley physics.[7][8][9] TMDs are often combined with other 2D materials like graphene and hexagonal boron nitride to make van der Waals heterostructures. These heterostructures need to be optimized to be possibly used as building blocks for many different devices such as transistors, solar cells, LEDs, photodetectors, fuel cells, photocatalytic and sensing devices. Some of these devices are already used in everyday life and can become smaller, cheaper and more efficient by using TMD monolayers.[15][16]
Crystal structure
[edit]
Transition-metal dichalcogenides (TMDs) are composed of three atomic planes and often two atomic species: a metal and two chalcogens. The honeycomb, hexagonal lattice has threefold symmetry and can permit mirror plane symmetry and/or inversion symmetry.[17] In the macroscopic bulk crystal, or more precisely, for an even number of monolayers, the crystal structure has an inversion center. In the case of a monolayer (or any odd number of layers), the crystal may or may not have an inversion center.
Broken inversion symmetry
[edit]Two important consequences of that are:
- nonlinear optical phenomena, such as second-harmonic generation. When the crystal is excited by a laser, the output frequency can be doubled.[18][19][20][21]
- an electronic band structure with direct energy gaps, where both conduction and valence band edges are located at the non-equivalent K points (K+ and K−) of the 2D hexagonal Brillouin zone. The interband transitions in the vicinity of the K+ (or K−) point are coupled to right (or left) circular photon polarization states. These so-called valley dependent optical selection rules arise from inversion symmetry breaking. This provides a convenient method to address specific valley states (K+ or K−) by circularly polarized (right or left) optical excitation.[9] In combination with strong spin-splitting, the spin and valley degree of freedom are coupled, enabling stable valley polarization.[22][23][24]
These properties indicate that TMD monolayers represent a promising platform to explore spin and valley physics with the corresponding possible applications.
Properties
[edit]Transport properties
[edit]
At submicron scales, 3D materials no longer have the same behavior as their 2D form, which can be an advantage. For example, graphene has a very high carrier mobility, and accompanying lower losses through the Joule effect. But graphene has zero bandgap, which results in a disqualifyingly low on/off ratio in transistor applications. TMD monolayers might be an alternative: they are structurally stable, display a band gap and show electron mobilities comparable to those of silicon, so they can be used to fabricate transistors.
Although thin-layer TMDs have been found to have a lower electron mobility than bulk TMDs, most likely because their thinness makes them more susceptible to damage, it has been found that coating the TMDs with HfO2 or hexagonal boron nitride (hBN) increases their effective carrier mobility.[25]
Optical properties
[edit]| A (eV) | A (nm) | B (eV) | B (nm) | |
|---|---|---|---|---|
| MoS2 | 1.78 | 695 | 1.96 | 632 |
| MoSe2 | 1.50 | 825 | 1.75 | 708 |
| MoTe2 | 1.06 | 1170 | 1.36 | 912 |
| WS2 | 1.84 | 673 | 2.28 | 544 |
| WSe2 | 1.52 | 815 | 2.00 | 620 |
A semiconductor can absorb photons with energy larger than or equal to its bandgap. This means that light with a shorter wavelength is absorbed. Semiconductors are typically efficient emitters if the minimum of the conduction band energy is at the same position in k-space as the maximum of the valence band, i.e., the band gap is direct. The band gap of bulk TMD material down to a thickness of two monolayers is still indirect, so the emission efficiency is lower compared to monolayered materials. The emission efficiency is about 104 greater for TMD monolayer than for bulk material.[4] The band gaps of TMD monolayers are in the visible range (between 400 nm and 700 nm). The direct emission shows two excitonic transitions called A and B, separated by the spin–orbit coupling energy. The lowest energy and therefore most important in intensity is the A emission.[2][27] Owing to their direct band gap, TMD monolayers are promising materials for optoelectronics applications.

Atomic layers of MoS2 have been used as a phototransistor and ultrasensitive detectors. Phototransistors are important devices: the first with a MoS2 monolayer active region shows a photoresponsivity of 7.5 mA W−1 which is similar to graphene devices that reach 6.1 mA W−1. Multilayer MoS2 show higher photoresponsivities, about 100 mA W−1, which is similar to silicon devices. Making a gold contact at the far edges of a monolayer allows an ultrasensitive detector to be fabricated.[5] Such a detector has a photoresponsivity reaching 880 A W−1, 106 greater than the first graphene photodetectors. This high degree of electrostatic control is due to the thin active region of the monolayer. Its simplicity and the fact that it has only one semiconductor region, whereas the current generation of photodetectors is typically a p–n junction, makes possible industrial applications such as high-sensitivity and flexible photodetectors. The only limitation for currently available devices is the slow photoresponse dynamics.[5] Utilizing WSe2 the photoresponse was improved to a bandwidth of over 230 MHz by device symmetry optimization[28]
Mechanical properties
[edit]Interest in the use of TMD monolayers such as MoS2, WS2, and WSe2 for the use in flexible electronics due to a change from an indirect band gap in 3D to a direct band gap in 2D emphasizes the importance of the mechanical properties of these materials.[29] Unlike in bulk samples it is much more difficult to uniformly deform 2D monolayers of material and as a result, taking mechanical measurements of 2D systems is more challenging. A method that was developed to overcome this challenge, called atomic force microscopy (AFM) nanoindentation, involves bending a 2D monolayer suspended over a holey substrate with an AFM cantilever and measuring the applied force and displacement.[30] Through this method, defect free mechanically exfoliated monolayer flakes of MoS2 were found to have a Young's modulus of 270 GPa with a maximum experienced strain of 10% before breaking.[31] In the same study, it was found that bilayer mechanically exfoliated MoS2 flakes have a lower Young's modulus of 200 GPa, which is attributed to interlayer sliding and defects in the monolayer.[31] With increasing flake thickness the bending rigidity of the flake plays a dominant role and it is found that the Young's modulus of multilayer, 5- 25 layers, mechanically exfoliated MoS2 flakes is 330 GPa.[32]
The mechanical properties of other TMDs such as WS2 and WSe2 have also been determined. The Young's modulus of multilayer, 5-14 layers, mechanically exfoliated WSe2 is found to be 167 GPa with a maximum strain of 7%.[33] For WS2, the Young's modulus of chemical vapor deposited monolayer flakes is 272 GPa.[34] From this same study the Young's modulus of CVD-grown monolayer flakes of MoS2 is found to be 264 GPa.[34] This is an interesting result as the Young's modulus of the exfoliated MoS2 flake is nearly the same as that of the CVD grown MoS2 flake. It is generally accepted that chemically vapor deposited TMDs will include more defects when compared with the mechanically exfoliated films that are obtained from bulk single crystals, which implies that defects (points defects, etc.) that are included in the flake do not drastically affect the strength of the flake itself.
Under the application of strain, a decrease in the direct and indirect band gap is measured that is approximately linear with strain.[35] Importantly, the indirect bandgap decreases faster with applied strain to the monolayer than the direct bandgap, resulting in a crossover from direct to indirect band gap at a strain level of around 1%.[36] As a result, the emission efficiency of monolayers is expected to decrease for highly strained samples.[37] This property allows mechanical tuning of the electronic structure and also the possibility of fabrication of devices on flexible substrates.
Fabrication of TMD monolayers
[edit]Exfoliation
[edit]Exfoliation is a top down approach. In the bulk form, TMDs are crystals made of layers, which are coupled by Van-der-Waals forces. These interactions are weaker than the chemical bonds between the Mo and S in MoS2, for example. So TMD monolayers can be produced by micromechanical cleavage, just as graphene.
The crystal of TMD is rubbed against the surface of another material (any solid surface). In practice, adhesive tape is placed on the TMD bulk material and subsequently removed. The adhesive tape, with tiny TMD flakes coming off the bulk material, is brought down onto a substrate. On removing the adhesive tape from the substrate, TMD monolayer and multilayer flakes are deposited. This technique produces small samples of monolayer material, typically about 5–10 micrometers in diameter.[38]
Large quantities of exfoliated material can also be produced using liquid-phase exfoliation by blending TMD materials with solvents and polymers.[39]
Chemical vapor deposition
[edit]Chemical vapor deposition (CVD) is another approach used to synthesize transition-metal dichalcogenides.[40] It has been used broadly to synthesize many different TMDs because it can be easily adapted for different TMD materials. Generally, CVD growth of TMDs is achieved by putting precursors to the material, typically a transition-metal oxide and pure chalcogen, into a furnace with the substrate on which the material will form.[41] The furnace is heated to high temperatures (anywhere from 650 to 1000 °C) with an inert gas, typically N2 or Ar, flowing through the tube.[41] Some materials require H2 gas as a catalyst for formation, so it may be flowed through the furnace in smaller quantities than the inert gas.[42]
Outside of traditional CVD, metal organic chemical vapor deposition (MOCVD) has been used to synthesize TMDs. Unlike traditional CVD described above, MOCVD uses gaseous precursors, as opposed to solid precursors and MOCVD is usually carried out at lower temperatures, anywhere from 300 to 900 °C.[43] MOCVD has been shown to provide more consistent wafer-scale growth than traditional CVD.
