Central Nervous System
 From Wikiversity - Reading time: 29 min
From Wikiversity - Reading time: 29 min
Introduction to the CNS
[edit | edit source]Most drugs that affect the central nervous system (CNS) act by altering some step in the neurotransmission process. Drugs may act presynaptically by influencing the production, storage, or termination of action of neurotransmitters. Other agents may activate or block postsynaptic receptors.
In many ways, the basic functioning of neurons in the CNS is similar to that of the autonomic nervous system. E.g. transmission of information in the CNS and in the periphery both involve the release of neurotransmitters that diffuse across the synaptic space to bind to specific receptors on the postsynaptic neuron. In both systems, the recognition of the transmitter by the membrane receptor of the postsynaptic neuron triggers intracellular changes. Several major differences exists between neurons in the peripheral autonomic nervous system and those of the CNS. The circuitry of the CNS is much more complex than the autonomic nervous system, and the number of synapses in the CNS is far greater. The CNS, unlike the peripheral nervous system, contains powerful networks of inhibitory neurons that are constantly active in modulating the rate of neuronal transmission. In addition, the CNS communicates through the use of more than 50 different neurotransmitters. In contrast, the autonomic system uses only two primary neurotransmitters, acetylcholine and noradrenaline.
General Anesthetics
[edit | edit source]General anesthesia is essential to surgical practice because it renders patients
(1) analgesics,
(2) amnesic,
(3) unconscious while causing
(4) muscle relaxation and
(5) suppression of undesirable reflexes.
No single drug is capable of achieving these effects rapidly and safely. Rather, several different categories of drugs are utilized to produce “balanced anesthesia”. E.g. adjuncts to anesthesia consist of preanesthetic medication and skeletal muscle relaxants. Preanesthetic medication serves to calm the patient, relieve pain, and protect against undesirable effects of the subsequently administered anesthetic. Skeletal muscle relaxants facilitate intubation and suppress muscle tone to the degree required for surgery. Potent general anesthetics are delivered via inhalation or intravenous injection. With the exception of nitrous oxide, modern inhaled anesthetics are all volatile, halogenated hydrocarbons that derive early research and clinical experience with diethyl ether and chloroform. On the other hand, intravenous general anesthetics consist of a number of chemically unrelated drug types that are commonly used for the rapid induction of anesthesia.
Concominant use of drugs
Quite often, surgical patients receive one or more of the following preanesthetic medications: benzodiazepines (e.g. diazepam) to relieve anxiety and facilitate amnesia; barbiturates (e.g. pentobarbital) for sedation; antihistamines for prevention of allergic reactions (e.g. dimedrol); antiemetics (e.g. droperidol); opioids (e.g. fentanyl) for analgesia; anticholinergics (e.g. scopolamine) to prevent bradycardia and secretion of fluids into the respiratory tract. These agents facilitate smooth induction of anesthesia, and lower the dose of anesthetic required to maintain the desired level of surgical (Stage III) anesthesia.
Induction, maintenance and recovery
Anesthesia can be divided into three stages: induction, maintenance, and recovery. Induction is defined as the period of time from onset of administration of the anesthetic to the development of effective surgical anesthesia in the patient. Maintenance provides a sustained surgical anesthesia. Recovery is the time from discontinuation of administration of anesthesia until consciousness is regained. Induction of anesthesia depends on how fast effective concentration of anesthetic drug reach the brain; recovery is the reverse of induction and depends on how fast the anesthetic drug is removed from the brain.
Depth of anesthesia
The depth of anesthesia can be divided into a series of four sequential stages; each is characterized by increased CNS depression that is caused by accumulation of the anesthetic drug in the brain. With ether, which produce a slow onset of anesthesia, all the stages are discernible. However, with halothane and many other commonly used anesthetics, the stages are difficult to clearly characterize because of the rapidity of onset of anesthesia.
Stage I–analgesia: Loss of pain sensation results from interference with sensory transmission in the spinothalamic tract. The patient is conscious and conversational. A reduced awareness of pain occurs as Stage II is approached.
Stage II–excitement: The patient experiences delirium and violent combative behaviour. There is a rise and irregularity in blood pressure. The respiratory rate may be increased. To avoid this stage of anesthesia, a short acting barbiturate, such as sodium thiopental, is given i.v. before inhalation anesthesia is administered.
Stage III–surgical anesthesia: Regular respiration and relaxation of the skeletal muscle occur in this stage. Eye reflexes decrease progressively, until the eye move-ments cease and the pupil is fixed. Surgery may proceed during this stage.
Stage IV–medulary paralysis: Severe depression of the respiratory center and vasomotor center occur during this stage. Death can rapidly ensue.
Inhalation Anesthetics
[edit | edit source]Inhaled gases are the mainstay of anesthesia and are primarily used for the maintenance of anesthesia after administration of an intravenous agent. Inhalation anesthetics have a benefit that is not available with intravenous agents, since the depth of anesthesia can be rapidly altered by changing the concentration of the inhaled anesthetic. Because most of these agents are rapidly eliminated from the body, they do not cause postoperative respiratory depression.
A. Common features of inhaled anesthetics: Modern inhalation anesthetics are non-explosive agents that include the gas nitrous oxide as well as a number of volatile halogenated hydrocarbons. As a group, these agents decrease cerebrovascular resistance, resulting in increased perfusion of the brain. They cause bronchodilation and decrease minute ventilation. Their clinical potency cannot be predicted by their chemical structure, but potency does correlate with their solubility in lipid. The movement of these agents from the lungs to the different body compartments depends upon their solubility in blood and various tissues. Recovery from their effects is due to their redistribution from the brain.
B. Potency: The potency of inhaled anesthetics is defined quantitatively as the minimum alveolar concentration (MAC), which is the concentration of anesthetic gas needed to eliminate movement among 50% of patients challenged by standardized skin incision. The MAC is usually expressed as the percent of gas in a mixture required to achieve the effect. Numerically, is small for potent anesthetics, such as halothane, and large for less potent agents, such as nitrous oxide (see diagram 6.1). The MAC values are useful in comparing pharmacologic effects of different anesthetics. The more lipid-soluble an anesthetic, the lower the concentration of anesthetic needed to produce anesthesia.
