Tuberculosis
 From Wikipedia - Reading time: 56 min
From Wikipedia - Reading time: 56 min
| Tuberculosis | |
|---|---|
| Other names | Phthisis, phthisis pulmonalis, consumption, great white plague |
 | |
| Chest X-ray of a person with advanced tuberculosis: Infection in both lungs is marked by white arrow-heads, and the formation of a cavity is marked by black arrows. | |
| Specialty | Infectious disease, pulmonology |
| Symptoms | Chronic cough, fever, cough with bloody mucus, weight loss. Latent TB infection is asymptomatic[1] |
| Causes | Mycobacterium tuberculosis[1] |
| Risk factors | Immunodeficiency[1] |
| Diagnostic method | CXR, microbial culture, TB skin test, interferon gamma release assay[1] |
| Differential diagnosis | Pneumonia, histoplasmosis, sarcoidosis, coccidioidomycosis[2] |
| Prevention | Screening those at high risk, treatment of those infected, vaccination with bacillus Calmette-Guérin (BCG)[1] |
| Treatment | Antibiotics[1] |
| Frequency | 10.8 million new infections per year[1] |
| Deaths | 1.25 million per year[1] |
Tuberculosis (TB), also known colloquially as the "white death", or historically as consumption,[3] is a contagious disease usually caused by Mycobacterium tuberculosis (MTB) bacteria.[4] Tuberculosis generally affects the lungs, but it can also affect other parts of the body.[1] Most infections show no symptoms, in which case it is known as inactive or latent tuberculosis.[4] A small proportion of latent infections progress to active disease that, if left untreated, can be fatal.[1] Typical symptoms of active TB are chronic cough with blood-containing mucus, fever, night sweats, and weight loss.[1] Infection of other organs can cause a wide range of symptoms.[5]
Tuberculosis is spread from one person to the next through the air when people who have active TB in their lungs cough, spit, speak, or sneeze.[1][4] People with latent TB do not spread the disease.[1] A latent infection is more likely to become active in those with weakened immune systems.[1] There are two principal tests for TB: interferon-gamma release assay (IGRA) of a blood sample, and the tuberculin skin test.[1][6]
Prevention of TB involves screening those at high risk, early detection and treatment of cases, and vaccination with the bacillus Calmette-Guérin (BCG) vaccine.[7][8][9] Those at high risk include household, workplace, and social contacts of people with active TB.[8] Treatment requires the use of multiple antibiotics over a long period of time.[1]
Tuberculosis has been present in humans since ancient times.[10] In the 1800s, when it was known as consumption, it was responsible for an estimated quarter of all deaths in Europe.[11] The incidence of TB decreased during the 20th century with improvement in sanitation and the introduction of drug treatments including antibiotics.[12] However, since the 1980s, antibiotic resistance has become a growing problem, with increasing rates of drug-resistant tuberculosis.[1][13] It is estimated that one quarter of the world's population have latent or active TB.[14] In 2023, TB is estimated to have newly infected 10.8 million people and caused 1.25 million deaths, making it the leading cause of death from an infectious disease.[1][15]
History
[edit]
Tuberculosis has existed since antiquity.[10] The oldest unambiguously detected M. tuberculosis gives evidence of the disease in the remains of bison in Wyoming dated to around 17,000 years ago.[16] However, whether tuberculosis originated in bovines, then transferred to humans, or whether both bovine and human tuberculosis diverged from a common ancestor, remains unclear.[17] A comparison of the genes of M. tuberculosis complex (MTBC) in humans to MTBC in animals suggests humans did not acquire MTBC from animals during animal domestication, as researchers previously believed. Both strains of the tuberculosis bacteria share a common ancestor, which could have infected humans even before the Neolithic Revolution.[18] Skeletal remains show some prehistoric humans (4000 BC) had TB, and researchers have found tubercular decay in the spines of Egyptian mummies dating from 3000 to 2400 BC.[19] Genetic studies suggest the presence of TB in the Americas from about AD 100.[20]
Identification
[edit]Although Richard Morton established the pulmonary form associated with tubercles as a pathology in 1689,[21][22] due to the variety of its symptoms, TB was not identified as a single disease until the 1820s. Benjamin Marten conjectured in 1720 that consumptions were caused by microbes which were spread by people living close to each other.[23] In 1819, René Laennec claimed that tubercles were the cause of pulmonary tuberculosis.[24] J. L. Schönlein first published the name "tuberculosis" (German: Tuberkulose) in 1832.[25][26]
Between 1838 and 1845, John Croghan, the owner of Mammoth Cave in Kentucky from 1839 onwards, brought a number of people with tuberculosis into the cave in the hope of curing the disease with the constant temperature and purity of the cave air; each died within a year.[27] Hermann Brehmer opened the first TB sanatorium in 1859 in Görbersdorf (now Sokołowsko) in Silesia.[28] In 1865, Jean Antoine Villemin demonstrated that tuberculosis could be transmitted, via inoculation, from humans to animals and among animals.[29] (Villemin's findings were confirmed in 1867 and 1868 by John Burdon-Sanderson.[30])

Robert Koch identified and described the bacillus causing tuberculosis, M. tuberculosis, on 24 March 1882.[31][32] In 1905, he was awarded the Nobel Prize in Physiology or Medicine for this discovery.[33]
Development of treatments
[edit]In Europe, rates of tuberculosis began to rise in the early 1600s to a peak level in the 1800s, when it caused nearly 25% of all deaths.[11] In the 18th and 19th century, tuberculosis had become epidemic in Europe, showing a seasonal pattern.[34][35] Tuberculosis caused widespread public concern in the 19th and early 20th centuries as the disease became common among the urban poor. In 1815, one in four deaths in England was due to "consumption". By 1918, TB still caused one in six deaths in France.[citation needed]
After TB was determined to be contagious, in the 1880s, it was put on a notifiable-disease list in Britain. Campaigns started to stop people from spitting in public places, and the infected poor were "encouraged" to enter sanatoria that resembled prisons. The sanatoria for the middle and upper classes offered excellent care and constant medical attention.[28] What later became known as the Alexandra Hospital for Children with Hip Disease (tuberculous arthritis) was opened in London in 1867.[36] Whatever the benefits of the "fresh air" and labor in the sanatoria, even under the best conditions, 50% of those who entered died within five years (c. 1916).[28]
Robert Koch did not believe the cattle and human tuberculosis diseases were similar, which delayed the recognition of infected milk as a source of infection. During the first half of the 1900s, the risk of transmission from this source was dramatically reduced after the application of the pasteurization process. Koch announced a glycerine extract of the tubercle bacilli as a "remedy" for tuberculosis in 1890, calling it "tuberculin". Although it was not effective, it was later successfully adapted as a screening test for the presence of pre-symptomatic tuberculosis.[37] World Tuberculosis Day is marked on 24 March each year, the anniversary of Koch's original scientific announcement. When the Medical Research Council formed in Britain in 1913, it initially focused on tuberculosis research.[38]
Albert Calmette and Camille Guérin achieved the first genuine success in immunization against tuberculosis in 1906, using attenuated bovine-strain tuberculosis. It was called bacille Calmette–Guérin (BCG). The BCG vaccine was first used on humans in 1921 in France,[39] but achieved widespread acceptance in the US, Great Britain, and Germany only after World War II.[40]
In 1946, the development of the antibiotic streptomycin made effective treatment and cure of TB a reality. Prior to the introduction of this medication, the only treatment was surgical intervention, including the "pneumothorax technique", which involved collapsing an infected lung to "rest" it and to allow tuberculous lesions to heal.[41]
By the 1950s mortality in Europe had decreased about 90%. Improvements in sanitation, vaccination, and other public-health measures began significantly reducing rates of tuberculosis even before the arrival of streptomycin and other antibiotics, although the disease remained a significant threat.[citation needed]
Drug resistant tuberculosis
[edit]However, a few years after the first antibiotic treatment for TB in 1943, some strains of the TB bacteria developed resistance to the standard drugs (streptomycin, para-aminosalicylic acid, and isoniazid).[42] Between 1970 and 1990, there were numerous outbreaks of drug-resistant tuberculosis involving strains resistant to two or more drugs; these strains are called multi-drug resistant TB (MDR-TB).[42] The resurgence of tuberculosis, caused in part by drug resistance and in part by the HIV pandemic, resulted in the declaration of a global health emergency by the World Health Organization (WHO) in 1993.[43][44]
Treatment of MDR-TB requires treatment with second-line drugs, which in general are less effective, more toxic and more expensive than first-line drugs.[45] Treatment regimes can run for two years, compared to the six months of first-line drug treatment.[46][47]
Signs and symptoms
[edit]

There is a popular misconception that tuberculosis is purely a disease of the lungs that manifests as coughing.[49] Tuberculosis may infect many organs, even though it most commonly occurs in the lungs (known as pulmonary tuberculosis).[5] Extrapulmonary TB occurs when tuberculosis develops outside of the lungs, although extrapulmonary TB may coexist with pulmonary TB.[5]
General signs and symptoms include fever, chills, night sweats, loss of appetite, weight loss, and fatigue.[5] In severe cases, nail clubbing may also occur.[50]
Latent tuberculosis
[edit]The majority of individuals with TB infection show no symptoms, a state known as inactive or latent tuberculosis.[4] This condition is not contagious, and can be detected by the tuberculin skin test (TST) and the interferon-gamma release assay (IGRA); other tests should be conducted to eliminate the possibility of active TB.[51] Without treatment, an estimated 5% to 15% of cases will progress into active TB during the person's lifetime.[51]
Pulmonary
[edit]If a tuberculosis infection does become active, it most commonly involves the lungs (in about 90% of cases).[10][52] Symptoms may include chest pain, a prolonged cough producing sputum which may be bloody, tiredness, temperature, loss of appetite, wasting and general malaise.[10][53] In very rare cases, the infection may erode into the pulmonary artery or a Rasmussen aneurysm, resulting in massive bleeding.[5][54]
Tuberculosis may cause extensive scarring of the lungs, which persists after successful treatment of the disease. Survivors continue to experience chronic respiratory symptoms such as cough, sputum production, and shortness of breath.[55][56]
Extrapulmonary
[edit]In 15–20% of active cases, the infection spreads outside the lungs, causing other kinds of TB.[57] These are collectively denoted as extrapulmonary tuberculosis.[58] Extrapulmonary TB occurs more commonly in people with a weakened immune system and young children. In those with HIV, this occurs in more than 50% of cases.[58] Notable extrapulmonary infection sites include the pleura (in tuberculous pleurisy), the central nervous system (in tuberculous meningitis), the lymphatic system (in scrofula of the neck), the genitourinary system (in urogenital tuberculosis), and the bones and joints (in Pott disease of the spine), among others. A potentially more serious, widespread form of TB is called "disseminated tuberculosis"; it is also known as miliary tuberculosis.[5] Miliary TB currently makes up about 10% of extrapulmonary cases.[59]
Symptoms of extrapulmonary TB include the general signs and symptoms as above, with additional symptoms related to the part of the body which is affected.[60]
Causes
[edit]Mycobacteria
[edit]
The main cause of TB is Mycobacterium tuberculosis (MTB), a small, aerobic, nonmotile bacillus.[5] It divides every 16 to 20 hours, which is slow compared with other bacteria, which usually divide in less than an hour.[61] Mycobacteria have a complex, lipid-rich cell envelope, with the high lipid content of the outer membrane acting as a robust barrier contributing to their drug resistance.[62][63] If a Gram stain is performed, MTB either stains very weakly "Gram-positive" or does not retain dye as a result of the high lipid and mycolic acid content of its cell wall.[64] MTB can withstand weak disinfectants and survive in a dry state for weeks. In nature, the bacterium can grow only within the cells of a host organism, but M. tuberculosis can be cultured in the laboratory.[65]
The term M. tuberculosis complex describes a genetically related group of Mycobacterium species that can cause tuberculosis in humans or other animals. It includes four other TB-causing mycobacteria: M. bovis, M. africanum, M. canettii, and M. microti.[66] M. bovis causes bovine TB and was once a common cause of human TB, but the introduction of pasteurized milk has almost eliminated this as a public health problem in developed countries.[67][68] M. africanum is not widespread, but it is a significant cause of human TB in parts of Africa.[69][70] M. canettii is rare and seems to be limited to the Horn of Africa, although a few cases have been seen in African emigrants.[71][72] M. microti appears to have a natural reservoir in small rodents such as mice and voles, but can infect larger mammals. It is rare in humans and is seen almost only in immunodeficient people, although its prevalence may be significantly underestimated.[73][74]