CVD is often used over mechanical exfoliation despite its added complexity because it can produce monolayers ranging anywhere from 5 to 100 microns in size as opposed to the surface areas of roughly 5-10 microns produced using the mechanical exfoliation method.[44] Not only do TMD monolayers produced by CVD have a larger surface area than those flakes produced by mechanical exfoliation, they are often more uniform. Monolayer TMD flakes with very little or no multilayer areas can be produced by chemical vapor deposition, in contrast to samples produced by mechanical exfoliation, which often have many multilayered areas.[38][41] An alternative method has demonstrated that transition metal sulfides, including those of Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, and W, can be synthesized through sulfurization of metal oxides in CS₂ vapor, achieving gram-scale production with simpler equipment and precursors.[45] Geometrically confined-growth techniques are also recently applied to realize wafer-scale single-domain TMD monolayer arrays and their heterostructures.[46]
Molecular-beam epitaxy
[edit]Molecular-beam epitaxy (MBE) is an established technique for growing semiconductor devices with atomic monolayer thickness control. MBE has been used to grow different TMDs, such as MoSe2, WSe2, and early transition metals, including titanium, vanadium, and chromium, tellurides,[47][48][49] resulting in extremely clean samples with a thickness of only 0.5 monolayer.[47][49]
The growth takes place in ultra-high vacuum (UHV). Precursors for the target materials are placed into evaporation cells, usually as powder (for example selenium), or as a rod (for example molybdenum).[47] Some elements, such as selenium and tellurium, both of which are chalcogens, can be used in pure solid form as precursors. Some elements, however, can only be used when extracted from solid compounds, such as sulfur from FeS2. The compound materials are broken down by heating up the material at UHV pressures.[50] The evaporation cells are either Knudsen cells or electron beam evaporation based, depending on the materials; electron beam evaporation works with rods and can be used to reach high temperatures without overheating heating filaments, while Knudsen cells are suitable for powders and materials with a lower evaporation point. The evaporated materials are then directed towards the substrate; some common ones are MoS2, HOPG, mica, or a sapphire substrate, such as Al2O3.[47][48][49][51] A specific substrate is chosen to fit the targeted growth the best. The substrate is kept heated during the process to enhance the growth, with the temperatures ranging from 300 °C to 700 °C. The temperature of the substrate is one key factor of the growth, and altering it can be used to grow different phases, such as 1T and 2H, of the same material.[47]
MBE holds some advantages in regards to both manual exfoliation and CVD. Use of reflection high-energy electron diffraction (RHEED) enables the in-situ monitoring of the growth, and this additionally with UHV and slow growth speed allows one to create clean, atomically thin monolayers.[47][52] The improvement in sample quality is considerable when compared to exfoliation, as MBE is more effective in getting rid of the large flakes and impurities. In contrast to CVD, MBE proves beneficial when single-layerd TMDs are required.[49][52] The disadvantage of MBE is that it is a relatively complicated process that requires large amounts of specialized equipment. Maintaining UHV can be difficult, and the preparation of samples is slower than in the other two methods.
Electrochemical Deposition
[edit]Electrodeposition is among the techniques that have emerged to produce TMDC semiconductors such as MoS2, WS2 and WSe2. Several reports have shown controlled electrodeposition of TMDC layers down to a monolayer.[53][54][55][56] The materials have so far shown continuous films of good uniformity but typically require annealing temperatures > 500 °C. Electrodepositions of TMDC films have been successfully reported over conducting films such as graphene and TiN, and over a SiO2 insulator by growing the TMDC laterally starting from a conductive film.[57]
Electronic band structure
[edit]Band gap
[edit]In the bulk form, TMD have an indirect gap in the center of the Brillouin zone, whereas in monolayer form the gap becomes direct and is located in the K points.[58][2]
Spin–orbit coupling
[edit]| Valence band
splitting (eV) |
Conduction band
splitting (eV) | |
|---|---|---|
| MoS2 | 0.148 | 0.003 |
| WS2 | 0.430 | 0.026 |
| MoSe2 | 0.184 | 0.007 |
| WSe2 | 0.466 | 0.038 |
| MoTe2 | 0.219 | 0.034 |
For TMDs, the atoms are heavy and the outer layers electronic states are from d-orbitals that have a strong spin–orbit coupling. This spin orbit coupling removes the spins degeneracy in both the conduction and valence band i.e. introduces a strong energy splitting between spin up and down states. In the case of MoS2, the spin splitting in conduction band is in the meV range, it is expected to be more pronounced in other material like WS2.[61][62][63] The spin orbit splitting in the valence band is several hundred meV.
Spin-valley coupling and the electron valley degree of freedom
[edit]

By controlling the charge or spin degree of freedom of carriers, as proposed by spintronics, novel devices have already been made. If there are different conduction/valence band extrema in the electronic band structure in k-space, the carrier can be confined in one of these valleys. This degree of freedom opens up a new field of physics: the controlling of carriers k-valley index, also called valleytronics.[22][64]
For TMD monolayers crystals, the parity symmetry is broken, there is no more inversion center. K valleys of different directions in the 2D hexagonal Brillouin zone are no longer equivalent. So there are two kinds of K valley called K+ and K−. Also there is a strong energy degeneracy of different spin states in valence band. The transformation of one valley to another is described by the time reversal operator. Moreover, crystal symmetry leads to valley dependent optical selection rules: a right circular polarized photon (σ+) initializes a carrier in the K+ valley and a left circular polarized photon (σ-) initializes a carrier in the K− valley.[7] Thanks to these two properties (spin-valley coupling and optical selection rules), a laser of specific polarization and energy allows to initialize the electron valley states (K+ or K−) and spin states (up or down).[1]
Emission and absorption of light: excitons
[edit]A single layer of TMD can absorb up to 20% of incident light,[5] which is unprecedented for such a thin material. When a photon of suitable energy is absorbed by a TMD monolayer, an electron is created in the conduction band; the electron now missing in the valence band is assimilated by a positively charged quasi-particle called a hole. The negatively charged electron and the positively charged hole are attracted via the Coulomb interaction, forming a bound state called an exciton which can be thought as a hydrogen atom (with some difference). This Bosonic-like quasi-particle is very well known and studied in traditional semiconductors, such as GaAs and ZnO but in TMD it provides exciting new opportunities for applications and for studying fundamental physics. Indeed, the reduced dielectric screening and the quantum size effect present in these ultrathin materials make the binding energy of excitons much stronger than those in traditional semiconductors. Binding energies of several hundreds of meV are observed for all the four principal members of the TMD family.[21][27][65][66][67]

As mentioned before, we can think about an exciton as if it were a hydrogen atom, with an electron bound to a hole. The main difference is that this system is not stable and tends to relax to the vacuum state, which is here represented by an electron in the valence band. The energy difference between the exciton 'ground state' (n=1) and the 'vacuum state' is called optical gap and is the energy of the photon emitted when an exciton recombines. This is the energy of the photons emitted by TMD monolayers and observed as huge emission peaks in photoluminescence (PL) experiments, such as the one labelled X0 in the figure. In this picture the binding energy EB is defined as the difference between the free particle band gap and the optical band gap and represent, as usual, the energy needed to take the hole and the electron apart. The existence of this energy difference is called band gap renormalization. The analogy with hydrogen atom doesn't stop here as excitonic excited states were observed at higher energies and with different techniques.[21][65]
Because of the spin–orbit splitting of the valence band two different series of excitons exist in TMD, called A- and B-excitons. In the A series the hole is located in the upper branch of the Valence band while for the B-exciton the hole is in the lower branch. As a consequence the optical gap for B-exciton is larger and the corresponding peak is found at higher energy in PL and reflectivity measurements.
Another peak usually appears in the PL spectra of TMD monolayers, which is associated to different quasi-particles called trions.[69][70] These are excitons bound to another free carrier which can be either an electron or a hole. As a consequence a trion is a negative or positively charged complex. The presence of a strong trion peak in a PL spectrum, eventually stronger than the peak associated with exciton recombination, is a signature of a doped monolayer. It is believed now that this doping is extrinsic, which means that it arises from charged trap states present in the substrate (generally SiO2). Positioning a TMD monolayer between two flakes of hBN removes this extrinsic doping and greatly increase the optical quality of the sample.[68][71]
At higher excitation powers biexcitons[72][73] have also been observed in monolayer TMDs. These complexes are formed by two bound excitons. Theory predicts that even larger charge-carrier complexes, such as charged biexcitons (quintons) and ion-bound biexcitons, are stable and should be visible in the PL spectra.[74] Additionally, quantum light has been observed to originate from point defects in these materials in a variety of configurations.[75][76][77][78][79][80]
Radiation effects of TMD monolayers
[edit]Common forms of radiation used to create defects in TMDs are particle and electromagnetic irradiation, impacting the structure and electronic performance of these materials. Scientist have been studying the radiation response of these materials to be used in high-radiation environments, such as space or nuclear reactors.[81] Damage to this unique class of materials occurs mainly through sputtering and displacement for metals or radiolysis and charging for insulators and semiconductors. To sputter away an atom, the electron must be able to transfer enough energy to overcome the threshold for knock-on damage.[82] Yet, the exact quantifiable determination of this energy still needs to be determined for TMDs. Consider MoS2 as an example, TEM exposure via sputtering creates vacancies in the lattice, these vacancies are then observed to be collected together in spectroscopic lines. Additionally, when looking at the radiation response of these materials, the three parameters that are proven to matter most are the choice of substrate,[83] the sample thickness,[84] and the sample preparation process.[85]
Janus TMD monolayers
[edit]A new type of asymmetric transitional metal dichalcogenide, the Janus TMDs monolayers, has been synthesized by breaking the out-of-plane structural symmetry via plasma assisted chemical vapor deposition.[86] Janus TMDs monolayers show an asymmetric structure MXY (M = Mo or W, X/Y = S, Se or Te)[87] exhibiting out-of-plane optical dipole[88] and piezoelectricity[89] due to the imbalance of the electronic wave-function between the dichalcogenides, which are absent in a non-polar TMDs monolayer, MX2. In addition, the asymmetric structure of Janus MoSSe provides an enhanced Rashba spin–orbit interaction, which suggests asymmetrically Janus TMDs monolayer can be a promising candidate for spintronic applications. In addition, Janus TMDs monolayer has been considered as an excellent material for electrocatalysis[90] or photocatalysis.[91]
Janus MoSSe can be synthesized by inductively coupled plasma CVD (ICP-CVD). The top layer of sulfur atoms on MoS2 is stripped using hydrogen ions, forming an intermediate state, MoSH. Afterward, the intermediate state is selenized by thermal annealing at 250 °C in an environment of hydrogen and argon gases.[86]
Aspirational uses
[edit]Electronics
[edit]A field-effect transistor (FET) made of monolayer MoS2 showed an on/off ratio exceeding 108 at room temperature owing to electrostatic control over the conduction in the 2D channel.[92] FETs made from MoS2, MoSe2, WS2, and WSe2 have been made. All show promise not just because of their electron mobility and band gap, but because their very thin structure makes them promising for use in thin, flexible electronics.[93]
Sensing
[edit]The band gap TMDs possess makes them attractive for sensors as a replacement for graphene. FET-based biosensors rely on receptors attached to the monolayer TMD. When target molecules attach to the receptors, it affects the current flowing through the transistor.[94]
However, it has been shown that one can detect nitrogenous bases in DNA when they pass through nanopores made in MoS2.[95] Nanopore sensors are based upon measuring ionic current through a nanopore in a material. When a single strand of DNA passes through the pore, there is a marked decrease in ionic current for each base. By measuring the current flowing through the nanopore, the DNA can then be sequenced.[95]
To this date, most sensors have been created from MoS2, although WS2 has been explored as well.[96]
Specific examples
[edit]Molybdenum disulfide
[edit]
2, with Mo in blue and S in yellow

Molybdenum disulfide monolayers consist of a unit of one layer of molybdenum atoms covalently bonded to two layers of sulfur atoms. While bulk molybdenum sulfide exists as 1T, 2H, or 3R polymorphs, molybdenum disulfide monolayers are found only in the 1T or 2H form.[97] The 2H form adopts a trigonal prismatic geometry[98] while the 1T form adopts an octahedral or trigonal antiprismatic geometry.[97] Molybdenum monolayers can also be stacked due to Van der Waals interactions between each layer.
