Fundamentals of chemistry
 From Wikiversity - Reading time: 10 min
From Wikiversity - Reading time: 10 min
| Subject classification: this is a chemistry resource. |
The Fundamentals of Chemistry is an introduction to the Periodic Table, stoichiometry, chemical states, chemical equilibria, acid & base, oxidation & reduction reactions, chemical kinetics, inorganic nomenclature and chemical bonding.
Chemical Element
[edit | edit source]Definition
[edit | edit source]Chemical Elements are fundamental ingredients of all matter in existence which can be combined in a reaction to create a chemical substance. Each chemical element in the universe has unique properties that distinguish it from all of the other chemical elements. They cannot be chemically interconverted or broken down into simpler substances and are the primary constituents of matter.
Chemical elements are usually notated with the symbol
- MZE
Where,
- E is the element name
- Z the Atomic Number
- M the element's mass
For example, Hydrogen is notated by
- 11H
Periodic Table of Elements
[edit | edit source]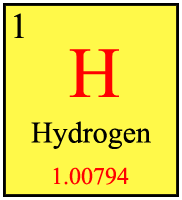
The periodic table groups the elements by properties. For its history, see Wikipedia's History of the Periodic Table. The Periodic Table is available here: Periodic Table on Wikimedia Commons and explanations will be based on this table. Print or order a hard copy of the periodic table for easy access and reference.
In the table, each box contains one element and additional information. For hydrogen, the "1" in the top corner is the atomic number, which deals with how many protons, or positive charges, are in the atom. The "H" is the symbol for Hydrogen. All the elements get a one or two letter symbol (there are a couple of exceptions with undeclared elements). The number at the bottom is the atomic weight or atomic mass. 1.00794 represents how many grams are in each mole (6.022×1023 entities) of hydrogen. The atomic mass is a very important part of chemistry and has many applications throughout.
The elements are organized in rows and columns. There are eighteen groups (or families or columns) on the periodic table. Each one represents how many electrons are attached to the elements and correlate to how many valence electrons are present.
The first two groups (1A and 2A) as well as the six on the very right (3A-8A). These are called representative elements. Group 1A are alkali metals (except Hydrogen which is a non-metal) and Group 2A are alkaline earth metals. Group 3A through 8A have mixed properties, but there are specific patterns.
Electrons are negatively charged subatomic particles that "orbit" around the nucleus of the element. Valence electrons are electrons that are on the very outside of the atom. There are seven periods (or horizontal rows) that describe electron shells.
Chemical compounds
[edit | edit source]Chemical compounds are pure substances composed of only one type of molecule (two or more atoms held together in a fixed ratio by Chemical bonds). The chemical compound generally has different properties than those of its constituent elements. The name of a chemical compound is usually identical to the name of the molecule that makes up the compound (such as Carbon Dioxide), but some compounds also have "common names" by which the substances are known outside of scientific discussion. For example, sodium bicarbonate is commonly known as "baking soda."
Atomic Model
[edit | edit source]Also see Dalton. The main points of Dalton's atomic theory are:
- Elements are made of extremely small particles called atoms.
- Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties.
- Atoms cannot be subdivided, created, or destroyed.
- Atoms of different elements combine in simple whole-number ratios to form chemical compounds.
- In chemical reactions, atoms are combined, separated, or rearranged.
Examples
[edit | edit source]For example, the compound Sodium Chloride (NaCl), is composed of one ion of Chlorine bonded to one ion of Sodium. Sodium, in its natural form, is a solid metal element which is highly reactive and produces a lot of effervescence when reacted with water.
Chlorine, in its natural form, is a non-metal element which is composed of many diatomic molecules of Cl2, and exists as a pale green gas that is toxic if inhaled in large amounts. The compound Sodium Chloride, however, is none other than the simple table salt applied to foods. The reason for the rise of these new properties lies in the type of bonding and the elements that make up the compound. This will be discussed in more detail in later sections.
Chemical Formulas
[edit | edit source]Chemical formula is used to represent a chemical compound . For example
- Water is
- Ozone is
- Salt is
Chemical bonding
[edit | edit source]Chemical bonding
[edit | edit source]A chemical bond is a lasting attraction between atoms that enables the formation of chemical compounds
For example, the compound Sodium Chloride (NaCl), is composed of one ion of Chlorine bonded to one ion of Sodium. Sodium, in its natural form, is a solid metal element which is highly reactive and produces a lot of effervescence when reacted with water.