. Uptake and Distribution: The partial of an anesthetic gas at the origin of the respiratory pathway is the driving force that moves the anesthetic into the alveolar space and thence into the blood, which delivers the drug to the brain and various other body compartments. Since gases move from one compartment to another within the body according to partial pressure gradients, a steady state is achieved when the partial pressure in each of these compartments is equivalent to that in the inspired mixture. The time course for attaining this steady state is determined by the following three factors:
1. alveolar wash-in: This term refers to the replacement of the normal lung gases with the inspired anesthetic mixture. The time required for this process is directly proportional to the functional residual capacity of the lung, and inversely proportional to the ventilatory rate; it is independent to the physical properties of the gas. Once the partial pressure builds within the lung, anesthetic uptake from the lung begins.
2. solubility in the blood: The first compartment that the anesthetic gas encountersis the blood. Solubility in blood is determined by a physical property of the anesthetic molecule called the blood/gas partion coefficient, which is the ratio of the total amount of gas in the blood relative to the gas equilibrium phase. Drugs with low versus high solubility in blood differ in their speed of induction of anesthesia. E.g. when an anesthetic gas with low blood solubility, such as nitrous oxide, diffuses from the alveoli into the circulation, little of the anesthetic dissolves in the blood. Therefore, the equilibrium between the inhaled anesthetic and arterial blood occur rapidly, and relative few additional molecules of anesthetic are required to raise arterial tension (that is, steady state is rapidly achieved). In contrast, an anesthetic gas with high blood solubility, such as halothane, dissolves more completely in the blood, and greater amount of the anesthetic and longer periods of time are required to raise arterial tension. This results in increased time of induction and recovery, and slower changes in the depth of anesthesia in response to changes in the concentration of the inhaled drug.
3. tissue uptake: The arterial circulation distributes the anesthetic to various tissues, and the pressure gradient drives free anesthetic gas into tissues. The time required for a particular tissue to achieve a steady-state is inversely proportional to the blood flow to that tissue (faster flow results in a more rapidly achieved steady-state), and directly proportional to the capacity to store anesthetic (larger capacity results in a longer time to achieve steady-state). Capacity, in turn, is directly proportional to the tissue’s volume, and the tissue/blood solubility coefficient of the anesthetic. On the basis of these considerations, four major compartments determine the time course of anesthetic uptake:
a. Brain, heart, liver, kidney, endocrine glands: These highly perfused tissues rapidly attain a steady-state with the partial pressure of the anesthetic in blood.
b. Skeletal muscles: These are poorly perfused during anesthesia. This fact prolongs the time required to achieve steady-state.
c. Fat: This tissue is also poorly perfused. However, potent general anesthetics are very lipid soluble. Therefore, fat has a large capacity to store anesthetic. This combination of slow delivery to a high capacity prolongs the time required to achieve steady state.
d. Bone, ligaments, and cartilage: These are poorly perfused and have a relatively low capacity to store anesthetic. Therefore, these tissues have only a slight impact on the time course of anesthetic distribution in the body.
4. washout: When the administration of an inhalation anesthetic is discontinued, the body now becomes the “source” that drives the anesthetic into the alveolar spece. The same factors that influence attainment of steady-state with an inspired anesthetic determine the time course of clearace of the drug from the body.
D. Specific inhalation anesthetics
Each of the halogenated gases has characteristics beneficial for selected clinical applications. No one anesthetic is superior to another under all circumstances. [Note: In a very small population of patients, all of the halogenated hydrocarbon anesthetics have the potential to induce malignant hyperthermia. While the etiology of this condition is unknown, it appears to be inherited. Should a patient exhibit the hyperthermia and muscle rigidity characteristic to malignant hyperthermia, dantrolene is given as the anesthetic mixture is withdrawn.]
1. Halothane (ftorothane): This agent is the prototype to which newer agents in this series of anesthetics are compared. While halothane is a potent anesthetic, it is a relatively weak analgesic. Thus, halothane is usually co-administered with nitrous oxide, opioids,or local anesthetics. Like other halogenated hydrocarbons, halothane is vagomimetic and will cause atropine-sensitive bradycardia. In addition, halothane has the undesirable property of causing cardiac arrhythmias. Halothane is oxidatively metabolized in the body to tissue-toxic hydrocarbons (e.g. trifluroethanol) and bromide ion. These substances may be responsible for the toxic reactions that same patients (especially females) develop after halothane anesthesia. This reaction begins as a fever, anorexia, nausea, and vomiting, and patients may exhibit signs of hepatitis. Although the incidence of this reaction is low––approximately 1 in 10,000 individuals––50% of such patients will die of hepatic necrosis. [Note: Halothane is not hepatotoxic in pediatric patients and that, combined with its pleasant odor, make it the agent of choice in children.] Halothane anesthesia is not repeated at intervals less than 2 to 3 weeks.
2. Enflurane: This gas is less potent than halothane but it produces rapid induction and recovery. About 2% of the agent is metabolized to fluoride ion, which is excreted by the kidney. Therefore, enflurane is contraindicated in patients with kidney failure. Enflurane anesthesia exhibits the following differences from halothane: fewer arrhythmias, less sensitization of the heart catecholamines, and greater potentiation of muscle relaxants. A disadvantage of enflurane is that it causes CNS excitation at twice the MAC and at lower doses if hyperventilation reduces the pCO2.
3. Isoflurane: This is a newer halogenated anesthetic that has low biotransformation and low organ toxicity. Unlike the other halogenated anesthetic gases, isoflurane does not induce cardiac arrhythmias and does not sensitize the heart to the action of catecholamines. Isoflurane is a vary stable molecule that undergoes little metabolism, as a result of which, less fluoride is produced. Isoflurane is not currently believed to be tissue toxic.
4. Methoxyflurane: The agent is the most potent inhalation anesthetic because of its high solubility in lipid. Prolonged administration of methoxyflurane is associated with metabolic release of fluoride, which is toxic to the kidneys. Therefore, methoxyflurane is rarely used outside obstetric practice. It finds use in child-birth because it does not relax the uterus when briefly inhaled.
5. Nitrous oxide: Whereas nitrous oxide (N2O or “laughing gas”) is a potent analgesic, it is a weak general anesthetic. Thus, it is frequently combined with other more potent agents. Because it moves very rapidly into and out of the body, nitrous oxide can increase the volume (pneumothorax) or pressure (sinuses) within closed body compartments. Furthermore, its speed of movement allows nitrous oxide to retard oxygen uptake during recovery, thus causing diffusion hypoxia. This anesthetic does not depress respiration nor does it produce muscle relaxation. It also has the least effect on cardiovasculaar system and on increasing cerebral blood flow, and is the least hepatotoxic of the inhalation anesthetics. It is therefore probably the safest of these anesthetics, provided that at least 20% of oxygen is always administered at the same time. [Note: Nitrous oxide at 80% (without other adjunct agents) cannot produce surgical anesthesia.] Nitrous oxide is often employed of 30% in combination with oxygen for analgesia, particularly in dental surgery.