Electrical
[edit]The electrical properties of molybdenum sulfide in electrical devices depends on factors such as the number of layers,[99] the synthesis method,[97] the nature of the substrate on which the monolayers are placed on,[100] and mechanical strain.[101]
As the number of layers decrease, the band gap begins to increase from 1.2eV in the bulk material up to a value of 1.9eV for a monolayer.[102] Odd number of molybdenum sulfide layers also produce different electrical properties than even numbers of molybdenum sulfide layers due to cyclic stretching and releasing present in the odd number of layers.[103] Molybdenum sulfide is a p-type material, but it shows ambipolar behavior when molybdenum sulfide monolayers that were 15 nm thick were used in transistors.[102] However, most electrical devices containing molybdenum sulfide monolayers tend to show n-type behavior.[98][104]
The band gap of molybdenum disulfide monolayers can also be adjusted by applying mechanical strain[101] or an electrical field.[102] Increasing mechanical strain shifts the phonon modes of the molybdenum sulfide layers.[101] This results in a decrease of the band gap and metal-to-insulator transition.[97] Applying an electric field of 2-3Vnm−1 also decreases the indirect bandgap of molybdenum sulfide bilayers to zero.[97]
Solution phase lithium intercalation and exfoliation of bulk molybdenum sulfide produces molybdenum sulfide layers with metallic and semiconducting character due to the distribution of 1T and 2H geometries within the material.[102][97] This is due to the two forms of molybdenum sulfide monolayers having different electrical properties. The 1T polymorph of molybdenum sulfide is metallic in character while the 2H form is more semiconducting.[98] However, molybdenum disulfide layers produced by electrochemical lithium intercalation are predominantly 1T and thus metallic in character as there is no conversion to the 2H form from the 1T form.[97]
Thermal
[edit]The thermal conductivity of molybdenum disulfide monolayers at room temperature is 34.5W/mK[105] while the thermal conductivity of few-layer molybdenum disulfide is 52W/mK.[105] The thermal conductivity of graphene, on the other hand, is 5300W/mK.[105] Due to the rather low thermal conductivity of molybdenum disulfide nanomaterials, it is not as promising material for high thermal applications as some other 2D materials.
Synthesis
[edit]Exfoliation
[edit]Exfoliation techniques for the isolating of molybdenum disulfide monolayers include mechanical exfoliation,[97] solvent assisted exfoliation,[98] and chemical exfoliation.[102]
Solvent assisted exfoliation is done by sonicating bulk molybdenum disulfide in an organic solvent such as isopropanol and N-methyl-2-pyrrolidone, which disperses the bulk material into nanosheets as the Van der Waals interactions between the layers in the bulk material are broken.[97] The amount of nanosheets produced is controlled by the sonication time,[98] the solvent-molybdenum disulfide interactions,[97] and the centrifuge speed.[97] Compared to other exfoliation techniques, solvent assisted exfoliation is the simplest method for large scale production of molybdenum disulfide nanosheets.[107]
The micromechanical exfoliation of molybdenum disulfide was inspired by the same technique used in the isolation of graphene nanosheets.[107] Micromechanical exfoliation allows for low defect molybdenum disulfide nanosheets but is not suitable for large scale production due to low yield.[98]
Chemical exfoliation involves functionalizing molybdenum difsulfide and then sonicating to disperse the nanosheets.[107] The most notable chemical exfoliation technique is lithium intercalation in which lithium is intercalated into bulk molybdenum disulfide and then dispersed into nanosheets by the addition of water.[102]
Chemical vapor deposition
[edit]Chemical vapor deposition of molybdenum disulfide nanosheets involves reacting molybdenum and sulfur precursors on a substrate at high temperatures.[107] This technique is often used in the preparing electrical devices with molybdenum disulfide components because the nanosheets are applied directly on the substrate; unfavorable interactions between the substrate and the nanosheets that would have occurred had they been separately synthesized are decreased.[98] In addition, since the thickness and area of the molybdenum disulfide nanosheets can be controlled by the selection of specific precursors, the electrical properties of the nanosheets can be tuned.[98]
- Electroplating
Among the techniques that have been used to deposit molybdenum disulfide is electroplating.[108] Ultra-thin films consisting of few-layers have been produced via this technique over graphene electrodes. In addition, other electrode materials were also electroplated with MoS2, such as Titanium Nitride (TiN), glassy carbon and polytetrafluoroethylene.[109][110][111] The advantage that this technique offers in producing 2D materials is its spatial growth selectivity and its ability to deposit over 3D surfaces. Controlling the thickness of electrodeposited materials can be achieved by adjusting the deposition time or current.
Laser ablation
[edit]Pulsed laser deposition involves the thinning of bulk molybdenum disulfide by laser to produce single or multi-layer molybdenum disulfide nanosheets.[97] This allows for synthesis of molybdenum disulfide nanosheets with a defined shape and size.[102] The quality of the nanosheets are determined by the energy of the laser and the irradation angle.[107]
Lasers can also be used to form molybdenum disulfide nanosheets from molybdenum disulfide fullerene-like molecules.[112]
Hafnium disulfide
[edit]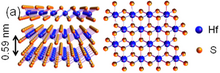
2 structure
Hafnium disulfide (HfS
2) has a layered structure with strong covalent bonding between the Hf and S atoms in a layer and weak van der Waals forces between layers. The compound has CdI
2 type structure and is an indirect band gap semiconducting material. The interlayer spacing between the layers is 0.56 nm, which is small compared to group VIB TMDs like MoS
2, making it difficult to cleave its atomic layers. However, recently its crystals with large interlayer spacing has grown using a chemical vapor transport route.[113] These crystals exfoliate in solvents like N-Cyclohexyl-2-pyrrolidone (CHP) in a time of just some minutes resulting in a high-yield production of its few-layers resulting in increase of its indirect bandgap from 0.9 eV to 1.3 eV. As an application in electronics, its field-effect transistors has been realised using its few layers as a conducting channel material offering a high current modulation ratio larger than 10000 at room temperature. Therefore, group IVB TMDs also holds potential applications in the field of opto-electronics.
Tungsten diselenide
[edit]
Tungsten diselenide is an inorganic compound with the formula WSe
2. The compound adopts a hexagonal crystalline structure similar to molybdenum disulfide. Every tungsten atom is covalently bonded to six selenium ligands in a trigonal prismatic coordination sphere, while each selenium is bonded to three tungsten atoms in a pyramidal geometry. The tungsten – selenium bond has a bond distance of 2.526 Å and the distance between selenium atoms is 3.34 Å.[114] Layers stack together via van der Waals interactions. WSe
2 is a stable semiconductor in the group-VI transition-metal dichalcogenides. The electronic bandgap of WSe
2 can be tuned by mechanical strain[115] which can also allow for conversion of the band type from indirect-to-direct in a WSe
2 bilayer.[116]
References
[edit]- ^ a b Eftekhari, A. (2017). "Tungsten dichalcogenides (WS2, WSe2, and WTe2): materials chemistry and applications". Journal of Materials Chemistry A. 5 (35): 18299–18325. doi:10.1039/C7TA04268J.
- ^ a b c d Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C. Y.; Galli, G.; Wang, F. (2010). "Emerging Photoluminescence in Monolayer MoS2". Nano Letters. 10 (4): 1271–5. Bibcode:2010NanoL..10.1271S. doi:10.1021/nl903868w. PMID 20229981.
- ^ a b c Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. (2011). "Single-layer MoS2 transistors". Nature Nanotechnology. 6 (3): 147–50. Bibcode:2011NatNa...6..147R. doi:10.1038/nnano.2010.279. PMID 21278752.