- →
Chlorine, in its natural form, is a non-metal element which is composed of many diatomic molecules of Cl2, and exists as a pale green gas that is toxic if inhaled in large amounts. The compound Sodium Chloride, however, is none other than the simple table salt applied to foods. The reason for the rise of these new properties lies in the type of bonding and the elements that make up the compound. This will be discussed in more detail in later sections.
- →
Types of chemical bonds
[edit | edit source]Chemical Reactions
[edit | edit source]A chemical reaction is an interaction between chemical subtances to form new substances. For example, an oxidization of a metal, or a de-oxidization of an oxidized metal.
Chemical Reactions' Types
[edit | edit source]The oxidization of a metal like copper (Cu) to create oxidized copper can be expressed as chemical equation as shown below:
Ionization describes the interaction between a metal and an acid to form ionized opposite polarity metals.
Chemical equations
[edit | edit source]Chemical equations are a way of expressing a chemical reaction. They present chemical species with the chemical symbols of the elements that compose them and subscripts which present the actual number of particles of that element, whether they be atoms or ions, which make up the compound. For example, consider the reaction shown below:
On the left side of the arrow, you can see two compounds represented. These are the reactants, the chemical species that rearrange to give the product, the chemical species represented on the right side of the arrow. The first reactant, H2, represents a hydrogen molecule. The subscript '2' shows that there are two atoms of Hydrogen that chemically combine to produce the molecule. Therefore, every molecule of H2 contains two Hydrogen atoms chemically-bonded to each other. The same concept applies to the reacting molecule of O2 to the right of it.
The (g) and (l) or state symbols represent what physical state of chemical species during the reaction. (g) means that the chemical species O2 and H2 both exist as gases before they react, and the subscript (l) means that the chemical species H2O exists as a liquid when it is formed by the reaction.
The coefficients in front of the molecules like H2O and the H2 represent the simplest whole number ratio the substance amount in the reaction mixture. For example, the above equation shows that every molecule of O2 reacts with two molecules of H2 to form two molecules of H2O.
Dimensional analysis
[edit | edit source]As a more complex example, the concentration of nitrogen oxides (i.e., ) in the flue gas from an industrial furnace can be converted to a mass flow rate expressed in grams per hour (i.e., g/h) of by using the following information as shown below:
- NOx concentration
- = 10 parts per million by volume = 10 ppmv = 10 volumes/106 volumes
- NOx molar mass
- = 46 kg/kgmol (sometimes also expressed as 46 kg/kmol)
- Flow rate of flue gas
- = 20 cubic meters per minute = 20 m³/min
- The flue gas exits the furnace at 0 °C temperature and 101.325 kPa absolute pressure.
- The molar volume of a gas at 0 °C temperature and 101.325 kPa is 22.414 m³/kgmol.
Stoichiometry
[edit | edit source]Stoichiometry is used to analyze quantitative measurements with relation to reactants and products of a chemical equation. The chemical equation is a symbolic representation of a chemical reaction. The reactants of a chemical equation are justified to the left which gives reference to its definition, the substance used or consumed in a chemical reaction. The products of a chemical equation are justified to the right, and is defined as the substance that is yielded or produced in a chemical reaction. In order to completely understand stoichiometric relationships, one must consider the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. Remember that mass or matter is neither created nor destroyed.
Among the properties of elements are states. There are 3 fundamental states of an element: solid, liquid, and a gas. They are indicated by subscript with (s), (l), and (g) respectively and assigned with the appropriate compound or element in the chemical equation. A substance dissolved in water is in indicated by (aq). Plasma can also exist, which is an ionized gas with special properties.
Stoichiometry allows chemists to quantitatively analyze relative relationships between substances in a chemical equation.
Balancing chemical equations
[edit | edit source]Ethyne (C2H2) is added to oxygen gas (O2) to yield carbon dioxide (CO2) and water (H2O). This reaction could be written as follows:
- Unbalanced equation
However, the above equation is not balanced.
- On the left side there are two Carbon atoms (C), two Hydrogen atoms (H) and two Oxygen atoms in total.
- On the right there is one Carbon atom, three Oxygen atoms, and two Hydrogen atoms.
Note that in order to properly count up the atoms in an equation, it must be noted to count up atoms with respect to the coefficient and subscripts. Careful notice should be made to compounds and polyatomic ions, since these are grouped together in relation.
In order to balance the equation correctly, a number, known as a coefficient must be added to the front of each representation in a chemical equation.
