Introduction to Functional Groups
 From Wikiversity - Reading time: 3 min
From Wikiversity - Reading time: 3 min
| Subject classification: this is a chemistry resource. |
Introduction
[edit | edit source]Functional groups are groups of atoms within a molecule that affect its function. Some basic ones are listed below.
Alkane
[edit | edit source]Every organic structure is based on alkanes. They contain only carbon and hydrogen, which are only connected through single bonds (C-H or C-C). These bonds are quite stable, non-polar, and therefore hydrophobic.
You can differ between linear, branched, and cyclic alkanes.
-list of linear alkanes with names, structure and semistructure will follow
Molecular Formula of Alkane is :CnH2n+2
Alkene
[edit | edit source]Alkenes are unsaturated hydrocarbons which have a general molecular formula of CnH2n. Hence, these are more reactive compared to their counterpart alkanes. Electrophilic addition reactions are a characteristic nature of alkenes. The first member is ethylene or ethene adds bromine to give 1,2-dibromoethane.
Alkyne
[edit | edit source]Triply bonded carbon atoms with the general structure CnH2n-2.
This is the SMILES for hexyne: H-C#C-C#C-C#C-H
Hydroxyl Group
[edit | edit source]
The hydroxyl group is formed by attaching an oxygen and a hydrogen atom to a molecule. Both the bond from the molecule to the oxygen and the bond from the oxygen to the hydrogen are single bonds; thus hydroxyl groups are usually denoted R-O-H. (where O is oxygen, H is hydrogen, and R is the molecule the hydroxyl group is attached to)
An organic compound that contains a hydroxyl group is called an alcohol.
Reactions of alcohols is covered in Section 4.2
Carbonyl Group
[edit | edit source]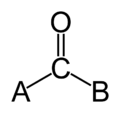
The Carbonyl group is formed by attaching a oxygen to a carbon by a double bond, then attaching that same carbon atom to a molecule. A carbonyl group is denoted X-C=O
Carboxyl Group
[edit | edit source]
The carboxyl group is formed by starting with a carbonyl group , then attaching a hydroxyl group to the same carbon atom in the carbonyl group. Finally, the carboxyl group is added to a molecule. Because carbon has four bonding sites, and three of them are taken up by the hydrogen and oxygen, the bond from the carbon to the molecule is a single bond. A carboxyl group is denoted X-COH=O
Aromatic
[edit | edit source]Aromatic compounds, also known as arenes or aromatics, are chemical compounds that contain conjugated planar ring systems with delocalised pi electron clouds instead of discrete alternating single and double bonds. Typical aromatic compounds are benzene and toluene. Considering benzene: Each carbon atom uses sp2 orbitals to form σ bonds to the other three atoms attached to it. This requires that all the atoms are in one plane. The carbon atoms have 1 electron in the p orbital which overlaps with its neighbours. This produces 6 new orbitals, three of which are bonding these are called π orbitals. Thus they form clouds of electrons above and below the ring in a torus shape and are known as the aromatic sextet. They can be represented as shown in figure 1. Figure 1: The aromatic sextet.
They should satisfy Hückel's law.
Heteroaromatic
[edit | edit source]Aromatic groups with more than one ring. An example of this is purine.
Haloalkane
[edit | edit source]Carbon-based motifs with a halogen, e.g. chloroethane.
Ether
[edit | edit source]R-O-R
Amine
[edit | edit source]R3-N, R2-NH, or R-NH2 are examples of amines.
Aldehyde
[edit | edit source]R-C(=O)-H
Ketone
[edit | edit source]R-C(=O)-R
Ester
[edit | edit source]R-C(=O)-O-R or H-C(=O)-O-R
Amide
[edit | edit source]R-C(=O)-N(-H)-R or R-C(-OH)=N-R Present in peptide bonds, which are bonds between amino acids in peptides and proteins.
Nitrile
[edit | edit source]R−C≡N This functional group is formed when nitrogen has a triple bond with carbon.
 KSF
KSF