Intravenous Anesthetics
[edit | edit source]Intravenous anesthetics are often used for the rapid induction of anesthesia, which is then maintained with an appropriate inhalation agent. They rapidly induce anesthesia, and must therefore be injected slowly. Recovery from intravenous anesthetics is due to redistribution from sites in the CNS.
A. Barbiturates
Thiopental is a potent anesthetic and a weak analgesic. It is the most widely used intravenously administered general anesthetic. It is an ultra-short-acting barbiturate and has a high lipid solubility. When thiopental is administered intravenously, it quickly enters the CNS and depresses function, often in less than 1 minute. However, diffusion out of the brain can occur very rapidly as well, because of redistribution of the drug to other body tissues, including skeletal muscle and ultimately adipose tissue. This latter site serves as a reservoir of drug from which the agent slowly leaks out and is metabolized and excreted. The short duration of action is due to the decrease of its concentration in the brain. Thus, metabolism of thiopental is much slower than tissue redistribution. The barbiturates are not significantly analgesic and require some type of supplementary analgesic administration during anesthesia. Thiopental has minor effects on the cardiovascular system, but it may contribute to severe hypotension in hypovolemic or shock patients. All barbiturates can cause apnea, coughing, chest wall spasm, laryngospasm, and bronchospasm. Barbiturates are contraindicated in patients with acute intermittent or variegate porphyria.
B. Benzodiazepines
Although diazepam is the prototype benzodiazepine, lorazepam and midazolam are more potent. All three facilitate amnesia while causing sedation. Midazolam has become popular because of its combination of water solubility, rapid onset and short duration of action. It produces amnesia with few side effects. Mental function returns to normal within 4 hours, making it popular choice for ambulatory surgery and during regional anesthesia.
C. Opioids
Because of their analgesic property, opioids are frequently used together with other anesthetics; e.g. the combination of morphine and nitrous oxide provide good anesthesia for cardiac surgery. However, opioids are not good amnesics and they can cause hypotension, respiratory depression, and muscle rigidity as well as post-anesthetic nausea and vomiting. Fentanyl is more frequently used than morphine.
The combination of droperidol and fentanyl is a fixed ration preparation called thalamonal (innovar). Since droperidol is a neuroleptic substance, THALAMONAL is said to produce neurolept analgesia. A neuroleptic has adrenergic blocking as well as sedative, antiemetic properties.
D. Ketamine
Ketamine, a short-acting nonbarbiturate anesthetic, induces a dissociated state in which the patient appears awake but is unconscious and does not feel pain. This dissociative anesthesia provides sedation, amnesia, and immobility. Ketamine stimulates the central sympathetic outflow, which in turn, causes stimulation of the heart and increases blood pressure and cardiac output. Ketamine is therefore used when circulatory depression is undesirable. On the other hand, these effects mitigate against the use of ketamine in hypertensive or stroke patients. The drug is lipophilic and enters the brain very quickly, but like the barbiturates, it can redistribute to other organs and tissues. It is metabolized in the liver. Ketamine is employed mainly in children and young adults for short procedures. It is not widely used because it increases cerebral blood flow and induces postoperative hallucinations (nightmares).
E. Propanidide
Propanidide (Sombrevin) is an ultra-short-acting intravenous anesthetic. Onset is smooth and occurs within about 40 seconds of administration. The emergence from anesthesia from propanidide is more rapid than that from thiopental and is characterized by minimal posoperative confusion.
F. Sodium oxybutyrate
Sodium oxybutirate is a derivative of -aminobutyric acid (GABA). GABA is an inhibitory neurotransmitter in the CNS. In contrast to GABA, sodium oxybutyrate readily crosses the blood-brain barier and produces sedative, hypnotic, anesthetic, anticonvulsive, and antihypoxic effects. However, sodium oxybutyrate is not good analgesic. The onset of action is slow (in 30-40 minutes after i.v. injection). It must be used with other anesthetics for surgical anesthesia.
Ethyl Alcohol (Ethanol)
[edit | edit source]Pharmacology of alcohol is important for its presence in beverages (which have been used since recorded history) and for alcohol intoxication, rather than as a drug. Alcohol is manufactured by fermentation of sugar:
C6H12O6 2CO2 + 2C2H5OH
Fermentation proceeds till alcohol content reaches 15%. Then reaction is inhibited by alcohol itself.
1. Local action: Rubbed on skin ethanol dissolves fat, precipitate surface proteins and harden skin. By precipitation bacterial proteins it acts as an antiseptic. The antiseptic action increases with concentration from 20% to 70%, remain constant from 70 to 90% and decreases above that. 100% ethanol is more dehydrating but poorer antiseptic than 90% ethanol. Applied to delicate skin or mucous membrane ethanol produces irritation and burning sensation. Injected s.c. it causes intense pain, inflammation and necrosis followed by fibrosis. Injected round a nerve it produces permanent damage.
2. Resorbtive action: Ethanol is a neuronal depressant. However, as highest areas are most sensitive to alcohol (these are primarily inhibitory),––excitation and euphoria are experienced at lower plasma concentrations (30-100mg/dl). Caution, self criticism and restraint are lost first. With the increasing concentration (100-150mg/dl) mental clouding, disorganization of thought and drowsiness occur. At 50-200mg/dl the person is sloppy, ataxic and drunk; 200-300mg/dl results in stupor and above this unconsciousness prevails, medulary centers are paralyzed and death may occur. Though, ethanol can produce anesthesia, margin of safety is narrow. Effects of alcohol are more marked when the concentration is rising than when it is falling.
Small doses of alcohol produce cutaneous and gastric vasodilation, a sense of warmth, but heat lost is increased in cold surroundings.
Alcoholic beverages have variable effect on gastric secretion depending on beverage itself and whether the individual likes it. Dilute alcohol (optimum 10%) is a strong stimulant of gastric secretion. Higher concentrations (above 20%) inhibit gastric secretion.