- ^ a b Sundaram, R. S.; Engel, M.; Lombardo, A.; Krupke, R.; Ferrari, A. C.; Avouris, Ph; Steiner, M. (2013). "Electroluminescence in Single Layer MoS2". Nano Letters. 13 (4): 1416–1421. arXiv:1211.4311. Bibcode:2013NanoL..13.1416S. doi:10.1021/nl400516a. PMID 23514373. S2CID 207581247.
- ^ a b c d e Lopez-Sanchez, O.; Lembke, D.; Kayci, M.; Radenovic, A.; Kis, A. (2013). "Ultrasensitive photodetectors based on monolayer MoS2". Nature Nanotechnology. 8 (7): 497–501. Bibcode:2013NatNa...8..497L. doi:10.1038/nnano.2013.100. PMID 23748194. S2CID 5435971.
- ^ Rycerz, A.; Tworzydło, J.; Beenakker, C. W. J. (2007). "Valley filter and valley valve in graphene". Nature Physics. 3 (3): 172–175. arXiv:cond-mat/0608533. Bibcode:2007NatPh...3..172R. doi:10.1038/nphys547. S2CID 119377206.
- ^ a b c Cao, T.; Wang, G.; Han, W.; Ye, H.; Zhu, C.; Shi, J.; Niu, Q.; Tan, P.; Wang, E.; Liu, B.; Feng, J. (2012). "Valley-selective circular dichroism of monolayer molybdenum disulphide". Nature Communications. 3 (6): 887. arXiv:1112.4013. Bibcode:2012NatCo...3..887C. doi:10.1038/ncomms1882. PMC 3621397. PMID 22673914.
- ^ a b Mak, K. F.; He, K.; Shan, J.; Heinz, T. F. (2012). "Control of valley polarization in monolayer MoS2 by optical helicity". Nature Nanotechnology. 7 (8): 494–8. arXiv:1205.1822. Bibcode:2012NatNa...7..494M. doi:10.1038/nnano.2012.96. PMID 22706698. S2CID 23248686.
- ^ a b c Zeng, H.; Dai, J.; Yao, W.; Xiao, D.; Cui, X. (2012). "Valley polarization in MoS2 monolayers by optical pumping". Nature Nanotechnology. 7 (8): 490–3. arXiv:1202.1592. Bibcode:2012NatNa...7..490Z. doi:10.1038/nnano.2012.95. PMID 22706701. S2CID 13228054.
- ^ Reyes-Retana, J.A.; Cervantes-Sodi, F. (2016). "Spin–orbital effects in metal-dichalcogenide semiconducting monolayers". Scientific Reports. 6: 24093. Bibcode:2016NatSR...624093R. doi:10.1038/srep24093. PMC 4837337. PMID 27094967.
- ^ Sallen, G.; Bouet, L.; Marie, X.; Wang, G.; Zhu, C.R.; Han, W.P.; Lu, P.; Tan, P.H.; Amand, T; Liu, B.L.; Urbaszek, B. (2012). "Robust optical emission polarization in MoS2 monolayers through selective valley excitation". Physical Review B. 86 (8): 3–6. arXiv:1206.5128. Bibcode:2012PhRvB..86h1301S. doi:10.1103/PhysRevB.86.081301. S2CID 62890713.
- ^ Husain, Sajid; Kumar, Abhishek; Kumar, Prabhat; Kumar, Ankit; Barwal, Vineet; Behera, Nilamani; Choudhary, Sudhanshu; Svedlindh, Peter; Chaudhary, Sujeet (2018). "Spin pumping in the Heusler alloy Co2FeAl/MoS2 heterostructure: Ferromagnetic resonance experiment and theory". Physical Review B. 98 (18): 180404. Bibcode:2018PhRvB..98r0404H. doi:10.1103/PhysRevB.98.180404. S2CID 125218447.
- ^ Husain, Sajid; Gupta, Rahul; Kumar, Ankit; Kumar, Prabhat; Behera, Nilamani; Brucas, Rimantas; Chaudhary, Sujeet; Svedlindh, Peter (2020-12-01). "Emergence of spin–orbit torques in 2D transition metal dichalcogenides: A status update". Applied Physics Reviews. 7 (4): 041312. Bibcode:2020ApPRv...7d1312H. doi:10.1063/5.0025318. S2CID 230546779.
- ^ Briggs, Natalie; Subramanian, Shruti; Lin, Zhong; Li, Xufan; Zhang, Xiaotian; Zhang, Kehao; Xiao, Kai; Geohegan, David; Wallace, Robert; Chen, Long-Qing; Terrones, Mauricio; Ebrahimi, Aida; Das, Saptarshi; Redwing, Joan; Hinkle, Christopher; Momeni, Kasra; van Duin, Adri; Crespi, Vin; Kar, Swastik; Robinson, Joshua A. (2019). "A roadmap for electronic grade 2D materials". 2D Materials. 6 (2): 022001. Bibcode:2019TDM.....6b2001B. doi:10.1088/2053-1583/aaf836. OSTI 1503991. S2CID 188118830.
- ^ "2-D materials enhance a 3-D world". phys.org. 2017-01-10.
- ^ Nealon, Cory (2016-05-13). "This 'nanocavity' may improve ultrathin solar panels, video cameras and more". phys.org.
- ^ Sung, S.H.; Schnitzer, N.; Brown, L.; Park, J.; Hovden, R. (2019). "Stacking, strain, and twist in 2D materials quantified by 3D electron diffraction". Physical Review Materials. 3 (6): 064003. arXiv:1905.11354. Bibcode:2019PhRvM...3f4003S. doi:10.1103/PhysRevMaterials.3.064003. S2CID 166228311.
- ^ Kumar, N.; Najmaei, S.; Cui, Q.; Ceballos, F.; Ajayan, P.; Lou, J.; Zhao, H. (2013). "Second harmonic microscopy of monolayer MoS2". Physical Review B. 87 (16): 161403. arXiv:1302.3935. Bibcode:2013PhRvB..87p1403K. doi:10.1103/PhysRevB.87.161403. S2CID 1796583.
- ^ Malard, L. M.; Alencar, T. V.; Barboza, A. P. M.; Mak, K. F.; De Paula, A. M. (2013). "Observation of intense second harmonic generation from MoS2 atomic crystals". Physical Review B. 87 (20): 201401. arXiv:1304.4289. Bibcode:2013PhRvB..87t1401M. doi:10.1103/PhysRevB.87.201401. S2CID 118392637.
- ^ Zeng, H.; Liu, G. B.; Dai, J.; Yan, Y.; Zhu, B.; He, R.; Xie, L.; Xu, S.; Chen, X.; Yao, W.; Cui, X. (2013). "Optical signature of symmetry variations and spin-valley coupling in atomically thin tungsten dichalcogenides". Scientific Reports. 3: 1608. arXiv:1208.5864. Bibcode:2013NatSR...3.1608Z. doi:10.1038/srep01608. PMC 3622914. PMID 23575911.
- ^ a b c Wang, G.; Marie, X.; Gerber, I.; Amand, T.; Lagarde, D.; Bouet, L.; Vidal, M.; Balocchi, A.; Urbaszek, B. (2015). "Giant Enhancement of the Optical Second-Harmonic Emission of WSe2 Monolayers by Laser Excitation at Exciton Resonances". Physical Review Letters. 114 (9): 097403. arXiv:1404.0056. Bibcode:2015PhRvL.114i7403W. doi:10.1103/PhysRevLett.114.097403. PMID 25793850. S2CID 119247783.
- ^ a b Xiao, D.; Liu, G. B.; Feng, W.; Xu, X.; Yao, W. (2012). "Coupled Spin and Valley Physics in Monolayers of MoS2 and Other Group-VI Dichalcogenides". Physical Review Letters. 108 (19): 196802. arXiv:1112.3144. Bibcode:2012PhRvL.108s6802X. doi:10.1103/PhysRevLett.108.196802. PMID 23003071. S2CID 13621082.
- ^ Jones, A. M.; Yu, H.; Ghimire, N. J.; Wu, S.; Aivazian, G.; Ross, J. S.; Zhao, B.; Yan, J.; Mandrus, D. G.; Xiao, D.; Yao, W.; Xu, X. (2013). "Optical generation of excitonic valley coherence in monolayer WSe2". Nature Nanotechnology. 8 (9): 634–8. arXiv:1303.5318. Bibcode:2013NatNa...8..634J. doi:10.1038/nnano.2013.151. PMID 23934096. S2CID 7090218.
- ^ Xu, X.; Yao, W.; Xiao, D.; Heinz, T. F. (2014). "Spin and pseudospins in layered transition metal dichalcogenides". Nature Physics. 10 (5): 343–350. Bibcode:2014NatPh..10..343X. doi:10.1038/nphys2942. S2CID 85510443.
- ^ Manzeli, Sajedeh; Ovchinnikov, Dmitry; Pasquier, Diego; Yazyev, Oleg V.; Kis, Andras (2017-06-13). "2D transition metal dichalcogenides". Nature Reviews Materials. 2 (8): 17033. Bibcode:2017NatRM...217033M. doi:10.1038/natrevmats.2017.33. ISSN 2058-8437.
- ^ Ramasubramaniam, A. (2012). "Large excitonic effects in monolayers of molybdenum and tungsten dichalcogenides". Physical Review B. 86 (11): 115409. Bibcode:2012PhRvB..86k5409R. doi:10.1103/PhysRevB.86.115409.
- ^ a b Qiu, D. Y.; Da Jornada, F. H.; Louie, S. G. (2013). "Optical Spectrum of MoS2: Many-Body Effects and Diversity of Exciton States". Physical Review Letters. 111 (21): 216805. arXiv:1311.0963. Bibcode:2013PhRvL.111u6805Q. doi:10.1103/PhysRevLett.111.216805. PMID 24313514. S2CID 19063715.
