Biochemistry for novices
 From Wikiversity - Reading time: 2 min
From Wikiversity - Reading time: 2 min
| Subject classification: this is a chemistry resource. |
| Subject classification: this is a biology resource. |
What will I get by learning Biochemistry?
[edit | edit source]You are going to enjoy learning biochemistry more if you have background in organic chemistry or cell biology. We will have parts that cater to these groups, marked ADVANCED.
If you had only high school chemistry, you're also welcome! Just recall your lessons, and we'll ride comfortably. There are parts marked REVIEW on this page, so you can re-study these parts if you forgot already. But I'm warning you, these parts are not intended to give a comprehensive review. If you need more review on the topics than these parts give, you may get some help from books and other chemistry texts.
The (bio)chemical basis of life
[edit | edit source]What is the basis of life as biochemists view it?
One word: MOLECULES!
Biochemists see life as a magnum opus, played by a giant orchestra composed of lots and lots of molecules. These molecules act on each other, or act together, so that all living matter can exercise the processes that define LIFE: the eating and drinking, the reproducing, the feeling, the loving, the moving, the breathing, every activity we consider as part of LIVING. So, be ready to encounter lots of molecules, big and small.
We will be dealing with five classes of molecules in biochemistry.
Small molecules
[edit | edit source]Small molecules are called "small" because they are small - comparing them to the next four molecule classes. They have molecular masses below 600 atomic mass units, which means that one mole of molecules of this kind would weigh less than 600 grams!
REVIEW
Atomic mass units are the units of the weight of a molecule.
An atomic mass unit is about 10-27 kilogram.
A mole of molecules is 6.022 x 1023 molecules.
The molar mass of a compound is the mass of one mole of molecules of that compound.
Almost all molecules enountered in high school chemistry are therefore considered small.
Benzene (MM = 72 g/mol) is small.
Sucrose (table sugar; MM = 180 g/mol) is small. 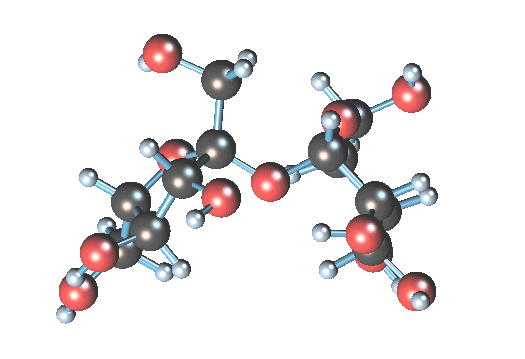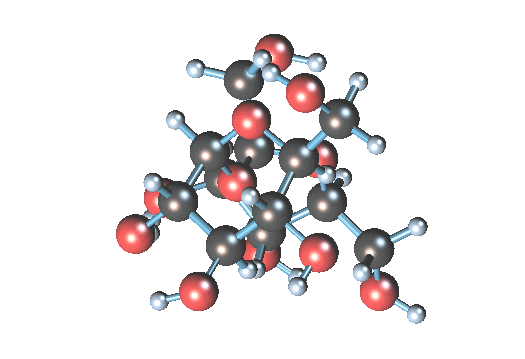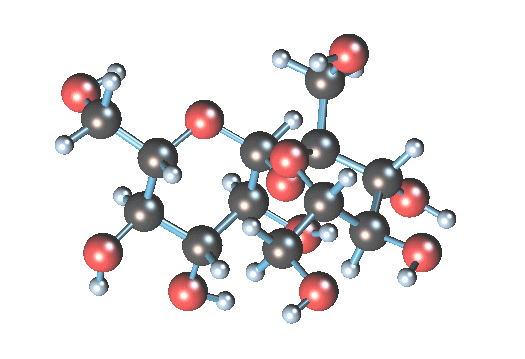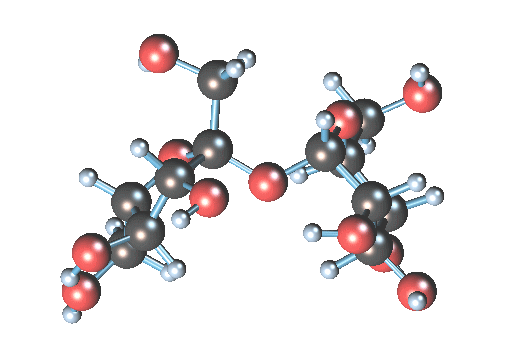
Even coronene (C24H12) is small. Can you calculate the molar mass of coronene?
Proteins
[edit | edit source]Proteins are made by joining together small molecules called amino acids.
Glycine is one such amino acid.
Glycine has two main parts, an -NH2 part and a -COOH part. The -NH2 part of one molecule of glycine can react with the -COOH part of another molecule of glycine to form glycylglycine. And glycylglycine, which has an -NH2 part and a -COOH part as well, can react with another molecule of glycylglycine in the same way! This way, long molecules called polypeptides are formed. And proteins are polypeptides that have been formed by the body naturally.
Some famous proteins are:
- Papain: used in whitening soaps. It's called a protein hydrolase because it's a protein that hydrolyzes other proteins. Another term for protein hydrolase is protease.
- Collagen: it forms the basis for connective tissues in animals' bodies (for example, the skin). It's a protein made up of mostly glycine and proline, another amino acid. Of course it has other amino acids in it.
 KSF
KSF