There are other known mycobacteria which cause lung disease resembling TB. M. avium complex is an environmental microorganism found in soil and water sources worldwide, which tends to present as an opportunistic infection in immunocompromised people.[75][76] The natural reservoir of M. kansasii is unknown, but it has been found in tap water; it is most likely to infect humans with lung disease or who smoke.[77] These two species are classified as "nontuberculous mycobacteria".[78]

Transmission
[edit]Tuberculosis spreads through the air when people with active pulmonary TB cough, sneeze, speak, or sing, releasing tiny airborne droplets containing the bacteria. Anyone nearby can breathe in these droplets and become infected. The droplets can remain airborne and infective for several hours, and are more likely to persist in poorly ventilated areas.[79]
Risk factors
[edit]Risk factors for TB include exposure to droplets from people with active TB and environmental-related and health-condition related factors that decrease a person's immune system response such as HIV or taking immunosuppressant medications.[80]
Close contact
[edit]Prolonged, frequent, or close contact with people who have active TB is a high high risk factor for becoming infected; this group includes health care workers and children where a family member is infected.[81][82] Transmission is most likely to occur from only people with active TB – those with latent infection are not thought to be contagious.[67] Environmental risk factors which put a person at closer contact with infective droplets from a person infected with TB are overcrowding, poor ventilation, or close proximity to a potentially infective person.[83][84]
Immunodeficiencies
[edit]The most important risk factor globally for developing active TB is concurrent human immunodeficiency virus (HIV) infection; in 2023, 6.1% of those becoming infected with TB were also infected with HIV.[15] Sub-Saharan Africa has a particularly high burden of HIV-associated TB.[1] Of those without HIV infection who are infected with tuberculosis, about 5–15% develop active disease during their lifetimes;[51] in contrast, 30% of those co-infected with HIV develop the active disease.[50] People living with HIV are estimated 16 times more likely to fall ill with TB than people without HIV; TB is the leading cause of death among people with HIV.[1]
Another important risk factor is use of medications which suppress the immune system; these include, chemotherapy, medication for lupus or rheumatoid arthritis, and medication after an organ transplant.[80] Other risk factors include: alcoholism, diabetes mellitus, silicosis, tobacco smoking, recreational drug use, severe kidney disease, head and neck cancer, low body weight.[80][85] Children, especially those under age five, have undeveloped immune systems and are at higher risk.[85]
Environmental factors which weaken the body's protective mechanisms and may put a person at additional risk of contracting TB include air pollution, exposure to smoke (including tobacco smoke), and exposure (often occupational) to dust or particulates.[83]
Pathogenesis
[edit]
TB infection begins when a M. tuberculosis bacterium, inhaled from the air, penetrates the lungs and reaches the alveoli. Here it encounters an alveolar macrophage, a cell which is part of the body's immune system, which attempts to destroy it.[86] However, M. tuberculosis is able to neutralise and colonise the macrophage, leading to persistent infection.[86]
The defence mechanism of the macrophage begins when a foreign body, such as a bacterial cell, binds to receptors on the surface of the macrophage. The macrophage then stretches itself around the bacterium and engulfs it. [87] Once inside this macrophage, the bacterium is trapped in a compartment called a phagosome; the phagosome subsequently merges with a lysosome to form a phagolysosome.[88] The lysosome is an organelle which contains digestive enzymes; these are released into the phagolysosome and kill the invader.[89]
The M. tuberculosis bacterium is able to subvert the normal process by inhibiting the development of the phagosome and preventing it from fusing with the lysosome.[88] The bacterium is able to survive and replicate within the phagosome; it will eventually destroy its host macrophage, releasing progeny bacteria which spread the infection.[86]
In the next stage of infection, macrophages, epithelioid cells, lymphocytes and fibroblasts aggregate to form a granuloma, which surrounds and isolates the infected macrophages.[86] This does not destroy the tuberculosis bacilli, but contains them, preventing spread of the infection to other parts of the body. They are nevertheless able to survive within the granuloma.[86][90] In tuberculosis, the granuloma contains necrotic tissue at its centre, and appears as a small white nodule, also known as a tubercle, from which the disease derives its name.[91]
Granulomas are most common in the lung, but they can appear anywhere in the body. As long as the infection is contained within granulomas, there are no outward symptoms and the infection is latent.[91] However, if the immune system is unable to control the infection, the disease can progress to active TB, which can cause significant damage to the lungs and other organs.[90]
If TB bacteria gain entry to the blood stream from an area of damaged tissue, they can spread throughout the body and set up many foci of infection, all appearing as tiny, white tubercles in the tissues.[92] This severe form of TB disease, most common in young children and those with HIV, is called miliary tuberculosis.[93] People with this disseminated TB have a high fatality rate even with treatment (about 30%).[59][94]
In many people, the infection waxes and wanes. Tissue destruction and necrosis are often balanced by healing and fibrosis.[95] Affected tissue is replaced by scarring and cavities filled with caseous necrotic material. During active disease, some of these cavities are joined to the air passages (bronchi) and this material can be coughed up. It contains living bacteria and thus can spread the infection. Treatment with appropriate antibiotics kills bacteria and allows healing to take place. Upon cure, affected areas are eventually replaced by scar tissue.[95]
Diagnosis
[edit]
Diagnosis of tuberculosis is often difficult. Symptoms manifest slowly, and are generally non-specific, e.g. cough, fatigue, fever which could be caused by a number of other factors.[96] The conclusive test for pulmonary TB is a bacterial culture taken from a sample of sputum, but this is slow to give a result, and does not detect latent TB. Extra-pulmonary TB infection can affect the kidneys, spine, brain, lymph nodes, or bones - a sample cannot easily be obtained for culture.[97] Tests based on the immune response are sensitive but are likely to give false negatives in those with weak immune systems such as very young patients and those coinfected with HIV. Another issue affecting diagnosis in many parts of the world is that TB infection is most common in resource-poor settings where sophisticated laboratories are rarely available.[98][99]
A diagnosis of TB should be considered in those with signs of lung disease or constitutional symptoms lasting longer than two weeks.[100] Diagnosis of TB, whether latent or active, starts with medical history and physical examination. Subsequently a number of tests can be performed to refine the diagnosis:[101] A chest X-ray and multiple sputum cultures for acid-fast bacilli are typically part of the initial evaluation.[100]
Mantoux test
[edit]
The Mantoux tuberculin skin test is often used to screen people at high risk for TB such as health workers or close contacts of TB patients, who may not display symptoms of infection.[100] In the Mantoux test, a small quantity of tuberculin antigen is injected intradermally on the forearm.[102][103] The result of the test is read after 48 to 72 hours. A person who has been exposed to the bacteria would be expected to mount an immune response; the reaction is read by measuring the diameter of the raised area.[104] Vaccination with Bacille Calmette-Guerin (BCG) may result in a false-positive result. Several factors may lead to false negatives; these include HIV infection, some viral illnesses, and overwhelming TB disease.[105][106]
Interferon-Gamma Release Assay
[edit]The Interferon-Gamma Release Assay (IGRA) is recommended in those who are positive to the Mantoux test.[107] This test mixes a blood sample with antigenic material derived from the TB bacterium. If the patient has developed an immune response to a TB infection, white blood cells in the sample will release interferon-gamma (IFN-γ), which can be measured.[108] This test is more reliable than the Mantoux test, and does not give a false positive after BCG vaccination; [108] however it may give a positive result in case of infection by the related bacteria M. szulgai, M. marinum, and M. kansasii.[109]
Chest radiograph
[edit]In active pulmonary TB, infiltrates (opaque areas) or scarring are visible in the lungs on a chest X-ray. Infiltrates are suggestive but not necessarily diagnostic of TB. Other lung diseases can mimic the appearance of TB; and this test will not detect extrapulmonary infection or a recent infection.[110]
Microbiological studies
[edit]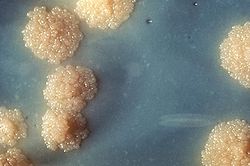
A definitive diagnosis of tuberculosis can be made by detecting Mycobacterium tuberculosis organisms in a specimen taken from the patient (most often sputum, but may also be pus, cerebrospinal fluid, biopsied tissue, etc.).[111] The specimen is examined by fluorescence microscopy.[112] The bacterium is slow growing so a cell culture may take several weeks to yield a result.[113]
Other tests
[edit]Nucleic acid amplification tests (NAAT) and adenosine deaminase testing may allow rapid diagnosis of TB.[114][115] In December 2010, the World Health Organization endorsed the Xpert MTB/RIF system (a NAAT) for diagnosis of tuberculosis in endemic countries.[116]
Blood tests to detect antibodies are not specific or sensitive, so they are not recommended.[117]
Prevention
[edit]
The main strategies to prevent infection with TB are treatment of both active and latent TB, as well as vaccination of children who are at risk.[10]
Although latent TB is not infective, it should be treated in order to prevent its development into active pulmonary TB, which is infective.[118] Active TB ceases to be infective after about 2 weeks of treatment; however it is important to complete the full course of treatment which is usually six months.[119]
Some countries have legislation to involuntarily detain or examine those suspected to have tuberculosis, or involuntarily treat them if infected.[120]
Vaccines
[edit]The only available vaccine as of 2021[update] is bacillus Calmette-Guérin (BCG).[121][122] In areas where tuberculosis is not common, only children at high risk are typically immunized, while suspected cases of tuberculosis are individually tested for and treated.[123] In countries where tuberculosis is common, one dose is recommended in healthy babies as soon after birth as possible.[123] A single dose is given by intradermal injection. Administered to children under 5, it decreases the risk of getting the infection by 20% and the risk of infection turning into active disease by nearly 60%.[124][125] It is not effective if administered to adults.[126]
Public health
[edit]Public health campaigns that have focused on overcrowding, public spitting and regular sanitation (including hand washing) during the 1800s helped to either interrupt or slow the spread of the disease. When combined with contact tracing, isolation and treatment thus helped to dramatically curb the transmission of both tuberculosis and other airborne diseases, which led to the elimination of tuberculosis as a major public health issue in most developed economies.[127][128] Other risk factors which worsened TB spread such as malnutrition were also ameliorated, but since the emergence of HIV a new population of immunocompromised individuals was available for TB to infect.
The cascade of person-to-person spread can be circumvented by segregating those with active ("overt") TB and putting them on anti-TB drug regimens. After about two weeks of effective treatment, subjects with nonresistant active infections generally do not remain contagious to others.[82] If someone does become infected, it typically takes three to four weeks before the newly infected person becomes infectious enough to transmit the disease to others.[129]
Worldwide campaigns
[edit]
The World Health Organization (WHO) declared TB a "global health emergency" in 1993,[10] and in 2006, the Stop TB Partnership developed a Global Plan to Stop Tuberculosis that aimed to save 14 million lives between its launch and 2015.[130] A number of targets they set were not achieved by 2015, mostly due to the increase in HIV-associated tuberculosis and the emergence of multi-drug resistant tuberculosis.[10]
In 2014, the WHO adopted the "End TB" strategy which aims to reduce TB incidence by 80% and TB deaths by 90% by 2030.[131] The strategy contains a milestone to reduce TB incidence by 20% and TB deaths by 35% by 2020.[132] However, by 2020 only a 9% reduction in incidence per population was achieved globally, with the European region achieving 19% and the African region achieving 16% reductions.[132] Similarly, the number of deaths only fell by 14%, missing the 2020 milestone of a 35% reduction, with some regions making better progress (31% reduction in Europe and 19% in Africa).[132] Correspondingly, also treatment, prevention and funding milestones were missed in 2020, for example only 6.3 million people were started on TB prevention short of the target of 30 million.[132]
The goal of tuberculosis elimination is being hampered by the lack of rapid testing, short and effective treatment courses, and completely effective vaccines.[133]
Management
[edit]
Treatment of TB uses antibiotics to kill the bacteria. Effective TB treatment is difficult, due to the unusual structure and chemical composition of the mycobacterial cell wall, which hinders the entry of drugs and makes many antibiotics ineffective.[134]
Active TB is best treated with combinations of several antibiotics to reduce the risk of the bacteria developing antibiotic resistance.[10]
Latent TB