Regular intake of small amounts of alcohol has been found to raise high density lipids and diminish low density lipids levels in plasma. This may be responsible for the somewhat lower incidence of coronary artery disease.
3. Pharmacokinetics: Rate of alcohol absorption from stomach is dependant on its concentration, presence of food, and other factors. Absorption from intestines is very fast. Thus, gastric emptying determines rate of absorption. Alcohol distributes widely in the body, crosses blood-brain barrier (concentration in the brain is very near to blood concentration); it also crosses placenta freely. It is oxidized in liver.
Metabolism of alcohol follows zero order kinetics, i.e. a constant amount is degraded in unit time, irrespective to blood concentration (8-10 ml of absolute alcohol/hour). Small amounts are excreted through kidney and lungs.
4. Acute alcoholic intoxication: Nausea, vomiting, hypotension, hypoglycemia, collapse, respiratory depression, coma and death.
Treatment: Gastric lavage, maintain patent airway and aspiration of vomitus. Most patient will recover with supportive treatment and maintenance of fluid and electrolyte balance. Insuline+fructose has been found to accelerate alcohol metabolism.
Disulfiram (teturam, esperal) has found some use in the patients seriously desiring to stop alcohol ingestion. The drug blocks the oxidation of acetaldehyde to acetic acid by inhibiting aldehyde dehydrogenase. This result in accumulation of acetaldehyde in the blood, causing flushing, tachycardia, hyperventilation, and nausea.
Hypnotic Drugs
[edit | edit source]Sleep is an active, circadian, physiological depression of consciousness. It is characterized by cyclical electroencephalographic (EEG) and eye movement changes. Normal sleep (categorized by eye movement) is of two kinds:
1. NREM (non-rapid eye movement), orthodox, forebrain or slow-wave EEG sleep. Heart rate, blood pressure and respiration are steady or decline and muscles are relaxed; growth hormone secretion is maximal.
2. REM (rapid eye movement), paradoxical, hindbrain of fast-wave EEG sleep; awakened subjects state they were ‘dreaming’. Heart rate, blood pressure and respiration are increased, skeletal muscles are profoundly relaxed.
Cycles of NREM sleep (about 90 min) alternate with REM sleep (about 20 min). thus, normal sleep contains 4-6 cycles (NREM+REM).
Classification of insomnia (sleep disorders):
a.Difficulty in getting to sleep, onset insomnia; this was associated with neurotic personality disorder. People who lie awake in bed for hours unable to relax, and then sleep well.
b.Difficulty in staying asleep, repeated awakenings.
c.Early waking in which sleep is shorter.
In general, a short-acting hypnotic drug is prescribed for patients who have prolonged sleep latency but who sleep well once sleep ensues; and a drug with a longer duration of action for those who awaken early and have difficulty in returning to sleep. Insomnia has many causes, and an accurate differential diagnosis is required before treatment should be considered. Prescription of a hypnotic without regard to the underlying disturbance subjects the patient to the risk of abuse, may mask the signs and symptoms of a pernicious disease etc.
Barbituates
[edit | edit source]Barbital was introduced in 1903 and phenobarbital in 1912. The barbiturates were formerly the mainstay of treatment used to sedate the patient or to induce and maintain sleep. Today, they have been largely replaced by the benzodiazepines, mainly because barbiturates induce tolerance, drug-metabolizing enzymes, physical dependence, and very severe withdrawal symptoms. Foremost is their ability to cause coma in toxic doses. Certain barbiturates, such as the very short-acting thiopental, are still used to induce anesthesia.
1. Mode of action
Barbiturates are thought to interfere with Na+ and K+ transport across cell membrane. This leads to inhibition of the mesencephalic reticular activation system. Polysynaptic transmission is inhibited in all areas of the CNS. Barbiturates also potentiate GABA action on chloride entry into the neuron, although they do not bind at the benzodiazepine receptor.
2.Actions
2.1 Depression of the CNS: The barbiturates can produce all degrees of depression of the CNS. Low doses produce sedation (calming effect, reducing excitement). At high doses, the drugs cause hypnosis, followed by anesthesia (loss of feeling or sensation), and finally coma and death. Thus, any degree of depression of the CNS is possible, depending on the dose. Barbiturates do not raise the pain threshold and have no analgesic properties. They may even exacerbate pain.
2.2 Respiratory depression: Barbiturates suppress the hypoxic and chemoreceptor response to CO2, and overdosage is followed by respiratory depression and death.
2.3 Enzyme induction: Barbiturates induce P-450 microsomal enzymes in the liver. Therefore, chronic barbiturate administration diminishes the action of many drugs that are dependant on P-450 metabolism (including barbiturates itself).
Barbiturates are classified according to their duration of action:
1. Long-acting (phenobarbital, barbital, barbital-sodium), which have a duration of action greater than a day; phenobarbital is useful in the treatment of seizures.
2. Intermediate-acting (pentobarbital, cyclobarbital, secobarbital), which are effective as sedative and hypnotic (but not antianxiety) agents.
3. Short-acting (hexobarbital).
4. Ultra short-acting (thiopental, hexenal), which act within seconds and have a duration of action of about 30 minutes, are used in the intravenous induction of anesthesia.
3. Therapeutic uses
3.1 Anesthesia: Selection of a barbiturate is strongly influenced by the desired duration of action. The ultra-short-acting barbiturates, such as thiopental, are used intravenously to induce anesthesia.
3.2 Anticonvulsant: Phenobarbital is used in long-term management of tonic-clonic seizures, status epilepticus, and eclampsia. Phenobarbital has been regarded as the drug of choice for treatment of young children with recurrent febrile seizures. However, phenobarbital can depress cognitive performance in children, and the drug should be used cautiously. Phenobarbital has specific anticonvulsant activity that is distinguished from the nonspecific CNS depression.
3.3 Sedation-hypnosis: Barbiturates have been used as mild sedative to relieve nervous tension, and insomnia. Most have been replaced by benzodiazepines.
4. Pharmacokinetics
Barbiturates are absorbed orally and distributed widely throughout the body. All barbiturates redistribute from the brain to the splanchnic areas, to skeletal muscle, adipose tissue. This movement is important in causing the short duration of action of thiopental. Barbiturates are metabolized in the liver, and inactive metabolites are excreted in the urine.