- ^ Strauß, Fabian; Kohlschreiber, Pia; Keck, Jakob; Michel, Patrick; Hiller, Jonas; Meixner, Alfred J.; Scheele, Marcus (2024). "A simple 230 MHz photodetector based on exfoliated WSe 2 multilayers". RSC Applied Interfaces. 1 (4): 728–733. doi:10.1039/D4LF00019F. PMC 11231687.
- ^ Akinwande, Deji; Petrone, Nicholas; Hone, James (2014-12-17). "Two-dimensional flexible nanoelectronics". Nature Communications. 5: 5678. Bibcode:2014NatCo...5.5678A. doi:10.1038/ncomms6678. PMID 25517105.
- ^ Lee, Changgu; Wei, Xiaoding; Kysar, Jeffrey W.; Hone, James (2008-07-18). "Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene". Science. 321 (5887): 385–388. Bibcode:2008Sci...321..385L. doi:10.1126/science.1157996. PMID 18635798. S2CID 206512830.
- ^ a b Bertolazzi, Simone; Brivio, Jacopo; Kis, Andras (2011-11-16). "Stretching and Breaking of Ultrathin MoS2". ACS Nano. 5 (12): 9703–9709. doi:10.1021/nn203879f. PMID 22087740.
- ^ Castellanos-Gomez, Andres; Poot, Menno; Steele, Gary A.; van der Zant, Herre S. J.; Agraït, Nicolás; Rubio-Bollinger, Gabino (2012). "Elastic Properties of Freely Suspended MoS2 Nanosheets". Advanced Materials. 24 (6): 772–775. arXiv:1202.4439. Bibcode:2012AdM....24..772C. doi:10.1002/adma.201103965. PMID 22231284. S2CID 205243099.
- ^ Zhang, Rui; Koutsos, Vasileious; Cheung, Cheung (January 2016). "Elastic properties of suspended multilayer WSe2". Applied Physics Letters. 108 (4): 042104. Bibcode:2016ApPhL.108d2104Z. doi:10.1063/1.4940982. hdl:20.500.11820/a69c599d-0b00-4fd5-80bd-1b1493ce56e1.
- ^ a b Liu, Kai; Yan, Qimin; Chen, Michelle; Fan, Wen; Sun, Yinghui; Suh, Joonki; Fu, Deyi; Lee, Sangwook; Zhou, Jian (2014). "Elastic Properties of Chemical-Vapor-Deposited Monolayer MoS2, WS2, and Their Bilayer Heterostructures". Nano Letters. 14 (9): 5097–5103. arXiv:1407.2669. Bibcode:2014NanoL..14.5097L. doi:10.1021/nl501793a. PMID 25120033. S2CID 2136100.
- ^ He, K.; Poole, C.; Mak, K. F.; Shan, J. (2013). "Experimental Demonstration of Continuous Electronic Structure Tuning via Strain in Atomically Thin MoS2". Nano Letters. 13 (6): 2931–6. arXiv:1305.3673. Bibcode:2013NanoL..13.2931H. doi:10.1021/nl4013166. PMID 23675872. S2CID 207691793.
- ^ Conley, H. J.; Wang, B.; Ziegler, J. I.; Haglund, R. F.; Pantelides, S. T.; Bolotin, K. I. (2013). "Bandgap Engineering of Strained Monolayer and Bilayer MoS2". Nano Letters. 13 (8): 3626–30. arXiv:1305.3880. Bibcode:2013NanoL..13.3626C. doi:10.1021/nl4014748. PMID 23819588. S2CID 8191142.
- ^ Zhu, C. R.; Wang, G.; Liu, B. L.; Marie, X.; Qiao, X. F.; Zhang, X.; Wu, X. X.; Fan, H.; Tan, P. H.; Amand, T.; Urbaszek, B. (2013). "Strain tuning of optical emission energy and polarization in monolayer and bilayer MoS2". Physical Review B. 88 (12): 121301. arXiv:1306.3442. Bibcode:2013PhRvB..88l1301Z. doi:10.1103/PhysRevB.88.121301. S2CID 119269184.
- ^ a b Novoselov, K. S.; Jiang, D; Schedin, F; Booth, T. J.; Khotkevich, V. V.; Morozov, S. V.; Geim, A. K. (2005). "Two-dimensional atomic crystals". Proceedings of the National Academy of Sciences. 102 (30): 10451–3. arXiv:cond-mat/0503533. Bibcode:2005PNAS..10210451N. doi:10.1073/pnas.0502848102. PMC 1180777. PMID 16027370.
- ^ Coleman, Jonathan N.; Lotya, Mustafa; O’Neill, Arlene; Bergin, Shane D.; King, Paul J.; Khan, Umar; Young, Karen; Gaucher, Alexandre; De, Sukanta; Smith, Ronan J.; Shvets, Igor V.; Arora, Sunil K.; Stanton, George; Kim, Hye-Young; Lee, Kangho; Kim, Gyu Tae; Duesberg, Georg S.; Hallam, Toby; Boland, John J.; Wang, Jing Jing; Donegan, John F.; Grunlan, Jaime C.; Moriarty, Gregory; Shmeliov, Aleksey; Nicholls, Rebecca J.; Perkins, James M.; Grieveson, Eleanor M.; Theuwissen, Koenraad; McComb, David W.; Nellist, Peter D.; Nicolosi, Valeria (2011). "Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials". Science. 331 (6017): 568–571. Bibcode:2011Sci...331..568C. doi:10.1126/science.1194975. hdl:2262/66458. PMID 21292974. S2CID 23576676.
- ^ Zhou, Jiadong; Lin, Junhao; Huang, Xiangwei; Zhou, Yao; Chen, Yu; Xia, Juan; Wang, Hong; Xie, Yu; Yu, Huimei; Lei, Jincheng; Wu, Di; Liu, Fucai; Fu, Qundong; Zeng, Qingsheng; Hsu, Chuang-Han (2018). "A library of atomically thin metal chalcogenides". Nature. 556 (7701): 355–359. Bibcode:2018Natur.556..355Z. doi:10.1038/s41586-018-0008-3. hdl:10356/84198. ISSN 1476-4687. PMID 29670263. S2CID 4945645.
- ^ a b c Kim, Se-Yang; Kwak, Jinsung; Ciobanu, Cristian V.; Kwon, Soon-Yong (February 2019). "Recent Developments in Controlled Vapor-Phase Growth of 2D Group 6 Transition Metal Dichalcogenides". Advanced Materials. 31 (20): 1804939. Bibcode:2019AdM....3104939K. doi:10.1002/adma.201804939. ISSN 0935-9648. PMID 30706541.
- ^ Shi, Yumeng; Li, Henan; Li, Lain-Jong (2015-04-28). "Recent advances in controlled synthesis of two-dimensional transition metal dichalcogenides via vapour deposition techniques". Chemical Society Reviews. 44 (9): 2744–2756. doi:10.1039/C4CS00256C. ISSN 1460-4744. PMID 25327436.
- ^ "AIXTRON Technologies: MOCVD :: AIXTRON". www.aixtron.com. Retrieved 2019-12-02.
- ^ Lee, Y. H.; Zhang, X. Q.; Zhang, W; Chang, M. T.; Lin, C. T.; Chang, K. D.; Yu, Y. C.; Wang, J. T.; Chang, C. S.; Li, L. J.; Lin, T. W. (2012). "Synthesis of large-area MoS2 atomic layers with chemical vapor deposition". Advanced Materials. 24 (17): 2320–5. arXiv:1202.5458. Bibcode:2012AdM....24.2320L. doi:10.1002/adma.201104798. PMID 22467187. S2CID 11713759.
- ^ Buravets, Vladislav; Hosek, Frantisek; Burtsev, Vasilii; Miliutina, Elena; Maixner, Jaroslav; Lapcak, Ladislav; Bajtosova, Lucia; Cieslar, Miroslav; Procházka, Michal; Minar, Jan; Kolska, Zdenka; Svorcik, Vaclav; Lyutakov, Oleksiy (2024-05-06). "Rapid and Universal Synthesis of 2D Transition Metal (Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, and W) Sulfides through Oxide Sulfurization in CS 2 Vapor". Inorganic Chemistry. 63 (18): 8215–8221. doi:10.1021/acs.inorgchem.4c00475. ISSN 0020-1669. PMC 11080058. PMID 38655681.
- ^ Kim, Ki Seok; Lee, Doyoon; Chang, Celesta S.; Seo, Seunghwan; Hu, Yaoqiao; Cha, Soonyoung; Kim, Hyunseok; Shin, Jiho; Lee, Ju-Hee; Lee, Sangho; Kim, Justin S.; Kim, Ki Hyun; Suh, Jun Min; Meng, Yuan; Park, Bo-In (2023-01-18). "Non-epitaxial single-crystal 2D material growth by geometric confinement". Nature. 614 (7946): 88–94. Bibcode:2023Natur.614...88K. doi:10.1038/s41586-022-05524-0. ISSN 1476-4687. PMID 36653458. S2CID 255970742.
- ^ a b c d e f Wei, Y; Hu, C; Li, Y; Hu, X; Yu, K; Su, L (Jul 2020). "Initial stage of MBE growth of MoSe2 monolayer". Nanotechnology. 31 (31): 315710. Bibcode:2020Nanot..31E5710W. doi:10.1088/1361-6528/ab884b. PMID 32272461. S2CID 215727487. Retrieved 21 July 2022.
- ^ a b Lasek, K; Coelho, P. M; Zberecki, K; Xin, Y; Kolekar, S. K; Li, J; Batzill, M (25 June 2020). "Molecular Beam Epitaxy of Transition Metal (Ti-, V-, and Cr-) Tellurides: From Monolayer Ditellurides to Multilayer Self-Intercalation Compounds". ACS Nano. 14 (7): 8473–8484. doi:10.1021/acsnano.0c02712. PMID 32584543. S2CID 220074214. Retrieved 21 July 2022.