[edit]Latent TB is treated with either isoniazid or rifampin alone, or a combination of isoniazid with either rifampicin or rifapentine.[135][136][137]
The treatment takes three to nine months depending on the medications used.[138][135][139][137] People with latent infections are treated to prevent them from progressing to active TB disease later in life.[140]
Education or counselling may improve the latent tuberculosis treatment completion rates.[141]
New onset
[edit]The recommended treatment of new-onset pulmonary tuberculosis, as of 2010[update], is six months of a combination of antibiotics containing rifampicin, isoniazid, pyrazinamide, and ethambutol for the first two months, and only rifampicin and isoniazid for the last four months.[10] Where resistance to isoniazid is high, ethambutol may be added for the last four months as an alternative.[10] Treatment with anti-TB drugs for at least 6 months results in higher success rates when compared with treatment less than 6 months, even though the difference is small. Shorter treatment regimen may be recommended for those with compliance issues.[142] There is also no evidence to support shorter anti-tuberculosis treatment regimens when compared to a 6-month treatment regimen.[143] However, results presented in 2020 from an international, randomized, controlled clinical trial indicate that a four-month daily treatment regimen containing high-dose, or "optimized", rifapentine with moxifloxacin (2PHZM/2PHM) is as safe and effective as the existing standard six-month daily regimen at curing drug-susceptible tuberculosis (TB) disease.[144]
Recurrent disease
[edit]If tuberculosis recurs, testing to determine which antibiotics it is sensitive to is important before determining treatment.[10] If multi-drug resistant TB (MDR-TB) is detected, treatment with at least four effective antibiotics for 18 to 24 months is recommended.[10]
Medication administration
[edit]Directly observed therapy, i.e., having a health care provider watch the person take their medications, is recommended by the World Health Organization (WHO) in an effort to reduce the number of people not appropriately taking antibiotics.[145] The evidence to support this practice over people simply taking their medications independently is of poor quality.[146] There is no strong evidence indicating that directly observed therapy improves the number of people who were cured or the number of people who complete their medicine.[146] Moderate quality evidence suggests that there is also no difference if people are observed at home versus at a clinic, or by a family member versus a health care worker.[146]
Methods to remind people of the importance of treatment and appointments may result in a small but important improvement.[147] There is also not enough evidence to support intermittent rifampicin-containing therapy given two to three times a week has equal effectiveness as daily dose regimen on improving cure rates and reducing relapsing rates.[148] There is also not enough evidence on effectiveness of giving intermittent twice or thrice weekly short course regimen compared to daily dosing regimen in treating children with tuberculosis.[149]
Medication resistance
[edit]Primary resistance occurs when a person becomes infected with a resistant strain of TB. A person with fully susceptible MTB may develop secondary (acquired) resistance during therapy because of inadequate treatment, not taking the prescribed regimen appropriately (lack of compliance), or using low-quality medication.[150] Drug-resistant TB is a serious public health issue in many developing countries, as its treatment is longer and requires more expensive drugs. MDR-TB is defined as resistance to the two most effective first-line TB drugs: rifampicin and isoniazid. Extensively drug-resistant TB is also resistant to three or more of the six classes of second-line drugs.[151] Totally drug-resistant TB is resistant to all currently used drugs.[152] It was first observed in 2003 in Italy,[153] but not widely reported until 2012,[152][154] and has also been found in Iran and India.[155] There is some efficacy for linezolid to treat those with XDR-TB but side effects and discontinuation of medications were common.[156][157] Bedaquiline is tentatively supported for use in multi-drug resistant TB.[158]
XDR-TB is a term sometimes used to define extensively resistant TB, and constitutes one in ten cases of MDR-TB. Cases of XDR TB have been identified in more than 90% of countries.[155]
For those with known rifampicin or MDR-TB, molecular tests such as the Genotype MTBDRsl Assay (performed on culture isolates or smear positive specimens) may be useful to detect second-line anti-tubercular drug resistance.[159][160]
Xpert MTB/XDR can be used to detect resistance of isoniazid, fluoroquinolones, and amikacin and can be helpful in selection of optimal medication.[161]
Prognosis
[edit]
| no data ≤10 10–25 25–50 50–75 75–100 100–250 | 250–500 500–750 750–1000 1000–2000 2000–3000 ≥ 3000 |
Progression from TB infection to overt TB disease occurs when the bacilli overcome the immune system defenses and begin to multiply. In primary TB disease (some 1–5% of cases), this occurs soon after the initial infection.[67] However, in the majority of cases, a latent infection occurs with no obvious symptoms.[67] These dormant bacilli produce active tuberculosis in 5–10% of these latent cases, often many years after infection.[50]
The risk of reactivation increases with immunosuppression, such as that caused by infection with HIV. In people coinfected with M. tuberculosis and HIV, the risk of reactivation increases to 10% per year.[67] Studies using DNA fingerprinting of M. tuberculosis strains have shown reinfection contributes more substantially to recurrent TB than previously thought,[163] with estimates that it might account for more than 50% of reactivated cases in areas where TB is common.[164] The chance of death from a case of tuberculosis is about 4% as of 2008[update], down from 8% in 1995.[10]
In people with smear-positive pulmonary TB (without HIV co-infection), after 5 years without treatment, 50–60% die while 20–25% achieve spontaneous resolution (cure). TB is almost always fatal in those with untreated HIV co-infection and death rates are increased even with antiretroviral treatment of HIV.[165]
Epidemiology
[edit]Roughly one-quarter of the world's population has been infected with M. tuberculosis,[166] with new infections occurring in about 1% of the population each year.[167] However, most infections with M. tuberculosis do not cause disease,[168] and 90–95% of infections remain asymptomatic.[169] In 2012, an estimated 8.6 million chronic cases were active.[170] In 2010, 8.8 million new cases of tuberculosis were diagnosed, and 1.20–1.45 million deaths occurred (most of these occurring in developing countries).[171][172] Of these, about 0.35 million occur in those also infected with HIV.[173] In 2018, tuberculosis was the leading cause of death worldwide from a single infectious agent.[1] The total number of tuberculosis cases has been decreasing since 2005, while new cases have decreased since 2002.[171]
Tuberculosis[clarification needed] incidence is seasonal, with peaks occurring every spring and summer.[174][175][176][177] The reasons for this are unclear, but may be related to vitamin D deficiency during the winter.[177][178] There are also studies linking tuberculosis to different weather conditions like low temperature, low humidity and low rainfall. It has been suggested that tuberculosis incidence rates may be connected to climate change.[179]
-
Number of new cases of tuberculosis per 100,000 people, 2016[180]
-
Tuberculosis deaths per million persons, 2012
-
Tuberculosis deaths by region, 1990 to 2017[181]
-
Deaths from tuberculosis, by age, World[182]
At-risk groups
[edit]Tuberculosis is closely linked to both overcrowding and malnutrition, making it one of the principal diseases of poverty.[10] Those at high risk thus include: people who inject illicit drugs, inhabitants and employees of locales where vulnerable people gather (e.g., prisons and homeless shelters), medically underprivileged and resource-poor communities, high-risk ethnic minorities, children in close contact with high-risk category patients, and health-care providers serving these patients.[183]
The rate of tuberculosis varies with age. In Africa, it primarily affects adolescents and young adults.[184] However, in countries where incidence rates have declined dramatically (such as the United States), tuberculosis is mainly a disease of the elderly and immunocompromised (risk factors are listed above).[67][185] Worldwide, 22 "high-burden" states or countries together experience 80% of cases as well as 83% of deaths.[155]
In Canada and Australia, tuberculosis is many times more common among the Indigenous peoples, especially in remote areas.[186][187] Factors contributing to this include higher prevalence of predisposing health conditions and behaviours, and overcrowding and poverty. In some Canadian Indigenous groups, genetic susceptibility may play a role.[84]
Socioeconomic status (SES) strongly affects TB risk. People of low SES are both more likely to contract TB and to be more severely affected by the disease. Those with low SES are more likely to be affected by risk factors for developing TB (e.g., malnutrition, indoor air pollution, HIV co-infection, etc.), and are additionally more likely to be exposed to crowded and poorly ventilated spaces. Inadequate healthcare also means that people with active disease who facilitate spread are not diagnosed and treated promptly; sick people thus remain in the infectious state and (continue to) spread the infection.[84]
Geographical epidemiology
[edit]The distribution of tuberculosis is not uniform across the globe; about 80% of the population in many African, Caribbean, South Asian, and eastern European countries test positive in tuberculin tests, while only 5–10% of the U.S. population test positive.[67] Hopes of totally controlling the disease have been dramatically dampened because of many factors, including the difficulty of developing an effective vaccine, the expensive and time-consuming diagnostic process, the necessity of many months of treatment, the increase in HIV-associated tuberculosis, and the emergence of drug-resistant cases in the 1980s.[10]
In developed countries, tuberculosis is less common and is found mainly in urban areas. In Europe, deaths from TB fell from 500 out of 100,000 in 1850 to 50 out of 100,000 by 1950. Improvements in public health were reducing tuberculosis even before the arrival of antibiotics, although the disease remained a significant threat to public health, such that when the Medical Research Council was formed in Britain in 1913 its initial focus was tuberculosis research.[188]
In 2010, rates per 100,000 people in different areas of the world were: globally 178, Africa 332, the Americas 36, Eastern Mediterranean 173, Europe 63, Southeast Asia 278, and Western Pacific 139.[173]
In 2023, tuberculosis overtook COVID-19 as the leading cause of infectious disease-related deaths globally, according to a World Health Organization.[15] Around 8.2 million people were newly diagnosed with TB last year, allowing them access to treatment—a record high since WHO's tracking began in 1995 and an increase from 7.5 million cases in 2022.[189] The report highlights ongoing obstacles in combating TB, including severe funding shortages that hinder efforts toward eradication. Although TB-related deaths decreased slightly to 1.25 million in 2023 from 1.32 million in 2022, the overall number of new cases rose marginally to an estimated 10.8 million.
Russia
[edit]Russia has achieved particularly dramatic progress with a decline in its TB mortality rate—from 61.9 per 100,000 in 1965 to 2.7 per 100,000 in 1993;[190][191] however, mortality rate increased to 24 per 100,000 in 2005 and then recoiled to 11 per 100,000 by 2015.[192]
China
[edit]China has achieved particularly dramatic progress, with about an 80% reduction in its TB mortality rate between 1990 and 2010.[173] The number of new cases has declined by 17% between 2004 and 2014.[155]
Africa
[edit]In 2007, the country with the highest estimated incidence rate of TB was Eswatini, with 1,200 cases per 100,000 people. In 2017, the country with the highest estimated incidence rate as a % of the population was Lesotho, with 665 cases per 100,000 people.[193]
In South Africa, 54,200 people died in 2022 from TB. The incidence rate was 468 per 100,000 people; in 2015, this was 988 per 100,000. The total incidence was 280,000 in 2022; in 2015, this was 552,000.[194]
India
[edit]As of 2017, India had the largest total incidence, with an estimated 2,740,000 cases.[193] According to the World Health Organization (WHO), in 2000–2015, India's estimated mortality rate dropped from 55 to 36 per 100,000 population per year with estimated 480 thousand people died of TB in 2015.[195][196] In India a major proportion of tuberculosis patients are being treated by private partners and private hospitals. Evidence indicates that the tuberculosis national survey does not represent the number of cases that are diagnosed and recorded by private clinics and hospitals in India.[197]
North America
[edit]In Canada, tuberculosis was endemic in some rural areas as of 1998.[198] The tuberculosis case rate in Canada in 2021 was 4.8 per 100,000 persons. The rates were highest among Inuit (135.1 per 100,000), First Nations (16.1 per 100,000) and people born outside of Canada (12.3 per 100,000).[199]
In the United States, Native Americans have a fivefold greater mortality from TB,[200] and racial and ethnic minorities accounted for 88% of all reported TB cases.[201] The overall tuberculosis case rate in the United States was 2.9 per 100,000 persons in 2023, representing a 16% increase in cases compared to 2022.[201]
In 2024, Long Beach, California authorized a public health emergency in response to a local outbreak of TB.[202]
Western Europe
[edit]In 2017, in the United Kingdom, the national average was 9 per 100,000 and the highest incidence rates in Western Europe were 20 per 100,000 in Portugal.