5. Adverse effects
5.1 CNS: Barbiturates cause drowsiness, impaired concentration, and mental and physical slugginess.
5.2 Drug hangover: Hypnotic doses of barbiturates produces feeling of tiredness well after the patient awakes. This drug hangover leads to impaired ability to function normally for many hours after waking.
5.3 Addiction: Abrupt withdrawal from barbiturates may cause tremors, anxiety, weakness, restlessness, nausea and vomiting, seizures, delirium, and cardiac arrest. Withdrawal is much more severe than that associated with opiates and can result in death.
5.4 Precautions: As noted previously, barbiturates induce the P-450 system and there-fore may decrease the effect of drugs that are metabolized by these hepatic enzymes. Barbiturates increase porphyrin synthesis, and are contraindicated in patients with acute intermittent porphyria.
6. Poisoning
Barbiturate poisoning has been a leading cause of death among drug overdoses for many decades. Severe depression of respiration is coupled with central cardiovascular depression, and results in a shock-like condition with shallow, infrequent breathing. Treatment includes artificial respiration and purging the stomach of its contents if the drug has been recently taken. Hemodialysis may be necessary if large quantities have been taken. Alkalinization of the urine often aids in the elimination of phenobarbital. Analeptics (bemegrid, corazole) may be useful if respiration is not depressed completely to restore breathing.
Benzodiazepines
[edit | edit source]Chlordiazepoxide was synthesized in 1957 by Sternbach. Randall discovered its unique pattern of actions. With its the introduction into clinical medicine in 1961, more than 3000 benzodiazepines have been synthesized, over 120 have been tested for biological activity, and about 35 are in clinical use in various parts of the world. They have largely replaced barbiturates and meprobamate as sedative-hypnotic agents mainly because of remarkably low capacity to produce fatal CNS depression.
Although the benzodiazepines exert qualitatively similar effects, important quantitative differences in their pharmacodinamic spectra and pharmacokinetic properties have led to varying patterns of therapeutic application. Benzodiazepines vary in the degrees of sedative-hypnotic, muscle relaxant, anxiolytic, and anticonvulsant effects.
Mode of action
Binding of -aminobutyric acid (GABA) to its receptor on the cell membrane triggers an opening of a chloride channel, which leads to an increase in chloride conductance.
The influx of chloride ions causes a small hyperpolarization that moves the postsynaptic potential away from its firing threshold and thus inhibits the formation of action potentials. Benzodiazepines bind to specific, high affinity sites on the cell membrane, which are separate from but adjacent to the receptor of GABA. The benzodiazepine receptors are found only in the CNS, and their location parallels that of GABA neurons. The binding of benzodiazepines enhances the affinity of GABA receptors for its neurotransmitter, resulting in a more frequent opening of adjacent chloride channels. This in turn results in enhanced hyperpolarization and further inhibition of neuronal firing.
Actions
The benzodiazepines are not general neuronal depressants, as are the barbiturates. All the benzodiazepines have quite similar pharmacological profiles. The most prominent of these effects are sedation, hypnosis, decreased anxiety, muscle relaxation, anterograde amnesia, and anticonvulsant activity. Nevertheless, the drugs differ in selectivity, and the clinical usefulness of individual benzodiazepines varies considerably.
Uses in sleep disorders
This lecture describes the benzodiazepines used primarily as hypnotic and anti-convulsant agents. Other applications will be discussed in details in a following lecture (see tranquilizers). Not all of the benzodiazepines are useful as hypnotic agents, although all have sedative or calming effects. The three most commonly prescribed benzodiazepines for sleep disorders are long-acting flurazepam, intermediate-acting temazepam, and short-acting triazolam. The principal factors that determine selection are pharmacokinetics.
a. Flurazepam: This long-acting benzodiazepine significantly reduces both sleep-induction time and the number of awakening, and increases the duration of sleep. With continued use, the drug has been shown to maintain its effectiveness for up to 4 weeks. Flurazepam has a half-life of approximately 85 hours, which may result in daytime sedation and accumulation of drug.
b. Temazepam: this drug is useful in patients who experience frequent awakening. However, the peak sedative effect occurs two to three hours after an oral dose, and therefore it may be given several hours before bedtime.
c. Triazolam: This benzodiazepine has a relatively short duration of action and is therefore used to induce sleep in patients with recurring insomnia. Whereas temazepam is useful for insomnia caused by the inability to stay asleep, triazolam is effective in treating individuals who have difficulty in going to sleep. Tolerance frequently develops within a few days, and withdrawal of the drug often results in rebound insomnia, leading the patient to demand another prescription. Therefore, this drug is best used intermittently rather than daily. In general, hypnotics should be given for only a limited time, usually less than 2 to 4 weeks.
Pharmacokinetics
1. Absorption and distribution: The benzodiazepines are lipophilic and are rapidly and completely absorbed after oral administration and are distributed throughout the body.
2. Duration of action: The half-lives of the benzodiazepines are very important clinically, since the duration of action may determine the therapeutic usefulness. The benzodiazepines can be roughly divided into short-, intermediate- and long-acting groups. The long-acting agents form active metabolites with long half-lives.
3. Fate: Most benzodiazepines, including chlordiazepoxide and diazepam, are metabolized by the hepatic microsomal metabolizing system to compounds that are also active. For these benzodiazepines, the apparent half-life of the drug represents the combined actions of the parent drug and its metabolites. The benzodiazepines are excreted in urine as glucuronides or oxidized metabolites.
Dependence
Psychological and physical dependence on benzodiazepines can develop if high doses of the drug are given over a prolonged period. Abrupt discontinuation of the benzo-diazepines results in withdrawal symptoms, including confusion, anxiety, agitation, restlessness, insomnia, and tension. Because of the long half-lives of some of the benzodiazepines, withdrawal symptoms may not occur until a number of days after discontinuation. Benzodiazepines with a short elimination half-life, such as triazolam, induce more abrupt and severe withdrawal reactions.
Adverse effects
Drowsiness and confusion are the two most common side effect of the benzo-diazepines. Ataxia occurs at high doses and precludes activities that require motor coordination, such as driving an automobile. Cognitive impairment (decreased long-term recall and acquisition of new knowledge) can occur. Triazolam, the benzodiazepine with most rapid elimination, often show a rapid development of tolerance, early morning insomnia and daytime anxiety, along with amnesia and confusion. Benzodiazepines potentiate alcohol and other CNS depressants. However, they are considerably less dangerous than other anxiolytic and hypnotic drugs. As a result, a drug overdose is seldom lethal, unless other central depressants, such as alcohol, are taken concurrently.