- ^ a b c d Nakano, M; Wang, Y; Kashiwabara, Y; Matsuoka, H; Iwasa, Y (2017). "Layer-by-Layer Epitaxial Growth of Scalable WSe2 on Sapphire by Molecular Beam Epitaxy". Nano Letters. 17 (9): 5595–5599. arXiv:1709.02912. Bibcode:2017NanoL..17.5595N. doi:10.1021/acs.nanolett.7b02420. PMID 28849935. S2CID 206742437. Retrieved 21 July 2022.
- ^ Hall, Joshua; Pielic, Borna; Murray, Clifford; Jolie, Wouter; Wekking, Tobias; Busse, Carsten; Kralj, Marko; Michley, Thomas (2018). "Molecular beam epitaxy of quasi-freestanding transition metal disulphide monolayers on van der Waals substrates: a growth study". 2D Mater. 5 (2): 025005. Bibcode:2018TDM.....5b5005H. doi:10.1088/2053-1583/aaa1c5. S2CID 103289660. Retrieved 28 November 2022.
- ^ Choudury, TH; Zhang, X; Balushi, ZY AL; Chubarov, M; Redwing, JM (Jul 2020). "Epitaxial Growth of Two-Dimensional Layered Transition Metal Dichalcogenides". Annual Review of Materials Research. 50: 155–177. arXiv:1909.03502. Bibcode:2020AnRMS..50..155C. doi:10.1146/annurev-matsci-090519-113456. S2CID 202540441. Retrieved 21 July 2022.
- ^ a b Singh, D. K; Gupta, G (2022). "van der Waals Epitaxy of Transition Metal Dichalcogenides via Molecular Beam Epitaxy: Looking Back and Moving Forward". Materials Advances. 3 (15): 6142–6156. doi:10.1039/D2MA00352J. S2CID 250156491.
- ^ Noori, Y J; Thomas, S; Ramadan, S; Greenacre, V K; Abdelazim, N M; Han, Y; Zhang, J; Beanland, R; Hector, A L; Klein, N; Reid, G; Bartlett, P N; de Groot, C H (2022-01-01). "Electrodeposited WS 2 monolayers on patterned graphene". 2D Materials. 9 (1): 015025. arXiv:2109.00083. Bibcode:2022TDM.....9a5025N. doi:10.1088/2053-1583/ac3dd6. ISSN 2053-1583. S2CID 244693600.
- ^ Noori, Yasir J.; Thomas, Shibin; Ramadan, Sami; Smith, Danielle E.; Greenacre, Vicki K.; Abdelazim, Nema; Han, Yisong; Beanland, Richard; Hector, Andrew L.; Klein, Norbert; Reid, Gillian; Bartlett, Philip N.; Kees de Groot, C. H. (2020-11-04). "Large-Area Electrodeposition of Few-Layer MoS 2 on Graphene for 2D Material Heterostructures". ACS Applied Materials & Interfaces. 12 (44): 49786–49794. arXiv:2005.08616. doi:10.1021/acsami.0c14777. ISSN 1944-8244. PMID 33079533. S2CID 224828493.
- ^ Wan, Xi; Chen, Kun; Chen, Zefeng; Xie, Fangyan; Zeng, Xiaoliang; Xie, Weiguang; Chen, Jian; Xu, Jianbin (May 2017). "Controlled Electrochemical Deposition of Large-Area MoS 2 on Graphene for High-Responsivity Photodetectors". Advanced Functional Materials. 27 (19): 1603998. doi:10.1002/adfm.201603998. S2CID 100120486.
- ^ Thomas, Shibin; Smith, Danielle E.; Greenacre, Victoria K.; Noori, Yasir J.; Hector, Andrew L.; Groot, C. H. (Kees) de; Reid, Gillian; Bartlett, Philip N. (2020-01-06). "Electrodeposition of MoS2 from Dichloromethane". Journal of the Electrochemical Society. 167 (10): 106511. Bibcode:2020JElS..167j6511T. doi:10.1149/1945-7111/ab9c88. ISSN 0013-4651. S2CID 225720683.
- ^ Abdelazim, Nema M.; Noori, Yasir J.; Thomas, Shibin; Greenacre, Victoria K.; Han, Yisong; Smith, Danielle E.; Piana, Giacomo; Zhelev, Nikolay; Hector, Andrew L.; Beanland, Richard; Reid, Gillian; Bartlett, Philip N.; Groot, Cornelis H. (September 2021). "Lateral Growth of MoS 2 2D Material Semiconductors Over an Insulator Via Electrodeposition". Advanced Electronic Materials. 7 (9): 2100419. doi:10.1002/aelm.202100419. ISSN 2199-160X. S2CID 232478824.
- ^ Mak, K. F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T. F. (2010). "Atomically Thin MoS2: A New Direct-Gap Semiconductor". Physical Review Letters. 105 (13): 136805. arXiv:1004.0546. Bibcode:2010PhRvL.105m6805M. doi:10.1103/PhysRevLett.105.136805. PMID 21230799. S2CID 40589037.
- ^ Cheng, Y. C.; Zhu, Z. Y.; Tahir, M.; Schwingenschlogl, U. (2012). "Spin–orbit–induced spin splittings in polar transition metal dichalcogenide monolayers". EPL. 102 (5): 57001. Bibcode:2013EL....10257001C. doi:10.1209/0295-5075/102/57001. S2CID 121978058.
- ^ a b Liu, G. B.; Shan, W. Y.; Yao, Y.; Yao, W.; Xiao, D. (2013). "Three-band tight-binding model for monolayers of group-VIB transition metal dichalcogenides". Physical Review B. 88 (8): 085433. arXiv:1305.6089. Bibcode:2013PhRvB..88h5433L. doi:10.1103/PhysRevB.88.085433. S2CID 119111681.
- ^ Zhu, Z.; Cheng, Y. C.; Schwingenschlogl, U. (2011). "Giant spin–orbit-induced spin splitting in two-dimensional transition-metal dichalcogenide semiconductors". Physical Review B. 84 (15): 153402. Bibcode:2011PhRvB..84o3402Z. doi:10.1103/PhysRevB.84.153402. hdl:10754/315771.
- ^ Kośmider, K.; González, J. W.; Fernández-Rossier, J. (2013). "Large spin splitting in the conduction band of transition metal dichalcogenide monolayers". Physical Review B. 88 (24): 245436. arXiv:1311.0049. Bibcode:2013PhRvB..88x5436K. doi:10.1103/PhysRevB.88.245436. S2CID 31176688.
- ^ Kormányos, A.; Zólyomi, V.; Drummond, N. D.; Burkard, G. (2014). "Spin–Orbit Coupling, Quantum Dots, and Qubits in Monolayer Transition Metal Dichalcogenides". Physical Review X. 4 (1): 011034. arXiv:1310.7720. Bibcode:2014PhRvX...4a1034K. doi:10.1103/PhysRevX.4.011034. S2CID 17909293.
- ^ Bussolotti, Fabio; Kawai, Hiroyo; Ooi, Zi En; Chellappan, Vijila; Thian, Dickson; Pang, Ai Lin Christina; Goh, Kuan Eng Johnson (2018). "Roadmap on finding chiral valleys: screening 2D materials for valleytronics". Nano Futures. 2 (3): 032001. Bibcode:2018NanoF...2c2001B. doi:10.1088/2399-1984/aac9d7. S2CID 139826293.
- ^ a b Chernikov, Alexey; Berkelbach, Timothy C.; Hill, Heather M.; Rigosi, Albert; Li, Yilei; Aslan, Ozgur Burak; Reichman, David R.; Hybertsen, Mark S.; Heinz, Tony F. (2014). "Exciton Binding Energy and Nonhydrogenic Rydberg Series in Monolayer WS2". Physical Review Letters. 113 (7): 076802. arXiv:1403.4270. Bibcode:2014PhRvL.113g6802C. doi:10.1103/PhysRevLett.113.076802. PMID 25170725. S2CID 23157872.
- ^ Ye, Ziliang; Cao, Ting; O’Brien, Kevin; Zhu, Hanyu; Yin, Xiaobo; Wang, Yuan; Louie, Steven G.; Zhang, Xiang (2014). "Probing excitonic dark states in single-layer tungsten disulphide". Nature. 513 (7517): 214–218. arXiv:1403.5568. Bibcode:2014Natur.513..214Y. doi:10.1038/nature13734. PMID 25162523. S2CID 4461357.
- ^ Ugeda, M. M.; Bradley, A. J.; Shi, S. F.; Da Jornada, F. H.; Zhang, Y.; Qiu, D. Y.; Ruan, W.; Mo, S. K.; Hussain, Z.; Shen, Z. X.; Wang, F.; Louie, S. G.; Crommie, M. F. (2014). "Giant bandgap renormalization and excitonic effects in a monolayer transition metal dichalcogenide semiconductor". Nature Materials. 13 (12): 1091–1095. arXiv:1404.2331. Bibcode:2014NatMa..13.1091U. doi:10.1038/nmat4061. PMID 25173579. S2CID 25491405.
- ^ a b Manca, M.; Glazov, M. M.; Robert, C.; Cadiz, F.; Taniguchi, T.; Watanabe, K.; Courtade, E.; Amand, T.; Renucci, P.; Marie, X.; Wang, G.; Urbaszek, B. (2017). "Enabling valley selective exciton scattering in monolayer WSe2 through upconversion". Nat. Commun. 8: 14927. arXiv:1701.05800. Bibcode:2017NatCo...814927M. doi:10.1038/ncomms14927. PMC 5382264. PMID 28367962.