India
[edit]India had the highest total number of TB cases worldwide in 2010, in part due to poor disease management within the private and public health care sector.[203] Programs such as the Revised National Tuberculosis Control Program are working to reduce TB levels among people receiving public health care.[204][205]
Society and culture
[edit]Names
[edit]Tuberculosis has been known by many names from the technical to the familiar.[206] Phthisis (φθίσις) in ancient Greek translates to decay or wasting disease, presumed to refer to pulmonary tuberculosis;[207] around 460 BCE, Hippocrates described phthisis as a disease of dry seasons.[208] The abbreviation TB is short for tubercle bacillus. Consumption was the most common nineteenth century English word for the disease, and was also in use well into the twentieth century.[3] The Latin root con meaning 'completely' is linked to sumere meaning 'to take up from under'.[209] In The Life and Death of Mr Badman by John Bunyan, the author calls consumption "the captain of all these men of death."[210] "Great white plague" has also been used.[206]
Art and literature
[edit]
Tuberculosis was for centuries associated with poetic and artistic qualities among those infected, and was also known as "the romantic disease".[206][211] Major artistic figures such as the poets John Keats, Percy Bysshe Shelley, and Edgar Allan Poe, the composer Frédéric Chopin,[212] the playwright Anton Chekhov, the novelists Franz Kafka, Katherine Mansfield,[213] Charlotte Brontë, Fyodor Dostoevsky, Thomas Mann, W. Somerset Maugham,[214] George Orwell,[215] and Robert Louis Stevenson, and the artists Alice Neel,[216] Jean-Antoine Watteau, Elizabeth Siddal, Marie Bashkirtseff, Edvard Munch, Aubrey Beardsley and Amedeo Modigliani either had the disease or were surrounded by people who did. A widespread belief was that tuberculosis assisted artistic talent. Physical mechanisms proposed for this effect included the slight fever and toxaemia that it caused, allegedly helping them to see life more clearly and to act decisively.[217][218][219]
Tuberculosis formed an often-reused theme in literature, as in Thomas Mann's The Magic Mountain, set in a sanatorium;[220] in music, as in Van Morrison's song "T.B. Sheets";[221] in opera, as in Puccini's La bohème and Verdi's La Traviata;[219] in art, as in Munch's painting of his ill sister;[222] and in film, such as the 1945 The Bells of St. Mary's starring Ingrid Bergman as a nun with tuberculosis.[223]
Folklore
[edit]In 19th century New England, tuberculosis deaths were associated with vampires. When one member of a family died from the disease, the other infected members would lose their health slowly. People believed this was caused by the original person with TB draining the life from the other family members.[224]
Public health efforts
[edit]In 2012, The World Health Organization (WHO), the Bill and Melinda Gates Foundation, and the U.S. government subsided a fast-acting diagnostic tuberculosis test, Xpert MTB/RIF, for use in low- and middle-income countries.[225][226][227] This is a rapid molecular test used to diagnose TB and simultaneously detect rifampicin resistance. It provides results in about two hours, which is much faster than traditional TB culture methods. The test is designed for use with the GeneXpert System.[115]
A 2014 EIU-healthcare report finds there is a need to address apathy and urges for increased funding. The report cites among others Lucica Ditui "[TB] is like an orphan. It has been neglected even in countries with a high burden and often forgotten by donors and those investing in health interventions."[155]
Slow progress has led to frustration, expressed by the executive director of the Global Fund to Fight AIDS, Tuberculosis and Malaria – Mark Dybul: "we have the tools to end TB as a pandemic and public health threat on the planet, but we are not doing it."[155] Several international organizations are pushing for more transparency in treatment, and more countries are implementing mandatory reporting of cases to the government as of 2014, although adherence is often variable. Commercial treatment providers may at times overprescribe second-line drugs as well as supplementary treatment, promoting demands for further regulations.[155]
The government of Brazil provides universal TB care, which reduces this problem.[155] Conversely, falling rates of TB infection may not relate to the number of programs directed at reducing infection rates but may be tied to an increased level of education, income, and health of the population.[155] Costs of the disease, as calculated by the World Bank in 2009 may exceed US$150 billion per year in "high burden" countries.[155] Lack of progress eradicating the disease may also be due to lack of patient follow-up – as among the 250 million rural migrants in China.[155]
There is insufficient data to show that active contact tracing helps to improve case detection rates for tuberculosis.[228] Interventions such as house-to-house visits, educational leaflets, mass media strategies, educational sessions may increase tuberculosis detection rates in short-term.[229] There is no study that compares new methods of contact tracing such as social network analysis with existing contact tracing methods.[230]
Stigma
[edit]Slow progress in preventing the disease may in part be due to stigma associated with TB.[155] Stigma may be due to the fear of transmission from affected individuals. This stigma may additionally arise due to links between TB and poverty, and in Africa, AIDS.[155] Such stigmatization may be both real and perceived; for example, in Ghana, individuals with TB are banned from attending public gatherings.[231]
Stigma towards TB may result in delays in seeking treatment,[155] lower treatment compliance, and family members keeping cause of death secret[231] – allowing the disease to spread further.[155] In contrast, in Russia stigma was associated with increased treatment compliance.[231] TB stigma also affects socially marginalized individuals to a greater degree and varies between regions.[231]
One way to decrease stigma may be through the promotion of "TB clubs", where those infected may share experiences and offer support, or through counseling.[231] Some studies have shown TB education programs to be effective in decreasing stigma, and may thus be effective in increasing treatment adherence.[231] Despite this, studies on the relationship between reduced stigma and mortality are lacking as of 2010[update], and similar efforts to decrease stigma surrounding AIDS have been minimally effective.[231] Some have claimed the stigma to be worse than the disease, and healthcare providers may unintentionally reinforce stigma, as those with TB are often perceived as difficult or otherwise undesirable.[155] A greater understanding of the social and cultural dimensions of tuberculosis may also help with stigma reduction.[232]
Research
[edit]The BCG vaccine has limitations and research to develop new TB vaccines is ongoing.[233] A number of potential candidates are currently in phase I and II clinical trials.[233][234] Two main approaches are used to attempt to improve the efficacy of available vaccines. One approach involves adding a subunit vaccine to BCG, while the other strategy is attempting to create new and better live vaccines.[233] MVA85A, an example of a subunit vaccine, is in trials in South Africa as of 2006, is based on a genetically modified vaccinia virus.[235] Vaccines are hoped to play a significant role in treatment of both latent and active disease.[236]
To encourage further discovery, researchers and policymakers are promoting new economic models of vaccine development as of 2006, including prizes, tax incentives, and advance market commitments.[237][238] A number of groups, including the Stop TB Partnership,[239] the South African Tuberculosis Vaccine Initiative, and the Aeras Global TB Vaccine Foundation, are involved with research.[240] Among these, the Aeras Global TB Vaccine Foundation received a gift of more than $280 million (US) from the Bill and Melinda Gates Foundation to develop and license an improved vaccine against tuberculosis for use in high burden countries.[241][242]
In 2012 a new medication regimen was approved in the US for multidrug-resistant tuberculosis, using bedaquiline as well as existing drugs. There were initial concerns about the safety of this drug,[243][244][245][246][247] but later research on larger groups found that this regimen improved health outcomes.[248] By 2017 the drug was used in at least 89 countries.[249] Another new drug is delamanid, which was first approved by the European Medicines Agency in 2013 to be used in multidrug-resistant tuberculosis patients,[250] and by 2017 was used in at least 54 countries.[249]
Steroids add-on therapy has not shown any benefits for active pulmonary tuberculosis infection.[251]
Other animals
[edit]Mycobacteria infect many different animals, including birds,[252] fish, rodents,[253] and reptiles.[254] The subspecies Mycobacterium tuberculosis, though, is rarely present in wild animals.[255] An effort to eradicate bovine tuberculosis caused by Mycobacterium bovis from the cattle and deer herds of New Zealand has been relatively successful.[256] Efforts in Great Britain have been less successful.[257][258]
As of 2015[update], tuberculosis appears to be widespread among captive elephants in the US. It is believed that the animals originally acquired the disease from humans, a process called reverse zoonosis. Because the disease can spread through the air to infect both humans and other animals, it is a public health concern affecting circuses and zoos.[259][260]
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t u "Tuberculosis (TB)". World Health Organization. 14 March 2025. Retrieved 14 March 2025.
- ^ Ferri FF (2010). Ferri's differential diagnosis: a practical guide to the differential diagnosis of symptoms, signs, and clinical disorders (2nd ed.). Philadelphia, PA: Elsevier/Mosby. p. Chapter T. ISBN 978-0-323-07699-9.
- ^ a b The Chambers Dictionary. New Delhi: Allied Chambers India Ltd. 1998. p. 352. ISBN 978-81-86062-25-8. Archived from the original on 6 September 2015.
- ^ a b c d "About Tuberculosis". Centers for Disease Control and Prevention. 27 February 2025. Retrieved 14 March 2025.
- ^ a b c d e f g Adkinson NF, Bennett JE, Douglas RG, Mandell GL (2010). Mandell, Douglas, and Bennett's principles and practice of infectious diseases (7th ed.). Philadelphia, PA: Churchill Livingstone/Elsevier. p. Chapter 250. ISBN 978-0-443-06839-3.
- ^ "Testing for Tuberculosis". Centers for Disease Prevention and Control. 17 June 2024. Retrieved 14 March 2025.
- ^ Hawn TR, Day TA, Scriba TJ, Hatherill M, Hanekom WA, Evans TG, et al. (December 2014). "Tuberculosis vaccines and prevention of infection". Microbiology and Molecular Biology Reviews. 78 (4): 650–71. doi:10.1128/MMBR.00021-14. PMC 4248657. PMID 25428938.
- ^ a b Implementing the WHO Stop TB Strategy: a handbook for national TB control programmes. Geneva: World Health Organization (WHO). 2008. p. 179. ISBN 978-92-4-154667-6. Archived from the original on 2 June 2021. Retrieved 17 September 2017.
- ^ Harris RE (2013). "Epidemiology of Tuberculosis". Epidemiology of chronic disease: global perspectives. Burlington, MA: Jones & Bartlett Learning. p. 682. ISBN 978-0-7637-8047-0. Archived from the original on 7 February 2024. Retrieved 17 September 2017.
- ^ a b c d e f g h i j k l m n o Lawn SD, Zumla AI (July 2011). "Tuberculosis". Lancet. 378 (9785): 57–72. doi:10.1016/S0140-6736(10)62173-3. PMID 21420161. S2CID 208791546.
- ^ a b Bloom BR (1994). Tuberculosis: pathogenesis, protection, and control. Washington, DC: ASM Press. ISBN 978-1-55581-072-6.
- ^ Persson S (2010). Smallpox, Syphilis and Salvation: Medical Breakthroughs That Changed the World. ReadHowYouWant.com. p. 141. ISBN 978-1-4587-6712-7. Archived from the original on 6 September 2015.
- ^ Wall R (9 July 2024). "Tuberculosis Drug Discovery: Navigating Resistance and Developing New Therapies". London School of Hygiene & Tropical Medicine. Retrieved 15 March 2025.
- ^ "10 facts on tuberculosis". World Health Organization. 29 October 2024. Retrieved 15 March 2025.
- ^ a b c Organization WH (2024). Global Tuberculosis Report 2024. Geneva. ISBN 978-92-4-010153-1. Retrieved 31 October 2024.
{{cite book}}:|website=ignored (help)CS1 maint: location missing publisher (link) - ^ Rothschild BM, Martin LD, Lev G, Bercovier H, Bar-Gal GK, Greenblatt C, et al. (August 2001). "Mycobacterium tuberculosis complex DNA from an extinct bison dated 17,000 years before the present". Clinical Infectious Diseases. 33 (3): 305–11. doi:10.1086/321886. PMID 11438894.
- ^ Pearce-Duvet JM (August 2006). "The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease". Biological Reviews of the Cambridge Philosophical Society. 81 (3): 369–82. doi:10.1017/S1464793106007020. PMID 16672105. S2CID 6577678.
- ^ Comas I, Gagneux S (October 2009). Manchester M (ed.). "The past and future of tuberculosis research". PLOS Pathogens. 5 (10): e1000600. doi:10.1371/journal.ppat.1000600. PMC 2745564. PMID 19855821.
- ^ Zink AR, Sola C, Reischl U, Grabner W, Rastogi N, Wolf H, et al. (January 2003). "Characterization of Mycobacterium tuberculosis complex DNAs from Egyptian mummies by spoligotyping". Journal of Clinical Microbiology. 41 (1): 359–67. doi:10.1128/JCM.41.1.359-367.2003. PMC 149558. PMID 12517873.
- ^ Konomi N, Lebwohl E, Mowbray K, Tattersall I, Zhang D (December 2002). "Detection of mycobacterial DNA in Andean mummies". Journal of Clinical Microbiology. 40 (12): 4738–40. doi:10.1128/JCM.40.12.4738-4740.2002. PMC 154635. PMID 12454182.
- ^ Léon Charles Albert Calmette at Whonamedit?
- ^ Trail RR (April 1970). "Richard Morton (1637–1698)". Medical History. 14 (2): 166–74. doi:10.1017/S0025727300015350. PMC 1034037. PMID 4914685.
- ^ Marten B (1720). A New Theory of Consumptions—More Especially a Phthisis or Consumption of the Lungs. London, England: T. Knaplock. Archived from the original on 26 March 2023. Retrieved 8 December 2020. P. 51: "The Original and Essential Cause ... may possibly be some certain Species of Animalcula or wonderfully minute living Creatures, ... " P. 79: "It may be therefore very likely, that by an habitual lying in the same Bed with a Consumptive Patient, constantly Eating and Drinking with him, or by very frequently conversing so nearly, as to draw in part of the Breath he emits from his Lungs, a Consumption may be caught by a sound Person; ... "
- ^ Laennec RT (1819). De l'auscultation médiate ... (in French). Vol. 1. Paris, France: J.-A. Brosson et J.-S Chaudé. p. 20. Archived from the original on 2 June 2021. Retrieved 6 December 2020. From p. 20: "L'existence des tubercules dans le poumon est la cause et constitue le charactère anatomique propre de la phthisie pulmonaire (a). (a) ... l'effet dont cette maladie tire son nom, c'est-à-dire, la consumption." (The existence of tubercles in the lung is the cause and constitutes the unique anatomical characteristic of pulmonary tuberculosis (a). (a) ... the effect from which this malady [pulmonary tuberculosis] takes its name, that is, consumption.)
- ^ Schönlein JL (1832). Allgemeine und specielle Pathologie und Therapie [General and Special Pathology and Therapy] (in German). Vol. 3. Würzburg, (Germany): C. Etlinger. p. 103. Archived from the original on 2 June 2021. Retrieved 6 December 2020.
- ^ The word "tuberculosis" first appeared in Schönlein's clinical notes in 1829. See: Jay SJ, Kırbıyık U, Woods JR, Steele GA, Hoyt GR, Schwengber RB, et al. (November 2018). "Modern theory of tuberculosis: culturomic analysis of its historical origin in Europe and North America". The International Journal of Tuberculosis and Lung Disease. 22 (11): 1249–1257. doi:10.5588/ijtld.18.0239. PMID 30355403. S2CID 53027676. See especially Appendix, p. iii.
- ^ "Kentucky: Mammoth Cave long on history". CNN. 27 February 2004. Archived from the original on 13 August 2006. Retrieved 8 October 2006.
- ^ a b c McCarthy OR (August 2001). "The key to the sanatoria". Journal of the Royal Society of Medicine. 94 (8): 413–17. doi:10.1177/014107680109400813. PMC 1281640. PMID 11461990. Archived from the original on 3 August 2012. Retrieved 28 September 2011.
- ^ Villemin JA (1865). "Cause et nature de la tuberculose" [Cause and nature of tuberculosis]. Bulletin de l'Académie Impériale de Médecine (in French). 31: 211–216. Archived from the original on 9 December 2021. Retrieved 6 December 2020.
- See also: Villemin JA (1868). Etudes sur la tuberculose: preuves rationnelles et expérimentales de sa spécificité et de son inoculabilité [Studies of tuberculosis: rational and experimental evidence of its specificity and inoculability] (in French). Paris, France: J.-B. Baillière et fils. Archived from the original on 7 February 2024. Retrieved 6 December 2020.
- ^ Burdon-Sanderson, John Scott. (1870) "Introductory Report on the Intimate Pathology of Contagion." Appendix to: Twelfth Report to the Lords of Her Majesty's Most Honourable Privy Council of the Medical Officer of the Privy Council [for the year 1869], Parliamentary Papers (1870), vol. 38, 229–256.
- ^ Koch R (24 March 1882). "Die Ätiologie der Tuberkulose (1882)". Robert Koch: Zentrale Texte [The Etiology of Tuberculosis]. Klassische Texte der Wissenschaft. Vol. 19. Berlin, Heidelberg: Springer Spektrum. pp. 221–30. doi:10.1007/978-3-662-56454-7_4. ISBN 978-3-662-56454-7. Archived from the original on 6 November 2018. Retrieved 15 June 2021.
{{cite book}}: ISBN / Date incompatibility (help) - ^ "History: World TB Day". Centers for Disease Control and Prevention (CDC). 12 December 2016. Archived from the original on 7 December 2018. Retrieved 23 March 2019.
- ^ "The Nobel Prize in Physiology or Medicine 1905". www.nobelprize.org. Archived from the original on 10 December 2006. Retrieved 7 October 2006.