Benzodiazepine antagonist flumazenil is a GABA receptor antagonist that can rapidly reverse the effects of benzodiazepines. The drug is available by i.v. administration only. Onset is rapid but duration is short, with a half-life of about one hour. Frequent administration may be necessary to maintain reversal of benzodiazepines.
Other Hypnotic Agents
[edit | edit source]Although the hypnotic zopiclone (imovan) is not a benzodiazepine, it acts on a subset of the benzodiazepine receptor family. Zopiclone has no anticonvulsive or muscle relaxing properties. It shows no withdrawal effects, exhibits minimal rebound insomnia and little or no tolerance occurs with prolonged use. Zopiclone has a rapid onset of action and short elimination. Although zopiclone potentially has advantage over the benzodiazepines, clinical experience with the drug is still limited.
Chloral hydrate is a trichlorinated derivative of acetaldehyde that is converted to trichlorethanol in the body. The drug is an effective sedative and hypnotic that induces sleep in about 30 minutes and lasts about 6 hours. Chloral hydrate is irritating to the gastrointestinal tract and causes epigastric distress. It also produces an unusual, unpleasant taste sensation.
Anticonvulsants
[edit | edit source]Antiepileptic Drugs
[edit | edit source]Epilepsy is widespread among the general population. Epilepsy is not a single entity; it is a family of different recurrent seizure disorders that have in common the sudden and excessive discharge of cerebral neurons. This results in abnormal movements or perceptions that are of short duration but that tend to recur. The site of electrical discharge determines the symptoms that are produced. E.g. epileptic seizures may cause convulsions if the motor cortex is involved. The seizures may include visual, auditory, or olfactory hallucinations if the parietal or occipital cortex plays a role. Drug therapy is the most widely effective mode of treatment for epilepsy. Seizures can be controlled completely in approximately 50% of epileptic patients, and meaningful improvement may be achieved in at least one half of the remaining patients.
A. Etiology
The neuronal discharge in epilepsy results from the firing of a small population of neurons in some specific area of the brain, referred to as the primary focus. These focal areas may be triggered into activity by changes in any of a variety of environmental factors, including alteration in blood gases, pH, electrolytes, or glucose availability.
1.Primary epilepsy: When no specific anatomic cause for the seizure, such as trauma or neoplasm, is evident the syndrome is called primary epilepsy. These seizures may be produced by an inherited abnormality in the CNS. Patients are treated chronically with antiepileptic drugs, often for life.
2.Secondary epilepsy: A number of reversible disturbances, such as tumors, head injury, hypoglycemia, meningeal infection, or rapid withdrawal of alcohol can precipitate seizures. Antiepileptic drugs are given until the primary cause can be corrected.
B. Classification of epilepsy
Seizures have been classified into two broad groups, partial (or focal), and generalized. Choice of drug treatment is based on the classification.
1. Partial: The symptoms of each seizure type depend on the site of neuronal discharge and on the extent to which the electrical activity spreads to other neurons in brain. Partial seizures may progress, becoming generalized tonic- clonic seizures.
1.1 Simple partial: These seizures are caused by a group of hyperactive neurons and are confined to a single locus in the brain; the abnormal activity does not spread. The patient does not lose consciousness and often exhibits abnormal activity of a single limb or muscle group that is controlled by the region the brain experiencing the disturbance. The patient may also show sensory distortions. Simple partial seizures may occur at any age.
1.2 Complex partial: These seizures exhibit complex sensory hallucinations, mental distortion, and loss of consciousness. Motor dysfunction may involve chewing movements, diarrhea, urination. Most (80%) of individuals experience their initial seizures before 20 years of age.
2. Generalized: These seizures begin locally, but they rapidly spread, producing abnormal electrical discharge throughout both hemispheres of the brain. Generalized seizures may be convulsive or nonconvulsive; the patient usually has an immediate loss of consciousness.
2.1 Tonic-clonic (grand mal): This is the most commonly encountered and the most dramatic form of epilepsy. Seizures result in loss of consciousness, followed by tonic, then clonic phases. The seizure is followed by a postictal period of confusion and exhaustion.
2.2 Absence (petit mal): These seizures involve a brief, abrupt, and self-limiting loss of consciousness. The onset occurs in patients at age 3 to 5 years and lasts until puberty. The patient stares and exhibits rapid eye-blinking, which lasts for 3 to 5 seconds.
2.3 Myoclonic: These seizures consist of short episodes of muscle contractions that may reoccur for several minutes. Myoclonic seizures are rare, occur at any age, and are often a result of hypoxia, uremia, encephalitis, or drug poisoning.
2.4 Febrile seizures: Young children (3 month to 5 years of age) frequently develop seizures with illness accompanied by high fever. The febrile consist of generalized tonic-clonic convulsions of short duration. Although febrile seizures may be frighten-ing, they are benign and do not cause death, neurologic damage, injury, or learning disorders, and they rarely require medication.
2.5 Status epilepticus: Seizures are rapidly recurrent.
3. Mechanism of action of antiepileptic drugs
Drugs that are effective in seizure reduction can either block the initiation of the electrical discharge from the focal area or, more commonly, prevent the spread of the abnormal electrical discharge to adjacent brain areas.
Initial drug treatment is based on the specific type of seizure. Thus, tonic-clonic (grand mal) seizures are treated differently than absence (petit mal). Several drugs may be equally effective, and the toxicity of the agent is often a major consideration in drug selection.
Diphenin (phenytoin) is effective in suppressing tonic-clonic and partial seizures, and is a drug of choice for initial therapy, particularly in treating adults.
1. Mechanism of action: Diphenin stabilizes neuronal membranes to depolarization by decreasing the flux of sodium ions in neurons in the resting state or during depolarization and suppresses repetitive firing of neurons.
2. Actions: Diphenin is not a generalized CNS depressant like the barbiturates, but it does produce some degree of drowsiness and lethargy. Diphenin reduces the propagation of abnormal impulses in the brain.
3. Therapeutic uses: Diphenin is highly effective for all partial seizures (simple and complex), for tonic-clonic seizures, and in status epilepticus. Diphenin is not effective for absence seizures, which often may worsen if such a patient is treated with this drug.
Carbamazepine
1. Action: Carbamazepine reduces the propagation of abnormal impulses in the brain by blocking sodium channels, thereby inhibiting the generation of repetitive action potentials in the epileptic focus.