- Correctly balanced equation
As can be seen, the subscripts were not touched, only whole numbers were added to the front of all the formulas, as needed. The coefficients may be fractions, which are generally used in thermochemistry but for all intents and purposes, whole numbers are generally used.
It would not be correct to balance it by changing the subscript numbers.
- Incorrectly balanced equation
By changing the subscripts you are changing the chemicals involved in the reaction. In the above, is ozone, not normal oxygen, and is not a stable compound. A small change in the subscripts and makeup of an individual compound yields a whole different set of properties.
Chemical States
[edit | edit source]We usually learn that there are four fundamental states of matter,
However, physics research suggests other states of matter as well (like the Bose-Einstein condensate), but this is usually taken as a coarse starting point.
Gases are made up of atoms and/or molecules that are freely moving and therefore have no definite shape. They morph uniformly to the shape of the container that they are in. If the container is not sealed, then the gas can move out. Therefore the volume of the gas is reliant on the temperature and/or pressure throughout the gas or environment. This is observed using the ideal gas laws, which are discussed later.
An important piece of information to know is what an aqueous solution is also. Aqueous solutions are not technically chemical states, but they appear often enough when dealing with stoichiometry and chemistry in general that they should be mentioned.
Acids and Bases
[edit | edit source]pH
[edit | edit source]The potential of hydrogen or pH (pronounced /piː.eitʃ/) is a measure of the acidity or alkalinity of a solution, numerically equal to 7 for neutral solutions, increasing pH with rising alkalinity and decreasing pH with more acidity. The pH scale commonly in use ranges from 0 to 14.
An alkali is sometimes called a "base".
| Substance | pH |
|---|---|
| Battery acid |
0.5
|
| Gastric acid |
1.5 – 2.0
|
| Lemon juice |
2.4
|
| Cola |
2.5
|
| Vinegar |
2.9
|
| Orange or apple juice |
3.5
|
| Beer |
4.5
|
| Acid Rain |
<5.0
|
| Coffee |
5.0
|
| Tea or healthy skin |
5.5
|
| Milk |
6.5
|
| Pure water |
7.0
|
| Healthy human saliva |
6.5 – 7.4
|
| Blood |
7.34 – 7.45
|
| Sea water |
8.0
|
| Hand soap |
9.0 – 10.0
|
| Household ammonia |
11.5
|
| Bleach |
12.5
|
| Household lye |
13.5
|
The pH is calculated by
A similar measure, called the pOH is defined as
Their sum is
Acids
[edit | edit source]Characteristics of acids:
- Aqueous acids can turn blue litmus towards red.
- React with bases and certain metals to form salts.
- Arrhenius' definition of acid: Yields hydrogen ions when dissolved in water.
- The Lewis definition of an acid: Can accept a pair of electrons to form a covalent bond.
- Brønsted-Lowry acid definition: A species that can lose or "donate" a hydrogen ion
- Can have a sour taste.
- Can give one or more than one protons (or simply, H+)
- Electrolytes, yet usually are not ionic compounds
Bases
[edit | edit source]Characteristics of bases:
- Aqueous bases (alkalis) can turn red litmus towards blue.
- React with acids to form salts.
- Arrehenius definition of base: produce OH− ions when dissolved in water.
- Lewis definition of Base: can donate a pair of electrons to form a covalent bond with an acid
- Brønsted-Lowry base definition: A species that can gain or "accept" a hydrogen ion
- Can have a bitter taste.
- Can accept one or more than one protons (or simpler H+)
- Conduct electricity
The difference between bases and alkalis is that alkalis dissolve in water and are considered basic salts of alkaline metals. An example of a base that is not an alkali is ammonia (NH3).
Nomenclature of inorganic chemistry
[edit | edit source]References
[edit | edit source]- Flowers, Paul, Klaus Theopold, Richard Langley, William R. Robinson, Mark Blaser, Simon Bott, Donald Carpenetti, Andrew Eklund, Emad El-Giar, Don Frantz, Paul Hooker, George Kaminski, Jennifer Look, Carol Martinez, Troy Milliken, Vicki Moravec, Jason D. Powell, Thomas Sorensen, and Allison Soult. Chemistry. N.p.: n.p., 2015. Chemistry. OpenStax College, Mar. 2015. Web.
 KSF
KSF















![{\displaystyle pH=-\log[H^{+}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b3cb063f8077d8b26ff8868e3d1d85c12ba8afdc)
![{\displaystyle pOH=-\log[OH^{-}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/55bff98f6d50bf9a84e9a46a9f778da08903c708)