- ^ Frith J. "History of Tuberculosis. Part 1 – Phthisis, consumption and the White Plague". Journal of Military and Veterans' Health. Archived from the original on 8 April 2021. Retrieved 26 February 2021.
- ^ Zürcher K, Zwahlen M, Ballif M, Rieder HL, Egger M, Fenner L (5 October 2016). "Influenza Pandemics and Tuberculosis Mortality in 1889 and 1918: Analysis of Historical Data from Switzerland". PLOS ONE. 11 (10): e0162575. Bibcode:2016PLoSO..1162575Z. doi:10.1371/journal.pone.0162575. PMC 5051959. PMID 27706149.
- ^ "Lost Hospitals of London". ezitis.myzen.co.uk. Retrieved 27 June 2024.
- ^ Waddington K (January 2004). "To stamp out 'so terrible a malady': bovine tuberculosis and tuberculin testing in Britain, 1890–1939". Medical History. 48 (1): 29–48. doi:10.1017/S0025727300007043. PMC 546294. PMID 14968644.
- ^ Hannaway C (2008). Biomedicine in the twentieth century: practices, policies, and politics. Amsterdam: IOS Press. p. 233. ISBN 978-1-58603-832-8. Archived from the original on 7 September 2015.
- ^ Bonah C (December 2005). "The 'experimental stable' of the BCG vaccine: safety, efficacy, proof, and standards, 1921–1933". Studies in History and Philosophy of Biological and Biomedical Sciences. 36 (4): 696–721. doi:10.1016/j.shpsc.2005.09.003. PMID 16337557.
- ^ Comstock GW (September 1994). "The International Tuberculosis Campaign: a pioneering venture in mass vaccination and research". Clinical Infectious Diseases. 19 (3): 528–40. doi:10.1093/clinids/19.3.528. PMID 7811874.
- ^ Shields T (2009). General thoracic surgery (7th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 792. ISBN 978-0-7817-7982-1. Archived from the original on 6 September 2015.
- ^ a b Keshavjee S, Farmer PE (6 September 2012). "Tuberculosis, Drug Resistance, and the History of Modern Medicine". New England Journal of Medicine. 367 (10): 931–936. doi:10.1056/NEJMra1205429. ISSN 0028-4793.
- ^ Chaisson RE, Frick M, Nahid P (March 2022). "The scientific response to TB - the other deadly global health emergency". The International Journal of Tuberculosis and Lung Disease. 26 (3): 186–189. doi:10.5588/ijtld.21.0734. PMC 8886961. PMID 35197158.
- ^ "Tuberculosis : a global emergency". World health. 46 (4): 3–31. July–August 1993.
- ^ Millard J, Ugarte-Gil C, Moore DA (26 February 2015). "Multidrug resistant tuberculosis". BMJ. 350: h882. doi:10.1136/bmj.h882. ISSN 1756-1833. PMID 25721508. S2CID 11683912.
- ^ Kaplan, Jeffrey. 2017. "Tuberculosis" American University. Lecture.
- ^ Adams and Woelke (2014). Understanding Global Health. Chapter 10: TB and HIV/AIDS (12th ed.). McGraw Hill. Retrieved 9 May 2015.
- ^ Schiffman G (15 January 2009). "Tuberculosis Symptoms". eMedicine Health. Archived from the original on 16 May 2009.
- ^ Kamboj A, Lause M, Kamboj K (2023). "The Problem of Tuberculosis: Myths, Stigma, and Mimics". In Rezaei N (ed.). Tuberculosis. Integrated Science. Vol. 11. Springer. pp. 1046–1062. doi:10.1007/978-3-031-15955-8_50. ISBN 978-3-031-15954-1.
- ^ a b c Gibson PG, Abramson M, Wood-Baker R, Volmink J, Hensley M, Costabel U, eds. (2005). Evidence-Based Respiratory Medicine (1st ed.). BMJ Books. p. 321. ISBN 978-0-7279-1605-1. Archived from the original on 8 December 2015.
- ^ a b c Price C, Nguyen AD (11 January 2024), "Latent Tuberculosis", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 38261712, retrieved 17 March 2025
- ^ Behera D (2010). Textbook of Pulmonary Medicine (2nd ed.). New Delhi: Jaypee Brothers Medical Publishers. p. 457. ISBN 978-81-8448-749-7. Archived from the original on 6 September 2015.
- ^ "Tuberculosis (TB)". National Health Service. 20 April 2023. Retrieved 17 March 2025.
- ^ Halezeroğlu S, Okur E (March 2014). "Thoracic surgery for haemoptysis in the context of tuberculosis: what is the best management approach?". Journal of Thoracic Disease. 6 (3): 182–85. doi:10.3978/j.issn.2072-1439.2013.12.25. PMC 3949181. PMID 24624281.
- ^ Gai X, Allwood B, Sun Y (August 2023). "Post-tuberculosis lung disease and chronic obstructive pulmonary disease". Chinese Medical Journal. 136 (16): 1923–1928. doi:10.1097/CM9.0000000000002771. PMC 10431356. PMID 37455331.
- ^ Basire D (23 April 2024). "Post-TB lung health: Lasting impact beyond treatment". Breathing Matters - UCL Respiratory. Retrieved 18 March 2025.
- ^ Jindal SK, ed. (2011). Textbook of Pulmonary and Critical Care Medicine. New Delhi: Jaypee Brothers Medical Publishers. p. 549. ISBN 978-93-5025-073-0. Archived from the original on 7 September 2015.
- ^ a b Golden MP, Vikram HR (November 2005). "Extrapulmonary tuberculosis: an overview". American Family Physician. 72 (9): 1761–68. PMID 16300038.
- ^ a b Habermann TM, Ghosh A (2008). Mayo Clinic internal medicine: concise textbook. Rochester, MN: Mayo Clinic Scientific Press. p. 789. ISBN 978-1-4200-6749-1. Archived from the original on 6 September 2015.
- ^ "Clinical Symptoms of Tuberculosis". Centers for Disease Control and Prevention. 8 May 2024. Retrieved 17 March 2025.
- ^ Jindal SK (2011). Textbook of Pulmonary and Critical Care Medicine. New Delhi: Jaypee Brothers Medical Publishers. p. 525. ISBN 978-93-5025-073-0. Archived from the original on 6 September 2015.
- ^ Southwick F (2007). "Chapter 4: Pulmonary Infections". Infectious Diseases: A Clinical Short Course, 2nd ed. McGraw-Hill Medical Publishing Division. pp. 104, 313–14. ISBN 978-0-07-147722-2.
- ^ Niederweis M, Danilchanka O, Huff J, Hoffmann C, Engelhardt H (March 2010). "Mycobacterial outer membranes: in search of proteins". Trends in Microbiology. 18 (3): 109–16. doi:10.1016/j.tim.2009.12.005. PMC 2931330. PMID 20060722.
- ^ Madison BM (May 2001). "Application of stains in clinical microbiology". Biotechnic & Histochemistry. 76 (3): 119–25. doi:10.1080/714028138. PMID 11475314.
- ^ Parish T, Stoker NG (December 1999). "Mycobacteria: bugs and bugbears (two steps forward and one step back)". Molecular Biotechnology. 13 (3): 191–200. doi:10.1385/MB:13:3:191. PMID 10934532. S2CID 28960959.
- ^ van Soolingen D, Hoogenboezem T, de Haas PE, Hermans PW, Koedam MA, Teppema KS, et al. (October 1997). "A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa". International Journal of Systematic Bacteriology. 47 (4): 1236–45. doi:10.1099/00207713-47-4-1236. PMID 9336935.
- ^ a b c d e f g Kumar V, Robbins SL (2007). Robbins Basic Pathology (8th ed.). Philadelphia: Elsevier. ISBN 978-1-4160-2973-1. OCLC 69672074.
- ^ Thoen C, Lobue P, de Kantor I (February 2006). "The importance of Mycobacterium bovis as a zoonosis". Veterinary Microbiology. 112 (2–4): 339–45. doi:10.1016/j.vetmic.2005.11.047. PMID 16387455.
- ^ Niemann S, Rüsch-Gerdes S, Joloba ML, Whalen CC, Guwatudde D, Ellner JJ, et al. (September 2002). "Mycobacterium africanum subtype II is associated with two distinct genotypes and is a major cause of human tuberculosis in Kampala, Uganda". Journal of Clinical Microbiology. 40 (9): 3398–405. doi:10.1128/JCM.40.9.3398-3405.2002. PMC 130701. PMID 12202584.
- ^ Niobe-Eyangoh SN, Kuaban C, Sorlin P, Cunin P, Thonnon J, Sola C, et al. (June 2003). "Genetic biodiversity of Mycobacterium tuberculosis complex strains from patients with pulmonary tuberculosis in Cameroon". Journal of Clinical Microbiology. 41 (6): 2547–53. doi:10.1128/JCM.41.6.2547-2553.2003. PMC 156567. PMID 12791879.
- ^ Acton QA (2011). Mycobacterium Infections: New Insights for the Healthcare Professional. ScholarlyEditions. p. 1968. ISBN 978-1-4649-0122-5. Archived from the original on 6 September 2015.
- ^ Pfyffer GE, Auckenthaler R, van Embden JD, van Soolingen D (1998). "Mycobacterium canettii, the smooth variant of M. tuberculosis, isolated from a Swiss patient exposed in Africa". Emerging Infectious Diseases. 4 (4): 631–4. doi:10.3201/eid0404.980414. PMC 2640258. PMID 9866740.
- ^ Panteix G, Gutierrez MC, Boschiroli ML, Rouviere M, Plaidy A, Pressac D, et al. (August 2010). "Pulmonary tuberculosis due to Mycobacterium microti: a study of six recent cases in France". Journal of Medical Microbiology. 59 (Pt 8): 984–989. doi:10.1099/jmm.0.019372-0. PMID 20488936.
- ^ Smith NH, Crawshaw T, Parry J, Birtles RJ (August 2009). "Mycobacterium microti: More diverse than previously thought". Journal of Clinical Microbiology. 47 (8): 2551–2559. doi:10.1128/jcm.00638-09. PMC 2725668. PMID 19535520.
- ^ "MAC Lung Disease". American Lung Association. Retrieved 18 March 2025.
- ^ Busatto C, Vianna JS, da Silva LV, Ramis IB, da Silva PE (January 2019). "Mycobacterium avium: an overview". Tuberculosis. 114: 127–134. doi:10.1016/j.tube.2018.12.004. PMID 30711152.
- ^ Johnston JC, Chiang L, Elwood K (January 2017). "Mycobacterium kansasii". Microbiology Spectrum. 5 (1): 10.1128/microbiolspec.tnmi7–0011–2016. doi:10.1128/microbiolspec.tnmi7-0011-2016. PMC 11687434. PMID 28185617.
- ^ "Diagnosis and treatment of disease caused by nontuberculous mycobacteria". American Journal of Respiratory and Critical Care Medicine. 156 (2 Pt 2): S1 – S25. August 1997. doi:10.1164/ajrccm.156.2.atsstatement. PMID 9279284.
- ^ "Tuberculosis: Causes and How It Spreads". Centers for Disease Control and Prevention. 5 February 2025. Retrieved 18 March 2025.
- ^ a b c "Tuberculosis (TB): Prevention and risks". Public Health Agency of Canada. 21 February 2024. Retrieved 20 March 2025.
- ^ "Clinical Overview of Latent Tuberculosis Infection". Centers for Disease Control and Prevention. 10 December 2024. Retrieved 19 March 2025.
- ^ a b Ahmed N, Hasnain SE (September 2011). "Molecular epidemiology of tuberculosis in India: moving forward with a systems biology approach". Tuberculosis. 91 (5): 407–13. doi:10.1016/j.tube.2011.03.006. PMID 21514230.
- ^ a b Schmidt CW (November 2008). "Linking TB and the Environment: An Overlooked Mitigation Strategy". Environmental Health Perspectives. 116 (11): A478 – A485. doi:10.1289/ehp.116-a478. PMC 2592293. PMID 19057686.
- ^ a b c Narasimhan P, Wood J, Macintyre CR, Mathai D (2013). "Risk factors for tuberculosis". Pulmonary Medicine. 2013: 828939. doi:10.1155/2013/828939. PMC 3583136. PMID 23476764.
- ^ a b "TB Risk Factors". CDC. 18 March 2016. Archived from the original on 30 August 2020. Retrieved 25 August 2020.
- ^ a b c d e Ahmad F, Rani A, Alam A, Zarin S, Pandey S, Singh H, et al. (6 May 2022). "Macrophage: A Cell With Many Faces and Functions in Tuberculosis". Frontiers in Immunology. 13. doi:10.3389/fimmu.2022.747799. ISSN 1664-3224. PMC 9122124. PMID 35603185.
- ^ Hampton MB, Vissers MC, Winterbourn CC (February 1994). "A single assay for measuring the rates of phagocytosis and bacterial killing by neutrophils". J. Leukoc. Biol. 55 (2): 147–52. doi:10.1002/jlb.55.2.147. PMID 8301210. S2CID 44911791. Archived from the original on 28 December 2012. Retrieved 19 December 2014.
- ^ a b Rohde K, Yates RM, Purdy GE, Russell DG (2007). "Mycobacterium tuberculosis and the environment within the phagosome". Immunological Reviews. 219 (1): 37–54. doi:10.1111/j.1600-065X.2007.00547.x. ISSN 1600-065X. PMID 17850480.
- ^ Delves et al. 2006, pp. 6–7
- ^ a b Silva Miranda M, Breiman A, Allain S, Deknuydt F, Altare F (2012). "The Tuberculous Granuloma: An Unsuccessful Host Defence Mechanism Providing a Safety Shelter for the Bacteria?". Journal of Immunology Research. 2012 (1): 139127. doi:10.1155/2012/139127. ISSN 2314-7156. PMC 3395138. PMID 22811737.