2. Therapeutic uses: Carbamazepine is highly effective for all partial seizures and is often the drug of first choice. In addition the drug is highly effective for tonic-clonic seizures and is used to treat trigeminal neuralgia. It has occasionally been used in manic-depressive patients to ameliorate the symptoms.
Phenobarbital provides a 50% favorable response rate for simple partial seizures, but it is not very effective for complex partial seizures. The drug has been regarded as the first choice in treating recurrent seizures in children, including febrile seizures. The drug is also used to treat tonic-clonic seizures, especially in patients who do not respond to diazepam plus diphenin. Doses required for antiepileptic action are lower than those that cause pronounced CNS depression. Phenobarbital is also used as a mild sedative to relieve anxiety, nervous tension and insomnia, although benzodiazepines are superior.
Hexamidin (Primidone) is structurally related to phenobarbital, and resembles phenobarbital in its anticonvulsive activity. Hexamidin is an alternative choice in partial seizures and tonic-clonic seizures. Much of hexamidin’s efficacy comes from its metabolites phenobarbital and phenylethylmalonamide.
Valproic acid is the most effective agent available for treatment myoclonic seizures. The drug diminishes absence seizures but is a second choice because of its hepatotoxic potential. Valproic acid also reduces the incidence and severity of tonic-clonic seizures.
Ethosuximide reduces propagation of abnormal electrical activity in the brain, and is the first choice in absence seizures.
Several of the benzodiazepines show antiepileptic activity. Clonazepam and clorazepate are used for chronic treatment, whereas diazepam is the drug of choice in the acute treatment of status epilepticus. Of all the antiepileptics, the benzodiazepines are the safest.
Drugs Used in Parkinson's Disease
[edit | edit source]Parkinsonism is a progressive neurologic disorder of muscle movement, characterized by tremors, muscular rigidity, and bradykinesia (slowness in initiating and carrying out voluntary movements). Parkinson’s disease is the fourth most common neurologic disorder among the elderly. Most cases involve people over the age of 65 among whom the incidence is about 1:100 individuals.
A. Etiology
The cause of Parkinson’s disease is unknown for most patients. The disease is correlated with a reduction in the activity of inhibitory dopaminergic neurons in the substantia nigra and corpus striatum–parts of the brain’s basal ganglia system that are responsible for motor control. Genetic factors do not play a dominant role in the etiology.
1. Substantia nigra: The substantia nigra, part of the extrapyramidal system, is the source of dopaminergic neurons that terminate in the striatum. Each neuron makes thousands synaptic contacts within the striatum and modulates the activity of a large number of cells. These dopaminergic projections from the substantia nigra fire tonically, rather than in response specific muscular movements or sensory input. Thus, dopaminergic system appears to serve as a tonic, sustaining influence on motor activity, rather than participating on specific movements.
2. Striatum: Normally, the striatum is connected to the substantia nigra by neurons that secrete the inhibitory transmitter GABA at their termini in the substantia nigra. In turn, cells of the substantia nigra sends neurons back to the striatum secreting the inhibitory transmitter dopamine. This mutual inhibitory pathway normally maintains a degree of inhibition of the two separate areas. Nerve fibers from the cerebral cortex and thalamus secrete acetylcholine in the neostriatum, causing excitatory effects that initiate and regulate gross intentional movements of the body. In Parkinson’s disease, destruction of cells in the substantia nigra results in the degeneration of neurons responsible for secreting dopamine in the neostriatum. Thus the normal modulating inhibitory influence of dopamine on the neostriatum is significantly diminished, resulting in the parkinsonian degeneration of the control of muscle movement.
3. Secondary parkinsonism: Parkinsonian symptoms infrequently follow viral encephalitis or multiple small vascular lesions. Drugs such as phenothiazines and haloperidol, whose major pharmacologic action is blockade of dopamine receptors in the brain, may also produce parkinsonian symptoms. These drugs should not be used in parkinsonian patients.
B. Strategy of treatment
In addition to an abundance of inhibitory dopaminergic neurons, the neostriatum is also rich in excitatory cholinergic neurons that oppose the action of dopamine. Many of the symptoms reflect an imbalance between the excitatory cholinergic neurons and the greatly diminished number of inhibitory dopaminergic neurons. Therapy is aimed at restoring dopamine in the basal ganglia and antagonizing the excitatory effect of cholinergic neurons, thus reestablishing the correct dopamine/acetylcholine balance.
Drugs
Currently available drugs offer temporary relief from the symptoms of the disorder, but do not arrest or reverse the neuronal degeneration caused by the disease.
Levodopa is a metabolic precursor of dopamine. It restores dopamine level in the extrapyramidal centers (substantia nigra) that atrophy in parkinsonism. In patients with early disease, the number of residual dopaminergic neurons (about 20% of normal) is adequate for conversion of levodopa to dopamine. Levodopa decreases the rigidity, tremor, and other symptoms of parkinsonism. Unfortunately, with time the number of neurons decreases and the drug effects “wear off”.
Dopamine itself does not cross the blood-brain barrier, but its immediate precursor levodopa is readily transported into the CNS and is converted to dopamine in the brain. Large doses of levodopa are required because much of the drug is decarboxylated to dopamine in the periphery, resulting in peripheral side effects (nausea, vomiting, cardiac arrhythmias, hypotension).
The effects of levodopa on the CNS can be greatly enhanced by coadministration carbidopa, a dopamine decarboxylase inhibitor that does not cross the blood-brain barrier. Carbidopa diminishes the metabolism of levodopa in the GI tract and peripheral tissues. The addition of carbidopa lowers the dose of levodopa needed by4-to 5- fold and, consequently, decreases the severity of the side effects of peripherally formed dopamine.
Bromocriptine, en ergotamine (an alkaloid with vasoconstrictor action) derivative, is a dopamine receptor agonist. The drug produces little response in patients who does not react to levodopa, but it is often used with levodopa in patients responding to drug therapy.
It was accidentally discovered that the antiviral drug, amantadine, effective in the treatment of influenza, has antiparkinsonian action. It appears to enhance the synthesis, release, or reuptake of dopamine from the surviving neurons. Amantadine is less efficacious than levodopa, but it has fewer side effects. The drug has little effect on tremor but is more effective than the anticholinergics against rigidity and bradykinesia.
