Microbiome and Mental Health
 From Wikiversity - Reading time: 20 min
From Wikiversity - Reading time: 20 min

The connection between microbiome and mental health has recently come to the attention of public media with articles about it published in the New York Times[1], Scientific American[2], Huffington Post[3], and Nature[4]. The history of research in this area has deep roots in the science field. Scientists have been pondering and writing on the connection between the brain and the body for centuries. For instance, in 1759, Laurence Sterne said in reference to “a man’s body and his mind" that if you "rumple the one, -you rumple the other" in his book The Life and Opinions of Tristan Sterne.
The human gastrointestinal tract contains a delicately balanced ecosystem of 100 trillion microorganisms, nearly ten times the number of cells in the entire human body. [5] These bacteria in our gut, which are collectively called the gut microbiome, play many physiological roles in the body: synthesizing vitamins, developing the immune system, aiding digestion, and managing the stress response.[5] Beyond involvement in somatic processes, bacteria within the body are so interwoven in our systems that impact our behavior and cognition. One study even found that when the gut contents of two mice were swapped, including all of their gut microbiome, the mice's personalities switched; for example, stress-prone mice became calm and calm mice became stress-prone.[5]
Both humans and animals naturally have very diverse compositions of their microbiomes. Thus, it has been difficult for researchers to discern the difference between unbalanced, or dysbiotic, microbiome and a healthy microbiome.[6] Over the past decades, researchers have found hundreds of bacteria strains in the human gut; however, only a handful amongst those identified are ubiquitous[6]. Some of these ubiquitous bacteria include: anaerobic cocci and Bacteroides--which are prevalent in high abundance--and Clostridium, Bifidobacterium, Eubacterium, Lactobacillus, Escherichia coli and Streptococcus--which are prevalent in lower abundance[6].
Acquisition and development in humans
[edit | edit source]
Bacteria begin to form an inextricable link to humans shortly before birth when they colonize the gut within the womb.[5] By the time humans are 3-5 years old, a full adult microbiome and a gut-brain axis has developed. Once the microbiome is established, it is relatively stable throughout life. [7]
The gut-brain axis
[edit | edit source]Many factors throughout the body have a significant impact on the state of one's mental health[8]:
- Excessive bodily inflammation
- Poor absorption of nutrients and medication in the gut
- Gut serotonin imbalance
- Leaky gut syndrome, which causes nutrients to leach into the blood stream rather than being absorbed by the intestines
- A dearth of brain serotonin, dopamine, and other neurotransmitters
- Hormonal imbalance
- Deficiencies in certain vitamins and minerals (A, B, C, D, E, K, Calcium, Iron, Magnesium, Phosphorus, Sodium, and Zinc, among others)
Although these varied systems may seem disparate, they are largely interconnected through the gut-brain axis. The gut and brain have dual-direction feedback and the gut-brain axis (GBA) is a set of mechanisms through which the gut and brain communicate bidirectionally. Three of the main communication routes are the immune system, the nervous system, and the endocrine system. Through these mechanisms, the information in the gut can affect behavior and cognition. For instance, one study found that when the gut bacteria from two different mice were swapped, the behavioral traits of those mice also swapped. Stress-prone mice became calm and calm mice became stress-prone[5]. Dually, changes in cognition and affect can result in differential gut microbiome composition[9][10][11].
Microbiome and mental illness
[edit | edit source]
Proper functioning of the gut microbiome is essential for maintaining physical and mental health. Dually, dysregulation of the enteric system has been linked to psychiatric illnesses.[11]
Gut permeability and psychosocial stressors
[edit | edit source]In a healthy human, bacterial metabolites cross the epithelium into the blood stream, and many are able to cross the blood brain barrier. These metabolites serve important roles in the brain, such as being precursors for different neurotransmitters. For instance, Eschericha, Bacillus, and Saccharomyces produce precursors for norepinephrine, and Bacillus produce precursors for dopamine. Additionally, microbial-derived tryptophan is a precursor for serotonin after it crosses the blood-brain-barrier. Dysregulation of all three of these neurotransmitters have been hypothesized to be associated with psychiatric illnesses such as depression.
If the gut’s permeability is disturbed, negative effects on cognition, affect, and behavior are observed due to modulation of important feedback systems, for instance, the production of neurotransmitters as described previously. Psychosocial stressors, which have great empirical support as risk factors for many psychiatric illnesses, modulate gut permeability. Interpersonal stress, which, relative to other forms of stress, is particularly predictive of depression and anxiety, is a particularly potent modulator of gut permeability. The increased permeability leads to bacterial translocation across the gut wall into the blood stream, conferring greater risk for psychiatric illness through neural, endocrine, and immune pathways. Stress has effect of gut permeability after both long exposure or short exposure. Psychosocial stress also changes gut microbiome composition by reducing quantities of bifidobaterium and lactobacillus—two important bacterial species in the gut. Stressors experienced in adulthood similarly appear to disturb the composition of the enteric microbiota. This model provides a way for life stressors, which increase risk for psychiatric illness, to impact cognition and behavior through modulating gut microbiome composition and gut permeability.