- ^ Ross, J. S.; et al. (2013). "Electrical control of neutral and charged excitons in a monolayer semiconductor". Nat. Commun. 4: 1474. arXiv:1211.0072. Bibcode:2013NatCo...4.1474R. doi:10.1038/ncomms2498. PMID 23403575. S2CID 9872370.
- ^ Mak, K. F.; et al. (2013). "Tightly bound trions in monolayer MoS2". Nat. Mater. 12 (3): 207–211. arXiv:1210.8226. Bibcode:2013NatMa..12..207M. doi:10.1038/nmat3505. PMID 23202371. S2CID 205408065.
- ^ Cadiz, F.; Courtade, E.; Robert, C.; Wang, G.; Shen, Y.; Cai, H.; Taniguchi, T.; Watanabe, K.; Carrere, H.; Lagarde, D.; Manca, M.; Amand, T.; Renucci, P.; Tongay, S.; Marie, X.; Urbaszek, B. (2017). "Excitonic linewidth approaching the homogeneous limit in MoS2 based van der Waals heterostructures : accessing spin-valley dynamics". Physical Review X. 7 (2): 021026. arXiv:1702.00323. Bibcode:2017PhRvX...7b1026C. doi:10.1103/PhysRevX.7.021026. S2CID 55508192.
- ^ Mai, C.; et al. (2014). "Many-Body Effects in Valleytronics: Direct Measurement of Valley Lifetimes in Single-Layer MoS2". Nano Lett. 14 (1): 202–206. Bibcode:2014NanoL..14..202M. doi:10.1021/nl403742j. PMID 24325650.
- ^ Shang, J.; et al. (2015). "Observation of Excitonic Fine Structure in a 2D Transition-Metal Dichalcogenide Semiconductor". ACS Nano. 9 (1): 647–655. doi:10.1021/nn5059908. PMID 25560634.
- ^ Mostaani, E.; et al. (2017). "Diffusion quantum Monte Carlo study of excitonic complexes in two-dimensional transition-metal dichalcogenides". Physical Review B. 96 (7): 075431. arXiv:1706.04688. Bibcode:2017PhRvB..96g5431M. doi:10.1103/PhysRevB.96.075431. S2CID 46144082.
- ^ Kern, Johannes; Niehues, Iris; Tonndorf, Philipp; Schmidt, Robert; Wigger, Daniel; Schneider, Robert; Stiehm, Torsten; Michaelis de Vasconcellos, Steffen; Reiter, Doris E. (September 2016). "Nanoscale Positioning of Single-Photon Emitters in Atomically Thin WSe2". Advanced Materials. 28 (33): 7101–7105. Bibcode:2016AdM....28.7101K. doi:10.1002/adma.201600560. PMID 27305430. S2CID 205268567.
- ^ He, Yu-Ming; Clark, Genevieve; Schaibley, John R.; He, Yu; Chen, Ming-Cheng; Wei, Yu-Jia; Ding, Xing; Zhang, Qiang; Yao, Wang (2015). "Single quantum emitters in monolayer semiconductors". Nature Nanotechnology. 10 (6): 497–502. arXiv:1411.2449. Bibcode:2015NatNa..10..497H. doi:10.1038/nnano.2015.75. PMID 25938571. S2CID 205454184.
- ^ Palacios-Berraquero, Carmen; Kara, Dhiren M.; Montblanch, Alejandro R.-P.; Barbone, Matteo; Latawiec, Pawel; Yoon, Duhee; Ott, Anna K.; Loncar, Marko; Ferrari, Andrea C. (August 2017). "Large-scale quantum-emitter arrays in atomically thin semiconductors". Nature Communications. 8 (1): 15093. arXiv:1609.04244. Bibcode:2017NatCo...815093P. doi:10.1038/ncomms15093. PMC 5458119. PMID 28530249.
- ^ Palacios-Berraquero, Carmen; Barbone, Matteo; Kara, Dhiren M.; Chen, Xiaolong; Goykhman, Ilya; Yoon, Duhee; Ott, Anna K.; Beitner, Jan; Watanabe, Kenji (December 2016). "Atomically thin quantum light-emitting diodes". Nature Communications. 7 (1): 12978. arXiv:1603.08795. Bibcode:2016NatCo...712978P. doi:10.1038/ncomms12978. PMC 5052681. PMID 27667022.
- ^ Wu, Wei; Dass, Chandriker K.; Hendrickson, Joshua R.; Montaño, Raul D.; Fischer, Robert E.; Zhang, Xiaotian; Choudhury, Tanushree H.; Redwing, Joan M.; Wang, Yongqiang (2019). "Locally defined quantum emission from epitaxial few-layer tungsten diselenide". Applied Physics Letters. 114 (21): 213102. Bibcode:2019ApPhL.114u3102W. doi:10.1063/1.5091779. hdl:10150/634575.
- ^ Dass, Chandriker Kavir; Khan, Mahtab A.; Clark, Genevieve; Simon, Jeffrey A.; Gibson, Ricky; Mou, Shin; Xu, Xiaodong; Leuenberger, Michael N.; Hendrickson, Joshua R. (2019). "Ultra-Long Lifetimes of Single Quantum Emitters in Monolayer WSe2/hBN Heterostructures". Advanced Quantum Technologies. 2 (5–6): 1900022. doi:10.1002/qute.201900022.
- ^ Srour, J.R.; McGarity, J.M. (1988). "Radiation effects on microelectronics in space". Proc. IEEE. 76 (11): 1443–1469. doi:10.1109/5.90114.
- ^ Walker, R.C.; Shi, T. (2016). "Radiation effects on two-dimensional materials". Proc. Physical Status Solida.
- ^ Freitag, M.; Low, T.; Avouris, P. (2013). "Increased responsivity of suspended graphene photodetectors". Nano Letters. 13 (4): 1644–1648. Bibcode:2013NanoL..13.1644F. doi:10.1021/nl4001037. PMID 23452264.
- ^ Lee, H.S. (2012). "MoS2 nanosheet phototransistors with thickness-modulated optical energy gap". Nano Letters. 12 (7): 3695–3700. Bibcode:2012NanoL..12.3695L. doi:10.1021/nl301485q. PMID 22681413.
- ^ Liu, F.; Shimotani, H.; Shang, H. (2014). "High-sensitivity photodetectors based on multilayer GaTe flakes". ACS Nano. 8 (1): 752–760. doi:10.1021/nn4054039. PMID 24364508.
- ^ a b Lu, Ang-Yu; Zhu, Hanyu; Xiao, Jun; Chuu, Chih-Piao; Han, Yimo; Chiu, Ming-Hui; Cheng, Chia-Chin; Yang, Chih-Wen; Wei, Kung-Hwa; Yang, Yiming; Wang, Yuan; Sokaras, Dimosthenis; Nordlund, Dennis; Yang, Peidong; Muller, David A.; Chou, Mei-Yin; Zhang, Xiang; Li, Lain-Jong (2017). "Janus monolayers of transition metal dichalcogenides". Nature Nanotechnology. 12 (8): 744–749. doi:10.1038/nnano.2017.100. PMID 28507333. S2CID 42620382.
- ^ Cheng, Y. C.; Zhu, Z. Y.; Tahir, M.; Schwingenschlögl, U. (2013). "Spin–orbit–induced spin splittings in polar transition metal dichalcogenide monolayers". EPL (Europhysics Letters). 102 (5): 57001. Bibcode:2013EL....10257001C. doi:10.1209/0295-5075/102/57001. S2CID 121978058.
- ^ Li, Fengping; Wei, Wei; Zhao, Pei; Huang, Baibiao; Dai, Ying (2017). "Electronic and Optical Properties of Pristine and Vertical and Lateral Heterostructures of Janus MoSSe and WSSe". The Journal of Physical Chemistry Letters. 8 (23): 5959–5965. doi:10.1021/acs.jpclett.7b02841. PMID 29169238.
- ^ Dong, Liang; Lou, Jun; Shenoy, Vivek B. (2017). "Large In-Plane and Vertical Piezoelectricity in Janus Transition Metal Dichalchogenides [sic]". ACS Nano. 11 (8): 8242–8248. doi:10.1021/acsnano.7b03313. PMID 28700210.
- ^ Zhang, Jing; Jia, Shuai; Kholmanov, Iskandar; Dong, Liang; Er, Dequan; Chen, Weibing; Guo, Hua; Jin, Zehua; Shenoy, Vivek B. (2017). "Janus Monolayer Transition-Metal Dichalcogenides". ACS Nano. 11 (8): 8192–8198. arXiv:1704.06389. doi:10.1021/acsnano.7b03186. PMID 28771310. S2CID 31445401.
- ^ Ma, Xiangchao; Wu, Xin; Wang, Haoda; Wang, Yucheng (2018). "A Janus MoSSe monolayer: a potential wide solar-spectrum water-splitting photocatalyst with a low carrier recombination rate". Journal of Materials Chemistry A. 6 (5): 2295–2301. doi:10.1039/c7ta10015a.
- ^ Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. (2011). "Single-layer MoS2 transistors". Nature Nanotechnology. 6 (3): 147–50. Bibcode:2011NatNa...6..147R. doi:10.1038/nnano.2010.279. PMID 21278752.
- ^ Choi, Wonbong; Choudhary, Nitin; Han, Gang Hee; Park, Juhong; Akinwande, Deji; Lee, Young Hee (2017-04-01). "Recent development of two-dimensional transition metal dichalcogenides and their applications". Materials Today. 20 (3): 116–130. doi:10.1016/j.mattod.2016.10.002. ISSN 1369-7021.
- ^ Syu, Yu-Cheng; Hsu, Wei-En; Lin, Chih-Ting (2018-01-01). "Review—Field-Effect Transistor Biosensing: Devices and Clinical Applications". ECS Journal of Solid State Science and Technology. 7 (7): Q3196–Q3207. doi:10.1149/2.0291807jss. ISSN 2162-8769.