- ^ a b Alzayer Z, Al Nasser Y (2025), "Primary Lung Tuberculosis", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 33620814, retrieved 26 March 2025
- ^ Crowley LV (2010). An introduction to human disease: pathology and pathophysiology correlations (8th ed.). Sudbury, MA: Jones and Bartlett. p. 374. ISBN 978-0-7637-6591-0. Archived from the original on 6 September 2015.
- ^ Harries AD, Maher D, Graham S (2005). TB/HIV a Clinical Manual (2nd ed.). Geneva: World Health Organization (WHO). p. 75. ISBN 978-92-4-154634-8. Archived from the original on 6 September 2015.
- ^ Jacob JT, Mehta AK, Leonard MK (January 2009). "Acute forms of tuberculosis in adults". The American Journal of Medicine. 122 (1): 12–17. doi:10.1016/j.amjmed.2008.09.018. PMID 19114163.
- ^ a b Grosset J (March 2003). "Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary". Antimicrobial Agents and Chemotherapy. 47 (3): 833–36. doi:10.1128/AAC.47.3.833-836.2003. PMC 149338. PMID 12604509.
- ^ Tobin EH, Tristram D (22 December 2024), "Tuberculosis Overview", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 28722945, retrieved 27 March 2025
- ^ CDC (30 January 2025). "Clinical Overview of Tuberculosis Disease". Tuberculosis (TB). Retrieved 29 March 2025.
- ^ Datta S, Evans CA (1 September 2020). "The uncertainty of tuberculosis diagnosis". The Lancet Infectious Diseases. 20 (9): 1002–1004. doi:10.1016/S1473-3099(20)30400-X. ISSN 1473-3099. PMC 7234790. PMID 32437698.
- ^ Hewison C, Gomez D, Deborggraeve S (24 October 2022). "The deadly gap in diagnosing children with tuberculosis". MSF Access Campaign. Retrieved 29 March 2025.
- ^ a b c Escalante P (June 2009). "In the clinic. Tuberculosis". Annals of Internal Medicine. 150 (11): ITC61-614, quiz ITV616. doi:10.7326/0003-4819-150-11-200906020-01006. PMID 19487708. S2CID 639982.
- ^ CDC (30 January 2025). "Clinical and Laboratory Diagnosis for Tuberculosis". Centers for Disease Control and Prevention. Retrieved 29 March 2025.
- ^ "TB Elimination - Tuberculin Skin Testing" (PDF). CDC.gov. CDC - National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention - Division of Tuberculosis Elimination. October 2011. Retrieved 5 June 2017.
- ^ "The Mantoux test: Administration, reading and interpretation" (PDF). NHS.uk. Archived from the original (PDF) on 15 February 2010. Retrieved 5 June 2017.
- ^ "Mantoux Tuberculin Skin Test" (PDF). Centers for Disease Control and Prevention. Retrieved 30 March 2025.
- ^ "Table A3.1, Causes of false-negative and false-positive tuberculin skin tests". www.ncbi.nlm.nih.gov. 2014. Retrieved 30 March 2025.
- ^ Nayak S, Acharjya B (April 2012). "Mantoux test and its interpretation". Indian Dermatology Online Journal. 3 (1): 2–6. doi:10.4103/2229-5178.93479. ISSN 2229-5178. PMC 3481914. PMID 23130251.
- ^ National Institute for Health and Clinical Excellence. Clinical guideline 117: Tuberculosis. London, 2011.
- ^ a b "Clinical Testing Guidance for Tuberculosis: Interferon Gamma Release Assay". Centers for Disease Control and Prevention. 12 September 2024. Retrieved 30 March 2025.
- ^ Jindal SK, ed. (2011). Textbook of Pulmonary and Critical Care Medicine. New Delhi: Jaypee Brothers Medical Publishers. p. 544. ISBN 978-93-5025-073-0. Archived from the original on 6 September 2015.
- ^ Sherrell Z (20 December 2023). "Chest X-ray for tuberculosis (TB): What to expect, results, and more". www.medicalnewstoday.com. Retrieved 30 March 2025.
- ^ Tobin EH, Tristram D (22 December 2024), "Tuberculosis Overview", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 28722945, retrieved 27 March 2025
- ^ Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, Cunningham J, et al. (September 2006). "Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review". The Lancet. Infectious Diseases. 6 (9): 570–81. doi:10.1016/S1473-3099(06)70578-3. PMID 16931408.
- ^ "Acid-Fast Bacillus (AFB) Tests". MedlinePlus. Retrieved 31 March 2025.
- ^ Bento J, Silva AS, Rodrigues F, Duarte R (2011). "[Diagnostic tools in tuberculosis]". Acta Médica Portuguesa. 24 (1): 145–54. doi:10.20344/amp.333. PMID 21672452. S2CID 76156550.
- ^ a b "Xpert MTB/RIF Assay - A Tool to Diagnose Tuberculosis". Centers for Disease Control and Prevention. 29 April 2024. Retrieved 15 April 2025.
- ^ "WHO endorses new rapid tuberculosis test" 8 December 2010. Retrieved on 12 June 2012
- ^ Steingart KR, Flores LL, Dendukuri N, Schiller I, Laal S, Ramsay A, et al. (August 2011). Evans C (ed.). "Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis". PLOS Medicine. 8 (8): e1001062. doi:10.1371/journal.pmed.1001062. PMC 3153457. PMID 21857806.
- ^ "Tuberculosis Vaccine". Centers for Disease Control and Prevention. 5 February 2025. Retrieved 21 April 2025.
- ^ "Tuberculosis (TB): migrant health guide". GOV.UK. 31 March 2025. Retrieved 21 April 2025.
- ^ Coker R, Thomas M, Lock K, Martin R (2007). "Detention and the evolving threat of tuberculosis: evidence, ethics, and law". The Journal of Law, Medicine & Ethics. 35 (4): 609–15, 512. doi:10.1111/j.1748-720X.2007.00184.x. PMID 18076512. S2CID 19924571.
- ^ McShane H (October 2011). "Tuberculosis vaccines: beyond bacille Calmette-Guerin". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 366 (1579): 2782–89. doi:10.1098/rstb.2011.0097. PMC 3146779. PMID 21893541.
- ^ "Vaccines | Basic TB Facts". CDC. 16 June 2021. Archived from the original on 30 December 2021. Retrieved 30 December 2021.
- ^ a b World Health Organization (February 2018). "BCG vaccines: WHO position paper – February 2018". Weekly Epidemiological Record. 93 (8): 73–96. hdl:10665/260307. PMID 29474026.
- ^ Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, et al. (August 2014). "Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis". BMJ. 349 (aug04 5): g4643. doi:10.1136/bmj.g4643. PMC 4122754. PMID 25097193.
- ^ Dias JV, Varandas L, Gonçalves L, Kagina B (June 2024). "Outcomes of childhood TB in countries with a universal BCG vaccination policy". The International Journal of Tuberculosis and Lung Disease. 28 (6): 273–277. doi:10.5588/ijtld.23.0321. PMID 38822485.
- ^ Martinez L, Cords O, Liu Q, Acuna-Villaorduna C, Bonnet M, Fox GJ, et al. (1 September 2022). "Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: a systematic review and individual participant data meta-analysis". The Lancet Global Health. 10 (9): e1307 – e1316. doi:10.1016/S2214-109X(22)00283-2. ISSN 2214-109X. PMID 35961354.
- ^ Clark M, Riben P, Nowgesic E (October 2002). "The association of housing density, isolation and tuberculosis in Canadian First Nations communities". International Journal of Epidemiology. 31 (5): 940–945. doi:10.1093/ije/31.5.940. PMID 12435764.
- ^ Barberis I, Bragazzi NL, Galluzzo L, Martini M (March 2017). "The history of tuberculosis: from the first historical records to the isolation of Koch's bacillus". Journal of Preventive Medicine and Hygiene. 58 (1): E9 – E12. PMC 5432783. PMID 28515626.
- ^ "Causes of Tuberculosis". Mayo Clinic. 21 December 2006. Archived from the original on 18 October 2007. Retrieved 19 October 2007.
- ^ "The Global Plan to Stop TB". World Health Organization (WHO). 2011. Archived from the original on 12 June 2011. Retrieved 13 June 2011.
- ^ "The End TB Strategy". who.int. Archived from the original on 22 July 2021. Retrieved 22 July 2021.
- ^ a b c d Global tuberculosis report 2020. World Health Organization. 2020. ISBN 978-92-4-001313-1. Archived from the original on 22 July 2021. Retrieved 22 July 2021.
- ^ Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, et al. (May 2015). "WHO's new end TB strategy". Lancet. 385 (9979): 1799–1801. doi:10.1016/S0140-6736(15)60570-0. PMID 25814376. S2CID 39379915.
- ^ Brennan PJ, Nikaido H (1995). "The envelope of mycobacteria". Annual Review of Biochemistry. 64 (1): 29–63. doi:10.1146/annurev.bi.64.070195.000333. PMID 7574484.
- ^ a b Latent tuberculosis infection. World Health Organization (WHO). 2018. p. 23. ISBN 978-92-4-155023-9. Archived from the original on 2 June 2021. Retrieved 25 July 2018.
- ^ Borisov AS, Bamrah Morris S, Njie GJ, Winston CA, Burton D, Goldberg S, et al. (June 2018). "Update of Recommendations for Use of Once-Weekly Isoniazid-Rifapentine Regimen to Treat Latent Mycobacterium tuberculosis Infection". MMWR. Morbidity and Mortality Weekly Report. 67 (25): 723–726. doi:10.15585/mmwr.mm6725a5. PMC 6023184. PMID 29953429.
- ^ a b Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, et al. (February 2020). "Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020". MMWR. Recommendations and Reports. 69 (1): 1–11. doi:10.15585/mmwr.rr6901a1. PMC 7041302. PMID 32053584.
- ^ "Core Curriculum on Tuberculosis: What the Clinician Should Know" (PDF) (5th ed.). Centers for Disease Control and Prevention (CDC), Division of Tuberculosis Elimination. 2011. p. 24. Archived (PDF) from the original on 19 May 2012.
- ^ Njie GJ, Morris SB, Woodruff RY, Moro RN, Vernon AA, Borisov AS (August 2018). "Isoniazid-Rifapentine for Latent Tuberculosis Infection: A Systematic Review and Meta-analysis". American Journal of Preventive Medicine. 55 (2): 244–252. doi:10.1016/j.amepre.2018.04.030. PMC 6097523. PMID 29910114.
- ^ Menzies D, Al Jahdali H, Al Otaibi B (March 2011). "Recent developments in treatment of latent tuberculosis infection". The Indian Journal of Medical Research. 133 (3): 257–66. PMC 3103149. PMID 21441678.
- ^ M'imunya JM, Kredo T, Volmink J, et al. (Cochrane Infectious Diseases Group) (May 2012). "Patient education and counselling for promoting adherence to treatment for tuberculosis". The Cochrane Database of Systematic Reviews. 2012 (5): CD006591. doi:10.1002/14651858.CD006591.pub2. PMC 6532681. PMID 22592714.
- ^ Gelband H, et al. (Cochrane Infectious Diseases Group) (25 October 1999). "Regimens of less than six months for treating tuberculosis". The Cochrane Database of Systematic Reviews. 1999 (2): CD001362. doi:10.1002/14651858.CD001362. PMC 6532732. PMID 10796641.
- ^ Grace AG, Mittal A, Jain S, Tripathy JP, Satyanarayana S, Tharyan P, et al. (Cochrane Infectious Diseases Group) (December 2019). "Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis". The Cochrane Database of Systematic Reviews. 12 (12): CD012918. doi:10.1002/14651858.CD012918.pub2. PMC 6953336. PMID 31828771.
- ^ "Landmark TB Trial Identifies Shorter-Course Treatment Regimen". CDC. NCHHSTP Media Team Centers for Disease Control and Prevention. 20 October 2020. Archived from the original on 27 November 2021. Retrieved 27 November 2021.
- ^ Mainous III AB (2010). Management of Antimicrobials in Infectious Diseases: Impact of Antibiotic Resistance. Totowa, NJ: Humana Press. p. 69. ISBN 978-1-60327-238-4. Archived from the original on 6 September 2015.
- ^ a b c Karumbi J, Garner P (May 2015). "Directly observed therapy for treating tuberculosis". The Cochrane Database of Systematic Reviews. 2015 (5): CD003343. doi:10.1002/14651858.CD003343.pub4. PMC 4460720. PMID 26022367.
- ^ Liu Q, Abba K, Alejandria MM, Sinclair D, Balanag VM, Lansang MA, et al. (Cochrane Infectious Diseases Group) (November 2014). "Reminder systems to improve patient adherence to tuberculosis clinic appointments for diagnosis and treatment". The Cochrane Database of Systematic Reviews. 2014 (11): CD006594. doi:10.1002/14651858.CD006594.pub3. PMC 4448217. PMID 25403701.
- ^ Mwandumba HC, Squire SB, et al. (Cochrane Infectious Diseases Group) (23 October 2001). "Fully intermittent dosing with drugs for treating tuberculosis in adults". The Cochrane Database of Systematic Reviews (4): CD000970. doi:10.1002/14651858.CD000970. PMC 6532565. PMID 11687088.
- ^ Bose A, Kalita S, Rose W, Tharyan P, et al. (Cochrane Infectious Diseases Group) (January 2014). "Intermittent versus daily therapy for treating tuberculosis in children". The Cochrane Database of Systematic Reviews. 2014 (1): CD007953. doi:10.1002/14651858.CD007953.pub2. PMC 6532685. PMID 24470141.