Systemic and neural inflammation
[edit | edit source]When the gut epithelium’s permeability is increased due to stress, bacteria in the gut can cross the gut epithelium and increase systemic inflammation and amount of proinflammatory cytokines in the blood stream. During regular immune response to an infection, there is an increase in pro-inflammatory cytokines, such as IL-1 and IL-2. In immune response, cells throughout the body release cytokines that mediate and regulate immunity, inflammation, and hematopoiesis (induced blood cell destruction in case of infection)[12]. Pro-inflammatory cytokines seek infected cells and signal other cells to destroy them, as well as induce other biological responses to infection like inflammation. Additionally, proinflammatory cytokines the blood-brain barrier, leading to neural inflammation.
Research has found that increased levels of pro-inflammatory cytokine activity in the brain lowers metabolism of neurotransmitters, specifically that of GABA; GABA, when in lower levels, hs been hypothesized to increase depressive symptoms[8]. Pro-inflammatory cytokines, in turn, have been implicated in the pathophysiology of stress-related psychiatric disorders such as depression. In a meta-analysis, two pro-inflammatory cytokines were consistently elevated in depressed relative to non-depressed individuals. Evidence of epithelial permeability and bacterial translocation has also been found for depression, with elevated serum immunoglobulin A and immunoglobulin M mediated immune responses to enterobacteria in depressed individuals relative to healthy controls.
Hypothalamus-Pituitary-Adrenal (HPA) axis
[edit | edit source]Reaction to psychosocial stressors is mediated by the HPA system. Exposure to psychosocial stressors stimulates the release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) from the paraventricular nucleus of the hypothalamus. These hormones, in turn, trigger the secretion of adrenocorticotropin (ACTH) from the anterior pituitary, leading to the downstream production and release of glucocorticoids (particularly cortisol in humans and corticosterone in rodents) from the adrenal cortex. Under normal circumstances, these glucocorticoids down-regulate CRH, AVP, and ACTH activity in a negative feedback loop, thereby maintaining neuroendocrinological equilibrium. This negative feedback cycle is mediated by mineralocotiocoid receptors and glucocorticoid receptors, situated particularly in the hippocampus, hypothalamus, and pituitary.
Pro-inflammatory cytokines cause dysregulation in the HPA system. As stress causes increased pro-inflammatory cytokine concentrations, exposure to chronic or severe stressors, and the corresponding prolonged elevation in glucocorticoid concentration, can result in hyperactivation of the HPA axis. Anomalous HPA axis activity has been observed for certain psychiatric conditions, particularly depression. This hyperactivity of the HPA axis observed in depression is believed to be a consequence of altered glucocorticoid receptor functioning and impaired negative feedback regulation of CRH by glucocorticoids.
Vagus nerve
[edit | edit source]Through various mechanisms, including the vagus nerve and through the release of neurotransmitter precursors, the enteric nervous system is connected bidirectionally with the central nervous system.[11] As previously mentioned, bacteria become incorporated in the human gut before birth in the womb[12]. Bacteria also become enmeshed within the nervous system in addition to the gut, strengthening specific neural pathways and lines of communication between the gut and brain and causing the development of signaling mechanisms in the central nervous system that irrevocably affect behavior and cognition[12]. Thus, healthy nervous system function is dependent upon the bacterial balance and correct functioning. Normally, information is sent from the heart, lungs, pancreas, liver, stomach, and intestines to the brain (including the cerebral cortex, medulla oblongata, limbic system, etc.) via sensory fibers in the vagus nerve[12]. From the medulla oblongata, the afferent inputs go to the locus ceruleus in the brain stem, from which the inputs send signals to widespread areas of the CNS that commence a stress response. If the locus ceruleus, an area responsible for coordinating stress response, is activated repeatedly, permanent changes occur in the way neurons activate and interact with one another that are in line with anxiety and depressive disorders[12]. This is also known as a hyperactive HPA axis, and it is activated similarly in the inflammatory aspect of the gut-brain axis, resulting in elevated stress response and anxiety. Elevated stress and anxiety has been shown to deplete ones microbiome of bacteria that produce anti-inflammatory cytokines, thus resulting in the biological effects inherent in inflammatory response and subsequent depression. Several examples of the significance of the vagal-mediated pathway between the gut and brain have been found in rodent experiments. One study found that anti-depressant and anxiolytic properties and associated neurochemical effects of Lactobacillus rhamnosus are absent in vagotomized rodents[13]. Additionally, vagotomy in rodents also appears to prevent colitis-induced anxiety-like behavior.[14] Furthermore, the probiotic Bifidobacterium longum requires an intact vagus nerve to elicit a reduction in anxiety.[11]
Other endocrine system interactions
[edit | edit source]Research has be conducted on the interactions between neuroendocrine hormones, and specifically on the relationships between hormones and the gut microbiota. Findings show that stress-induced neuroendocrine hormones can influence bacterial growth[15] and that gut microbiota may play an important role in hormone regulation such that endocrine effects of bacteria may influence host responses ranging from behavior to metabolism and appetite, and even immune responses.[16]
Anxiety and depression
[edit | edit source]Depression is known to be closely related to elevations in C-reactive proteins, inflammatory cytokines, and oxidative stress.[17] Current research is being done on the relationship between fecal bacteria (which served as a proxy to analyze the gut microbes) and depression, showing that the presence of certain bacteria are correlated to symptoms of depression.[18] One such study examined the role of switching the gut contents of two mice with distinct behavioral characteristics, with one very stress prone and the other not. Researchers found that when the gut contents were switched, the non-stressed mouse became stress prone and stressed mouse became more calm[5].