The antimuscarinic agents (cyclodol, noracin etc.) are much less efficacious than levodopa and play only adjuvant role in the antiparkinsonian therapy. Blockage of the cholinergic transmission produces similar to augmentation of dopaminergic transmission (again, because of the creation of an imbalance in the dopamine/acetylcholine ratio). All these drugs can induce mood changes and produce xerostomia (dryness of the mouth) and visual problems, as do all muscarinic blockers. They interfere with gastrointestinal peristaltics, may cause urinary retention and increase in intraocular pressure.
Hemodialysis
[edit | edit source]|
Medical disclaimer: This page is for educational and informational purposes only and may not be construed as medical advice. The information is not intended to replace medical advice offered by physicians. Please refer to the full text of the Wikiversity medical disclaimer. |
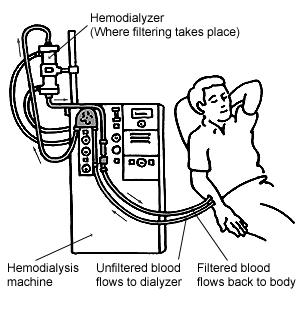
Starting w:hemodialysis is often a frightening experience. Hemodialysis machines are complicated and dialysis sessions often are punctuated by alarms. At the beginning of dialysis and at the end of dialysis a lot of things happen. Not knowing what it is can be anxiety provoking. The following step-by-step description of hemodialysis will hopefully clarify some things for people starting dialysis and allow others to gain a better understanding of what dialysis entails.
Pre-dialysis
[edit | edit source]- Before or around the time the patient arrives for his/her scheduled session, a dialysis machine will be prepared. There are many models of dialysis machines, but typically in modern machines there will be a computer, CRT, a pump, and facility for disposable tubing and filters. The filters (the actual artificial kidneys) are cylindrical, clear plastic outside with the filter material visible inside (looks like thick paper). They are perhaps 15-18 w:inches long, and 2-3 inches thick. They have tubing connectors at both ends. The technician or nurse will set up plumbing on the machine in a moderately complex pattern that has been worked out to move blood through the filter, allow for saline drip (or not), allow for various other medications/chemicals to be administered. How the plumbing is set up may vary between models of machine and the types of filters. For some filters, it is necessary to clear sterilizing fluid from the filter before connecting the patient. This is done by altering the plumbing to push saline through the filter, and carefully checked with a type of w:litmus test.
- The pump does not directly contact the blood or fluid in the plumbing — it works by applying pressure to the tubing, then moving that pressure point around. Think of a disk with a protrusion in it. Put this into a close fitting 270 degree enclosure. Put plastic tubing between the enclosure and the disk, entering and exiting in the 90 open degrees. Now imagine the disk turning. It will put pressure on the tubing, and the pressure point will roll around through the 270 degrees, forcing the fluid to move (see also w:Peristaltic pump). It is characteristic of dialysis machines that most of the blood out of the patients body at any given time is visible. This facilitates troubleshooting, particularly detection of clotting.
- The patient arrives and is carefully weighed. Standing and sitting blood pressures are taken. Temperature is taken.
- Access is set up. For patients with a w:fistula (a surgical modification to an arm or leg vein to make it more robust, and therefore usable for high capacity blood movement required by dialysis) this means inserting two large gauge needles into the fistula. This is painful for the patient but there are various methods of numbing the entry sites before the needles are inserted — the two most common are lignocaine (lidocaine), a local anaesthetic injected under the skin, and there is also a cream called EMLA which is applied to the skin 45 minutes before the needles are inserted. Fistulas are widely considered the desirable way to get access for hemodialysis, but they take time to set up and mature (anywhere between 5 weeks to 15 weeks). For other patients, access may be via a w:catheter installed to connect to large veins in the chest. Other arrangements can be made as well.
- When access has been set up, the patient is then connected to the preconfigured plumbing, creating a complete loop through the pump and filter.
Dialysis
[edit | edit source]- The pump and a timer are started. Hemodialysis is underway.
- Periodically (every half-hour, nominally) blood pressure is taken. As a practical matter, fluid is also removed during dialysis. Most dialysis patients are on moderate to severe fluid restrictive diets (in addition to other dietary restrictions), since kidney failure usually includes an inability to properly regulate fluid levels in the body. A session of hemodialysis may typically remove 2-5 w:kilograms (5-10 pounds) of fluid from the patient. The amount of fluid to be removed is set by the dialysis nurse according to the patient's "estimated dry weight." This is a weight that the care staff believes represents what the patient should weigh without fluid built up because of kidney failure. Removing this much fluid can cause or exacerbate w:low blood pressure. Monitoring is intended to detect this before it becomes too severe. Low blood pressure can cause cramping or loss of consciousness. Often this is temporary and passes after the head is placed down (w:Trendelenburg position) for a short time.
Post-dialysis
[edit | edit source]- At the end of the prescribed time, the patient is disconnected from the plumbing - blood lines (which is removed and discarded, except perhaps for the filter, which may be sterilized and reused for the same patient at a later date). Needle wounds (in case of fistula) are bandaged with gauze, held for 10 to 15 minutes with direct pressure to stop bleeding, and then taped in place. The process is similar to getting blood drawn, only it is lengthier, and more fluid or blood is lost.
- Temperature, standing and sitting blood pressure, and weight are all measured again. Temperature changes may indicate infection. BP discussed above. Weighing is to confirm the removal of the desired amount of fluid.
- Care staff verifies that the patient is in condition suitable for leaving. The patient must be able to stand (if previously able), maintain a reasonable blood pressure, and be coherent (if normally coherent). Different rules apply for in-patient treatment.
Post-dialysis washout
[edit | edit source]Following hemodialysis, patients may experience a syndrome called "washout". The patient feels weak, tremulous, and may suffer extreme fatigue. Patients report they "are too tired, too weak to converse, hold a book or even a newspaper." It may also vary in intensity ranging from whole body aching, stiffness in joints and other flu-like symptoms including headaches, nausea and loss of appetite. The syndrome may begin toward the end of treatment or minutes following the treatment. It may last 30 minutes or 12-14 hours in a dissipating form. Patients though exhausted have difficulty falling asleep. Eating a light meal, rest and quiet help the patient cope with washout until it has 'worn away.'
References
[edit | edit source]External links
[edit | edit source]- What is dialysis? - Kidney Foundation of Canada
 KSF
KSF