- ^ O'Brien RJ (June 1994). "Drug-resistant tuberculosis: etiology, management and prevention". Seminars in Respiratory Infections. 9 (2): 104–12. PMID 7973169.
- ^ Centers for Disease Control and Prevention (CDC) (March 2006). "Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000–2004". MMWR. Morbidity and Mortality Weekly Report. 55 (11): 301–5. PMID 16557213. Archived from the original on 22 May 2017.
- ^ a b McKenna M (12 January 2012). "Totally Resistant TB: Earliest Cases in Italy". Wired. Archived from the original on 14 January 2012. Retrieved 12 January 2012.
- ^ Migliori GB, De Iaco G, Besozzi G, Centis R, Cirillo DM (May 2007). "First tuberculosis cases in Italy resistant to all tested drugs". Euro Surveillance. 12 (5): E070517.1. doi:10.2807/esw.12.20.03194-en. PMID 17868596.
- ^ "Totally Drug-Resistant TB: a WHO consultation on the diagnostic definition and treatment options" (PDF). World Health Organization (WHO). Archived (PDF) from the original on 21 October 2016. Retrieved 25 March 2016.
- ^ a b c d e f g h i j k l m n o p Kielstra P (30 June 2014). Tabary Z (ed.). "Ancient enemy, modern imperative – A time for greater action against tuberculosis" (PDF). The Economist. Economist Intelligence Unit. Archived from the original (PDF) on 10 August 2014. Retrieved 22 January 2022.
- ^ Singh B, Cocker D, Ryan H, Sloan DJ, et al. (Cochrane Infectious Diseases Group) (March 2019). "Linezolid for drug-resistant pulmonary tuberculosis". The Cochrane Database of Systematic Reviews. 3 (3): CD012836. doi:10.1002/14651858.CD012836.pub2. PMC 6426281. PMID 30893466.
- ^ Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, ZiaZarifi AH, et al. (August 2009). "Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran". Chest. 136 (2): 420–425. doi:10.1378/chest.08-2427. PMID 19349380.
- ^ "Provisional CDC Guidelines for the Use and Safety Monitoring of Bedaquiline Fumarate (Sirturo) for the Treatment of Multidrug-Resistant Tuberculosis". Archived from the original on 4 January 2014.
- ^ Theron G, Peter J, Richardson M, Warren R, Dheda K, Steingart KR, et al. (Cochrane Infectious Diseases Group) (September 2016). "® MTBDRsl assay for resistance to second-line anti-tuberculosis drugs". The Cochrane Database of Systematic Reviews. 2016 (9): CD010705. doi:10.1002/14651858.CD010705.pub3. PMC 5034505. PMID 27605387.
- ^ "The use of molecular line probe assays for the detection of resistance to second-line anti-tuberculosis drugs" (PDF). World Health Organization. Archived (PDF) from the original on 22 September 2021. Retrieved 18 June 2021.
- ^ Pillay S, Steingart KR, Davies GR, Chaplin M, De Vos M, Schumacher SG, et al. (18 May 2022). Cochrane Infectious Diseases Group (ed.). "Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin". Cochrane Database of Systematic Reviews. 2022 (5). doi:10.1002/14651858.CD014841.pub2. PMC 9115865. PMID 35583175.
- ^ "WHO Disease and injury country estimates". World Health Organization (WHO). 2004. Archived from the original on 11 November 2009. Retrieved 11 November 2009.
- ^ Lambert ML, Hasker E, Van Deun A, Roberfroid D, Boelaert M, Van der Stuyft P (May 2003). "Recurrence in tuberculosis: relapse or reinfection?". The Lancet. Infectious Diseases. 3 (5): 282–7. doi:10.1016/S1473-3099(03)00607-8. PMID 12726976.
- ^ Wang JY, Lee LN, Lai HC, Hsu HL, Liaw YS, Hsueh PR, et al. (July 2007). "Prediction of the tuberculosis reinfection proportion from the local incidence". The Journal of Infectious Diseases. 196 (2): 281–8. doi:10.1086/518898. PMID 17570116.
- ^ "1.4 Prognosis – Tuberculosis". medicalguidelines.msf.org. Archived from the original on 2 June 2021. Retrieved 25 August 2020.
- ^ "Tuberculosis (TB)". World Health Organization (WHO). 16 February 2018. Archived from the original on 30 December 2013. Retrieved 15 September 2018.
- ^ "Tuberculosis". World Health Organization (WHO). 2002. Archived from the original on 17 June 2013.
- ^ "Fact Sheets: The Difference Between Latent TB Infection and Active TB Disease". Centers for Disease Control and Prevention (CDC). 20 June 2011. Archived from the original on 4 August 2011. Retrieved 26 July 2011.
- ^ Skolnik R (2011). Global health 101 (2nd ed.). Burlington, MA: Jones & Bartlett Learning. p. 253. ISBN 978-0-7637-9751-5.
- ^ "Global tuberculosis report 2013". World Health Organization (WHO). 2013. Archived from the original on 12 December 2006.
- ^ a b "The sixteenth global report on tuberculosis" (PDF). World Health Organization (WHO). 2011. Archived from the original (PDF) on 6 September 2012.
- ^ Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. (December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. hdl:10536/DRO/DU:30050819. PMC 10790329. PMID 23245604. S2CID 1541253. Archived from the original on 19 May 2020. Retrieved 18 March 2020.
- ^ a b c "Global Tuberculosis Control 2011" (PDF). World Health Organization (WHO). Archived from the original (PDF) on 17 June 2012. Retrieved 15 April 2012.
- ^ Douglas AS, Strachan DP, Maxwell JD (September 1996). "Seasonality of tuberculosis: the reverse of other respiratory diseases in the UK". Thorax. 51 (9): 944–946. doi:10.1136/thx.51.9.944. PMC 472621. PMID 8984709.
- ^ Martineau AR, Nhamoyebonde S, Oni T, Rangaka MX, Marais S, Bangani N, et al. (November 2011). "Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa". Proceedings of the National Academy of Sciences of the United States of America. 108 (47): 19013–19017. doi:10.1073/pnas.1111825108. PMC 3223428. PMID 22025704.
- ^ Parrinello CM, Crossa A, Harris TG (January 2012). "Seasonality of tuberculosis in New York City, 1990-2007". The International Journal of Tuberculosis and Lung Disease. 16 (1): 32–37. doi:10.5588/ijtld.11.0145. PMID 22236842.
- ^ a b Korthals Altes H, Kremer K, Erkens C, van Soolingen D, Wallinga J (May 2012). "Tuberculosis seasonality in the Netherlands differs between natives and non-natives: a role for vitamin D deficiency?". The International Journal of Tuberculosis and Lung Disease. 16 (5): 639–644. doi:10.5588/ijtld.11.0680. PMID 22410705.
- ^ Koh GC, Hawthorne G, Turner AM, Kunst H, Dedicoat M (2013). "Tuberculosis incidence correlates with sunshine: an ecological 28-year time series study". PLOS ONE. 8 (3): e57752. Bibcode:2013PLoSO...857752K. doi:10.1371/journal.pone.0057752. PMC 3590299. PMID 23483924.
- ^ Kuddus MA, McBryde ES, Adegboye OA (September 2019). "Delay effect and burden of weather-related tuberculosis cases in Rajshahi province, Bangladesh, 2007–2012". Scientific Reports. 9 (1): 12720. Bibcode:2019NatSR...912720K. doi:10.1038/s41598-019-49135-8. PMC 6722246. PMID 31481739.
- ^ "Tuberculosis incidence (per 100,000 people)". Our World in Data. Archived from the original on 26 September 2019. Retrieved 7 March 2020.
- ^ "Tuberculosis deaths by region". Our World in Data. Archived from the original on 8 May 2020. Retrieved 7 March 2020.
- ^ "Deaths from tuberculosis, by age". Our World in Data. Retrieved 8 April 2025.
- ^ Griffith DE, Kerr CM (August 1996). "Tuberculosis: disease of the past, disease of the present". Journal of PeriAnesthesia Nursing. 11 (4): 240–45. doi:10.1016/S1089-9472(96)80023-2. PMID 8964016.
- ^ "Global Tuberculosis Control Report, 2006 – Annex 1 Profiles of high-burden countries" (PDF). World Health Organization (WHO). Archived from the original (PDF) on 26 July 2009. Retrieved 13 October 2006.
- ^ "2005 Surveillance Slide Set". Centers for Disease Control and Prevention. 12 September 2006. Archived from the original on 23 November 2006. Retrieved 13 October 2006.
- ^ FitzGerald JM, Wang L, Elwood RK (February 2000). "Tuberculosis: 13. Control of the disease among aboriginal people in Canada". Canadian Medical Association Journal. 162 (3): 351–55. PMC 1231016. PMID 10693593.
- ^ Quah SR, Carrin G, Buse K, Heggenhougen K (2009). Health Systems Policy, Finance, and Organization. Boston: Academic Press. p. 424. ISBN 978-0-12-375087-7. Archived from the original on 6 September 2015.
- ^ "Origins of the MRC". Medical Research Council. Archived from the original on 11 April 2008. Retrieved 7 October 2006.
- ^ "WHO report shows global tuberculosis cases are rising | CIDRAP". www.cidrap.umn.edu. 29 October 2024. Retrieved 31 October 2024.
- ^ Shkolnikov VM, Meslé F (1996). "The Russian Epidemiological Crisis as Mirrored by Mortality Trends". In DaVanzo J, Farnsworth G (eds.). Russia's Demographic "Crisis". RAND Corporation. p. 142. ISBN 0-8330-2446-9. Archived from the original on 20 February 2023. Retrieved 20 February 2023.
- ^ "Global Tuberculosis Control". World Health Organization. 2011. Archived from the original on 12 December 2006.
- ^ "WHO global tuberculosis report 2016. Annex 2. Country profiles: Russian Federation". Archived from the original on 14 July 2017. Retrieved 22 August 2020.
- ^ a b "Global Tuberculosis Report 2018" (PDF). Archived (PDF) from the original on 7 August 2020. Retrieved 27 September 2019.
- ^ Tomlinson C (10 November 2023). "In-depth: What new WHO TB numbers mean for South Africa". Spotlight. Retrieved 27 March 2024.
- ^ "WHO Global tuberculosis report 2016: India". Archived from the original on 6 February 2018. Retrieved 22 August 2020.
- ^ "Govt revisits strategy to combat tuberculosis". Daily News and Analysis. 8 April 2017. Archived from the original on 3 June 2021. Retrieved 22 August 2020.
- ^ Mahla RS (August 2018). "Prevalence of drug-resistant tuberculosis in South Africa". The Lancet. Infectious Diseases. 18 (8): 836. doi:10.1016/S1473-3099(18)30401-8. PMID 30064674.
- ^ Al-Azem A, Kaushal Sharma M, Turenne C, Hoban D, Hershfield E, MacMorran J, et al. (1998). "Rural outbreaks of Mycobacterium tuberculosis in a Canadian province". Abstr Intersci Conf Antimicrob Agents Chemother. 38: 555. abstract no. L-27. Archived from the original on 18 November 2011.
- ^ "Tuberculosis (TB): Monitoring". Government of Canada. 4 March 2024. Archived from the original on 26 March 2024. Retrieved 30 October 2024.
- ^ Birn AE (2009). Textbook of International Health: Global Health in a Dynamic World. Oxford University Press. p. 261. ISBN 978-0-19-988521-3. Archived from the original on 6 September 2015.
- ^ a b Williams PM, Pratt RH, Walker WL, Price SF, Stewart RJ, Feng PI (2024). "Tuberculosis — United States, 2023". MMWR. Morbidity and Mortality Weekly Report. 73 (12): 265–270. doi:10.15585/mmwr.mm7312a4. PMC 10986816. PMID 38547024.
- ^ Bendix A (7 May 2024). "California city declares a public health emergency after tuberculosis sickens 14". NBC News.
- ^ Sandhu GK (2011). "Tuberculosis: Current Situation, Challenges and Overview of its Control Programs in India". Journal of Global Infectious Diseases. 3 (2): 143–150. doi:10.4103/0974-777X.81691. ISSN 0974-777X. PMC 3125027. PMID 21731301.
- ^ Bhargava A, Pinto L, Pai M (2011). "Mismanagement of tuberculosis in India: Causes, consequences, and the way forward" (PDF). Hypothesis. 9 (1): e7. Archived from the original (PDF) on 24 October 2022.
- ^ Amdekar Y (July 2009). "Changes in the management of tuberculosis". Indian Journal of Pediatrics. 76 (7): 739–42. doi:10.1007/s12098-009-0164-4. PMID 19693453. S2CID 41788291.
- ^ a b c Lawlor C. "Katherine Byrne, Tuberculosis and the Victorian Literary Imagination". British Society for Literature and Science. Archived from the original on 6 November 2020. Retrieved 11 June 2017.
- ^ "φθίσις", Wiktionary, the free dictionary, 26 February 2025, retrieved 16 April 2025
- ^ "Hippocrates 3.16 Classics, MIT". Archived from the original on 11 February 2005. Retrieved 15 December 2015.
- ^ Caldwell M (1988). The Last Crusade. New York: Macmillan. p. 21. ISBN 978-0-689-11810-4.
- ^ Bunyan J (1808). The Life and Death of Mr. Badman. London: W. Nicholson. p. 244. Retrieved 28 September 2016 – via Internet Archive.
captain.
- ^ Byrne K (2011). Tuberculosis and the Victorian Literary Imagination. Cambridge University Press. ISBN 978-1-107-67280-2.