- ^ a b Barua, Shaswat; Dutta, Hemant Sankar; Gogoi, Satyabrat; Devi, Rashmita; Khan, Raju (2018-01-26). "Nanostructured MoS2-Based Advanced Biosensors: A Review". ACS Applied Nano Materials. 1 (1): 2–25. doi:10.1021/acsanm.7b00157.
- ^ Hu, Yanling; Huang, Ying; Tan, Chaoliang; Zhang, Xiao; Lu, Qipeng; Sindoro, Melinda; Huang, Xiao; Huang, Wei; Wang, Lianhui; Zhang, Hua (2016-11-30). "Two-dimensional transition metal dichalcogenide nanomaterials for biosensing applications". Materials Chemistry Frontiers. 1 (1): 24–36. doi:10.1039/C6QM00195E. ISSN 2052-1537.
- ^ a b c d e f g h i j k l Rao, C. N. R; Maitra, Urmimala (2015-01-01). "Inorganic Graphene Analogs". Annual Review of Materials Research. 45 (1): 29–62. Bibcode:2015AnRMS..45...29R. doi:10.1146/annurev-matsci-070214-021141.
- ^ a b c d e f g h Li, Xiao; Zhu, Hongwei (2015-03-01). "Two-dimensional MoS2: Properties, preparation, and applications". Journal of Materiomics. 1 (1): 33–44. doi:10.1016/j.jmat.2015.03.003.
- ^ Mak, Kin Fai; Lee, Changgu; Hone, James; Shan, Jie; Heinz, Tony F. (2010). "Atomically ThinMoS2: A New Direct-Gap Semiconductor". Physical Review Letters. 105 (13): 136805. arXiv:1004.0546. Bibcode:2010PhRvL.105m6805M. doi:10.1103/physrevlett.105.136805. PMID 21230799. S2CID 40589037.
- ^ Najmaei, Sina; Zou, Xiaolong; Er, Dequan; Li, Junwen; Jin, Zehua; Gao, Weilu; Zhang, Qi; Park, Sooyoun; Ge, Liehui (2014-03-12). "Tailoring the Physical Properties of Molybdenum Disulfide Monolayers by Control of Interfacial Chemistry". Nano Letters. 14 (3): 1354–1361. Bibcode:2014NanoL..14.1354N. CiteSeerX 10.1.1.642.1938. doi:10.1021/nl404396p. PMID 24517325.
- ^ a b c Conley, Hiram J.; Wang, Bin; Ziegler, Jed I.; Haglund, Richard F.; Pantelides, Sokrates T.; Bolotin, Kirill I. (2013). "Bandgap Engineering of Strained Monolayer and Bilayer MoS2". Nano Letters. 13 (8): 3626–3630. arXiv:1305.3880. Bibcode:2013NanoL..13.3626C. doi:10.1021/nl4014748. PMID 23819588. S2CID 8191142.
- ^ a b c d e f g Rao, C. N. R.; Ramakrishna Matte, H. S. S.; Maitra, Urmimala (2013-12-09). "Graphene Analogues of Inorganic Layered Materials". Angewandte Chemie International Edition. 52 (50): 13162–13185. doi:10.1002/anie.201301548. PMID 24127325.
- ^ Wu, Wenzhuo; Wang, Lei; Li, Yilei; Zhang, Fan; Lin, Long; Niu, Simiao; Chenet, Daniel; Zhang, Xian; Hao, Yufeng (2014-10-23). "Piezoelectricity of single-atomic-layer MoS2 for energy conversion and piezotronics". Nature. 514 (7523): 470–474. Bibcode:2014Natur.514..470W. doi:10.1038/nature13792. PMID 25317560. S2CID 4448528.
- ^ Lee, Kangho; Kim, Hye-Young; Lotya, Mustafa; Coleman, Jonathan N.; Kim, Gyu-Tae; Duesberg, Georg S. (2011-09-22). "Electrical Characteristics of Molybdenum Disulfide Flakes Produced by Liquid Exfoliation". Advanced Materials. 23 (36): 4178–4182. Bibcode:2011AdM....23.4178L. doi:10.1002/adma.201101013. PMID 21823176. S2CID 205240634.
- ^ a b c Yan, Rusen; Simpson, Jeffrey R.; Bertolazzi, Simone; Brivio, Jacopo; Watson, Michael; Wu, Xufei; Kis, Andras; Luo, Tengfei; Walker, Angela R. Hight (2014). "Thermal Conductivity of Monolayer Molybdenum Disulfide Obtained from Temperature-Dependent Raman Spectroscopy". ACS Nano. 8 (1): 986–993. doi:10.1021/nn405826k. PMID 24377295.
- ^ Backes, Claudia; et al. (2020). "Production and processing of graphene and related materials". 2D Materials. 7 (2): 022001. Bibcode:2020TDM.....7b2001B. doi:10.1088/2053-1583/ab1e0a. hdl:2262/91730.
- ^ a b c d e Kannan, Padmanathan Karthick; Late, Dattatray J.; Morgan, Hywel; Rout, Chandra Sekhar (2015-08-06). "Recent developments in 2D layered inorganic nanomaterials for sensing". Nanoscale. 7 (32): 13293–13312. Bibcode:2015Nanos...713293K. doi:10.1039/c5nr03633j. PMID 26204797.
- ^ Noori, Yasir J.; Thomas, Shibin; Ramadan, Sami; Smith, Danielle E.; Greenacre, Vicki K.; Abdelazim, Nema; Han, Yisong; Beanland, Richard; Hector, Andrew L.; Klein, Norbert; Reid, Gillian (2020-11-04). "Large-Area Electrodeposition of Few-Layer MoS2 on Graphene for 2D Material Heterostructures". ACS Applied Materials & Interfaces. 12 (44): 49786–49794. arXiv:2005.08616. doi:10.1021/acsami.0c14777. ISSN 1944-8244. PMID 33079533. S2CID 224828493.
- ^ Thomas, Shibin; Smith, Danielle E.; Greenacre, Victoria K.; Noori, Yasir J.; Hector, Andrew L.; Groot, C. H. (Kees) de; Reid, Gillian; Bartlett, Philip N. (2020-06-23). "Electrodeposition of MoS2 from Dichloromethane". Journal of the Electrochemical Society. 167 (10): 106511. Bibcode:2020JElS..167j6511T. doi:10.1149/1945-7111/ab9c88. ISSN 1945-7111.
- ^ Murugesan, Sankaran; Akkineni, Arunkumar; Chou, Brendan P.; Glaz, Micah S.; Vanden Bout, David A.; Stevenson, Keith J. (2013-09-24). "Room Temperature Electrodeposition of Molybdenum Sulfide for Catalytic and Photoluminescence Applications". ACS Nano. 7 (9): 8199–8205. doi:10.1021/nn4036624. ISSN 1936-0851. PMID 23962095.
- ^ Wang, Tanyuan; Zhuo, Junqiao; Du, Kuangzhou; Chen, Bingbo; Zhu, Zhiwei; Shao, Yuanhua; Li, Meixian (2014). "Electrochemically Fabricated Polypyrrole and MoSx Copolymer Films as a Highly Active Hydrogen Evolution Electrocatalyst". Advanced Materials. 26 (22): 3761–3766. Bibcode:2014AdM....26.3761W. doi:10.1002/adma.201400265. ISSN 1521-4095. PMID 24638848. S2CID 205254714.
- ^ Wu, Haihua; Yang, Rong; Song, Baomin; Han, Qiusen; Li, Jingying; Zhang, Ying; Fang, Yan; Tenne, Reshef; Wang, Chen (2011). "Biocompatible Inorganic Fullerene-Like Molybdenum Disulfide Nanoparticles Produced by Pulsed Laser Ablation in Water". ACS Nano. 5 (2): 1276–1281. doi:10.1021/nn102941b. PMID 21230008.
- ^ Kaur, Harneet (2016). "High Yield Synthesis and Chemical Exfoliation of Two-Dimensional Layered Hafnium Disulphide". Nano Research. 11: 343–353. arXiv:1611.00895. doi:10.1007/s12274-017-1636-x. S2CID 99414438.
- ^ Schutte, W.J.; De Boer, J.L.; Jellinek, F. (1986). "Crystal Structures of Tungsten Disulfide and Diselenide". Journal of Solid State Chemistry. 70 (2): 207–209. Bibcode:1987JSSCh..70..207S. doi:10.1016/0022-4596(87)90057-0.
- ^ Schmidt, Robert; Niehues, Iris; Schneider, Robert; Drüppel, Matthias; Deilmann, Thorsten; Rohlfing, Michael; Michaelis de Vasconcellos, Steffen; Castellanos-Gomez, Andres; Bratschitsch, Rudolf (2016). "Reversible Uniaxial Strain Tuning in Atomically thin WSe2". 2D Materials. 3 (2): 021011. Bibcode:2016TDM.....3b1011S. doi:10.1088/2053-1583/3/2/021011. S2CID 138241397.
- ^ Wu, Wei; Wang, Jin; Ercius, Peter; Wright, Nicomario; Leppert-Simenauer, Danielle; Burke, Robert; Dubey, Madan; Dongare, Avinash; Pettes, Michael (2018). "Giant Mechano-Optoelectronic Effect in an Atomically Thin Semiconductor". Nano Letters. 18 (4): 2351–2357. Bibcode:2018NanoL..18.2351W. doi:10.1021/acs.nanolett.7b05229. OSTI 1432708. PMID 29558623. S2CID 206746478.
External links
[edit]- Wood, Charlie (2022-08-16). "Physics Duo Finds Magic in Two Dimensions". Quanta Magazine.
 KSF
KSF