- ^ "About Chopin's illness". Icons of Europe. Archived from the original on 28 September 2017. Retrieved 11 June 2017.
- ^ Vilaplana C (March 2017). "A literary approach to tuberculosis: lessons learned from Anton Chekhov, Franz Kafka, and Katherine Mansfield". International Journal of Infectious Diseases. 56: 283–85. doi:10.1016/j.ijid.2016.12.012. PMID 27993687.
- ^ Rogal SJ (1997). A William Somerset Maugham Encyclopedia. Greenwood Publishing. p. 245. ISBN 978-0-313-29916-2. Archived from the original on 2 June 2021. Retrieved 4 October 2017.
- ^ Eschner K. "George Orwell Wrote '1984' While Dying of Tuberculosis". Smithsonian. Archived from the original on 24 March 2019. Retrieved 25 March 2019.
- ^ "Tuberculosis (whole issue)". Journal of the American Medical Association. 293 (22): cover. 8 June 2005. Archived from the original on 24 August 2020. Retrieved 4 October 2017.
- ^ Lemlein RF (1981). "Influence of Tuberculosis on the Work of Visual Artists: Several Prominent Examples". Leonardo. 14 (2): 114–11. doi:10.2307/1574402. JSTOR 1574402. S2CID 191371443.
- ^ Wilsey AM (May 2012). 'Half in Love with Easeful Death:' Tuberculosis in Literature. Humanities Capstone Projects (PhD Thesis thesis). Pacific University. Archived from the original on 11 October 2017. Retrieved 28 September 2017.
- ^ a b Morens DM (November 2002). "At the deathbed of consumptive art". Emerging Infectious Diseases. 8 (11): 1353–8. doi:10.3201/eid0811.020549. PMC 2738548. PMID 12463180.
- ^ "Pulmonary Tuberculosis/In Literature and Art". McMaster University History of Diseases. Retrieved 9 June 2017.
- ^ Thomson G (1 June 2016). "Van Morrison – 10 of the best". The Guardian. Archived from the original on 14 August 2020. Retrieved 28 September 2017.
- ^ "Tuberculosis Throughout History: The Arts" (PDF). United States Agency for International Development (USAID). Archived from the original (PDF) on 30 June 2017. Retrieved 12 June 2017.
- ^ Corliss R (22 December 2008). "Top 10 Worst Christmas Movies". Time. Archived from the original on 22 September 2020. Retrieved 28 September 2017.
'If you don't cry when Bing Crosby tells Ingrid Bergman she has tuberculosis', Joseph McBride wrote in 1973, 'I never want to meet you, and that's that.'
- ^ Sledzik PS, Bellantoni N (June 1994). "Brief communication: bioarcheological and biocultural evidence for the New England vampire folk belief" (PDF). American Journal of Physical Anthropology. 94 (2): 269–74. doi:10.1002/ajpa.1330940210. PMID 8085617. Archived (PDF) from the original on 18 February 2017.
- ^ "Public–Private Partnership Announces Immediate 40 Percent Cost Reduction for Rapid TB Test" (PDF). World Health Organization (WHO). 6 August 2012. Archived (PDF) from the original on 29 October 2013.
- ^ Lawn SD, Nicol MP (September 2011). "Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance". Future Microbiology. 6 (9): 1067–82. doi:10.2217/fmb.11.84. PMC 3252681. PMID 21958145.
- ^ "WHO says Cepheid rapid test will transform TB care". Reuters. 8 December 2010. Archived from the original on 11 December 2010.
- ^ Fox GJ, Dobler CC, Marks GB (September 2011). "Active case finding in contacts of people with tuberculosis". The Cochrane Database of Systematic Reviews. 2011 (9): CD008477. doi:10.1002/14651858.CD008477.pub2. PMC 6532613. PMID 21901723.
- ^ Mhimbira FA, Cuevas LE, Dacombe R, Mkopi A, Sinclair D, et al. (Cochrane Infectious Diseases Group) (November 2017). "Interventions to increase tuberculosis case detection at primary healthcare or community-level services". The Cochrane Database of Systematic Reviews. 2017 (11): CD011432. doi:10.1002/14651858.CD011432.pub2. PMC 5721626. PMID 29182800.
- ^ Braganza Menezes D, Menezes B, Dedicoat M, et al. (Cochrane Infectious Diseases Group) (August 2019). "Contact tracing strategies in household and congregate environments to identify cases of tuberculosis in low- and moderate-incidence populations". The Cochrane Database of Systematic Reviews. 2019 (8): CD013077. doi:10.1002/14651858.CD013077.pub2. PMC 6713498. PMID 31461540.
- ^ a b c d e f g Courtwright A, Turner AN (July–August 2010). "Tuberculosis and stigmatization: pathways and interventions". Public Health Reports. 125 (4_suppl): 34–42. doi:10.1177/00333549101250S407. PMC 2882973. PMID 20626191.
- ^ Mason PH, Roy A, Spillane J, Singh P (March 2016). "Social, Historical and Cultural Dimensions of Tuberculosis". Journal of Biosocial Science. 48 (2): 206–32. doi:10.1017/S0021932015000115. PMID 25997539.
- ^ a b c Martín Montañés C, Gicquel B (March 2011). "New tuberculosis vaccines". Enfermedades Infecciosas y Microbiologia Clinica. 29 (Suppl 1): 57–62. doi:10.1016/S0213-005X(11)70019-2. PMID 21420568.
- ^ Zhu B, Dockrell HM, Ottenhoff TH, Evans TG, Zhang Y (April 2018). "Tuberculosis vaccines: Opportunities and challenges". Respirology. 23 (4): 359–368. doi:10.1111/resp.13245. hdl:1887/77156. PMID 29341430.
- ^ Ibanga HB, Brookes RH, Hill PC, Owiafe PK, Fletcher HA, Lienhardt C, et al. (August 2006). "Early clinical trials with a new tuberculosis vaccine, MVA85A, in tuberculosis-endemic countries: issues in study design". The Lancet. Infectious Diseases. 6 (8): 522–8. doi:10.1016/S1473-3099(06)70552-7. PMID 16870530.
- ^ Kaufmann SH (October 2010). "Future vaccination strategies against tuberculosis: thinking outside the box". Immunity. 33 (4): 567–77. doi:10.1016/j.immuni.2010.09.015. PMID 21029966.
- ^ Webber D, Kremer M (2001). "Stimulating Industrial R&D for Neglected Infectious Diseases: Economic Perspectives" (PDF). Bulletin of the World Health Organization. 79 (8): 693–801. Archived (PDF) from the original on 26 September 2007.
- ^ Barder O, Kremer M, Williams H (2006). "Advance Market Commitments: A Policy to Stimulate Investment in Vaccines for Neglected Diseases". The Economists' Voice. 3 (3). doi:10.2202/1553-3832.1144. S2CID 154454583. Archived from the original on 5 November 2006.
- ^ Department of Economic and Social Affairs (2009). Achieving the global public health agenda: dialogues at the Economic and Social Council. New York: United Nations. p. 103. ISBN 978-92-1-104596-3. Archived from the original on 6 September 2015.
- ^ Jong EC, Zuckerman JN (2010). Travelers' vaccines (2nd ed.). Shelton, CT: People's Medical Publishing House. p. 319. ISBN 978-1-60795-045-5. Archived from the original on 6 September 2015.
- ^ Bill and Melinda Gates Foundation Announcement (12 February 2004). "Gates Foundation Commits $82.9 Million to Develop New Tuberculosis Vaccines". Archived from the original on 10 October 2009.
- ^ Nightingale K (19 September 2007). "Gates foundation gives US$280 million to fight TB". Archived from the original on 1 December 2008.
- ^ Zumla A, Hafner R, Lienhardt C, Hoelscher M, Nunn A (March 2012). "Advancing the development of tuberculosis therapy". Nature Reviews. Drug Discovery. 11 (3): 171–172. doi:10.1038/nrd3694. PMID 22378254. S2CID 7232434. Archived from the original on 12 January 2020. Retrieved 8 May 2020.
- ^ "J&J Sirturo Wins FDA Approval to Treat Drug-Resistant TB". Bloomberg News. 31 December 2012. Archived from the original on 4 January 2013. Retrieved 1 January 2013.
- ^ Avorn J (April 2013). "Approval of a tuberculosis drug based on a paradoxical surrogate measure". JAMA. 309 (13): 1349–1350. doi:10.1001/jama.2013.623. PMID 23430122.
- ^ US Food and Drug Administration. "Briefing Package: NDA 204–384: Sirturo" (PDF). Food and Drug Administration. Archived (PDF) from the original on 4 January 2014.
- ^ Zuckerman D, Yttri J (January 2013). "Antibiotics: When science and wishful thinking collide". Health Affairs. doi:10.1377/forefront.20130125.027503. Archived from the original on 29 March 2022. Retrieved 29 March 2022.
- ^ Mbuagbaw L, Guglielmetti L, Hewison C, Bakare N, Bastard M, Caumes E, et al. (May 2019). "Outcomes of Bedaquiline Treatment in Patients with Multidrug-Resistant Tuberculosis". Emerging Infectious Diseases. 25 (5): 936–943. doi:10.3201/eid2505.181823. PMC 6478224. PMID 31002070.
- ^ a b Khoshnood S, Taki E, Sadeghifard N, Kaviar VH, Haddadi MH, Farshadzadeh Z, et al. (7 October 2021). "Mechanism of Action, Resistance, Synergism, and Clinical Implications of Delamanid Against Multidrug-Resistant Mycobacterium tuberculosis". Frontiers in Microbiology. 12: 717045. doi:10.3389/fmicb.2021.717045. PMC 8529252. PMID 34690963.
- ^ "European Medicines Agency – News and Events – European Medicines Agency recommends two new treatment options for tuberculosis". 3 December 2013. Archived from the original on 3 December 2013. Retrieved 9 April 2024.
- ^ Critchley JA, Orton LC, Pearson F (November 2014). "Adjunctive steroid therapy for managing pulmonary tuberculosis". The Cochrane Database of Systematic Reviews. 2014 (11): CD011370. doi:10.1002/14651858.CD011370. PMC 6532561. PMID 25387839.
- ^ Shivaprasad HL, Palmieri C (January 2012). "Pathology of mycobacteriosis in birds". The Veterinary Clinics of North America. Exotic Animal Practice. 15 (1): 41–55, v–vi. doi:10.1016/j.cvex.2011.11.004. PMID 22244112.
- ^ Reavill DR, Schmidt RE (January 2012). "Mycobacterial lesions in fish, amphibians, reptiles, rodents, lagomorphs, and ferrets with reference to animal models". The Veterinary Clinics of North America. Exotic Animal Practice. 15 (1): 25–40, v. doi:10.1016/j.cvex.2011.10.001. PMID 22244111.
- ^ Mitchell MA (January 2012). "Mycobacterial infections in reptiles". The Veterinary Clinics of North America. Exotic Animal Practice. 15 (1): 101–11, vii. doi:10.1016/j.cvex.2011.10.002. PMID 22244116.
- ^ Wobeser GA (2006). Essentials of disease in wild animals (1st ed.). Ames, IO [u.a.]: Blackwell Publishing. p. 170. ISBN 978-0-8138-0589-4. Archived from the original on 6 September 2015.
- ^ Ryan TJ, Livingstone PG, Ramsey DS, de Lisle GW, Nugent G, Collins DM, et al. (February 2006). "Advances in understanding disease epidemiology and implications for control and eradication of tuberculosis in livestock: the experience from New Zealand". Veterinary Microbiology. 112 (2–4): 211–19. doi:10.1016/j.vetmic.2005.11.025. PMID 16330161.
- ^ White PC, Böhm M, Marion G, Hutchings MR (September 2008). "Control of bovine tuberculosis in British livestock: there is no 'silver bullet'". Trends in Microbiology. 16 (9): 420–7. CiteSeerX 10.1.1.566.5547. doi:10.1016/j.tim.2008.06.005. PMID 18706814.
- ^ Ward AI, Judge J, Delahay RJ (January 2010). "Farm husbandry and badger behaviour: opportunities to manage badger to cattle transmission of Mycobacterium bovis?". Preventive Veterinary Medicine. 93 (1): 2–10. doi:10.1016/j.prevetmed.2009.09.014. PMID 19846226.
- ^ Holt N (24 March 2015). "The Infected Elephant in the Room". Slate. Archived from the original on 14 April 2016. Retrieved 5 April 2016.
- ^ Mikota SK. "A Brief History of TB in Elephants" (PDF). Animal and Plant Health Inspection Service (APHIS). Archived (PDF) from the original on 6 October 2016. Retrieved 5 April 2016.
External links
[edit]- "Tuberculosis (TB)". Centers for Disease Control and Prevention (CDC). 24 October 2018.
- "Tuberculosis (TB)". London: Health Protection Agency. Archived from the original on 5 July 2007.
- WHO global 2016 TB report (infographic)
- WHO tuberculosis country profiles
- "Tuberculosis Among African Americans", 1990-11-01, In Black America; KUT Radio, American Archive of Public Broadcasting (WGBH and the Library of Congress)
- Working Group on New TB drugs, tracking clinical trials and drug candidates
 KSF
KSF

![Tuberculosis deaths by region, 1990 to 2017[181]](https://upload.wikimedia.org/wikipedia/commons/thumb/b/bb/Tuberculosis_deaths_by_region%2C_OWID.svg/250px-Tuberculosis_deaths_by_region%2C_OWID.svg.png)
![Deaths from tuberculosis, by age, World[182]](https://upload.wikimedia.org/wikipedia/commons/thumb/b/bd/Tuberculosis-deaths-by-age.svg/250px-Tuberculosis-deaths-by-age.svg.png)