Schizophrenia and bipolar disorder
[edit | edit source]Schizophrenia is a neuropsychiatric disorder which can appear during adolescence and usually persists throughout an individuals' life. There are varying degrees of Schizophrenia, with characteristic symptoms such as hallucinations, delusions, apathy, and social withdrawal. Bipolar disorder (BD) is a complex and multifaceted disorder with a wide range of manifestations. Bipolar Disorder varies greatly and is defined by the presence of mania or depression in different regards. Previous studies have demonstrated that both schizophrenia and bipolar disorder are associated with alterations of the systemic immune system including low-grade chronic inflammation (increased plasma cytokines, soluble cytokine receptors, chemokines, acute phase reactants) and T-cell activation features.[19][20][21] In addition, elevated antibodies to S. cerevisiae were also found in increased levels in individuals with schizophrenia and bipolar disorder [22] The gut microbiome can influence brain function, thus playing a role in mental diseases such as Schizophrenia. Specifically, humoral immunity to food antigens, intestinal inflammation, exposure to the parasite Toxoplasma gondii, endothelial barrier defects and microbial dysbiosis consistent with a physiological model where gut-bases processes create a systematic state of immune dysregulation.[23][24] A variety of factors influence GI function and environment, and while no known medication exists to completely suppress GI trauma, practicing psychiatrists should consider complementing treatment with probiotics, herbal remedies, vitamins, and minerals that improve GI symptoms in individuals with schizophrenia and bipolar disorder.[25]
Autism spectrum disorder
[edit | edit source]Links between particular bacteria and phenotypes relevant to ASD raise the question of whether microbial dysbiosis (imbalances of the microbiome) plays a role in the development or presentation of ASD symptoms.[26] Studies of fecal DNA have found over represented clusters of Clostridium or Desulfovibrio in children with ASD and gastrointestinal complaints as compared to children with typical neuro-behavioral development and similar GI complaints.[27][28][29] A study found that children with autism have increased incidences of GI problems such as constipation and food selectivity, suggesting that neurobehavioral etiology may account for the higher incidences of the GI symptoms in children with autism.[30]
Anorexia and bulimia
[edit | edit source]Because of the growing evidence suggesting the importance of the microbiome in weight regulation and its relationship to anxiety and depression, research into gut-brain interactions may be important to the treatment of anorexia and bulimia. [31]
Potential interventions
[edit | edit source]"Crapsules" and fecal transplant
[edit | edit source]It is challenging to find ways to deliver desirable biota in a way where they can thrive in a new body. Eating or drinking them means that the bacteria need to survive exposure to stomach acid. Two solutions are currently being explored: fecal transplant, and enteric coating of pills to protect them until they move into the intestine. The enteric coated capsules have been nicknamed "crapsules." The delivery system appears effective. The remaining challenge is for the Food and Drug Administration and other regulatory agencies to decide how to monitor the quality, purity, and efficacy of the microbiome material.
Testing benefits of specific microbes
[edit | edit source]The potential clinical utility of probiotics, or microorganisms that incorporate into gut flora when consumed, and prebiotics, chemical compounds that provide impact the growth of gut flora when consumed, has become clearer in light of the accumulation of epical support.[32] Research into the microbiome-gut-brain axis has not only revealed the potential anxiogenic affects of specific bacteria and parasites and gut dysbiosis, but also the anxiolytic (anxiety-reducing) effects of certain microbial species.[11] Two bacterial genera, Lactobacillus and Bifidobacteria, are common anti-inflammatory probiotics that have been shown to reduce anxiety and behavioral signs of distress in both human and rodent studies.[33] Other genera, including Campylobacteria, Citrobacter, and Trichuris have also shown such anxiolytic affects.[11] The following table, adapted from a review by Liu et al., compiles results of many different studies on the effects of certain microbiota on cognitive and behavioral dimensions.[11]
| Type of study | Probiotic microbe(s) | Clinical outcome | Citation |
|---|---|---|---|
| Rodent study | Campylobacter jejuni, Citrobacter rodentium, Trichuris muris, high-fat microbiota | Increased anxiety symptoms/behavior | Bruce-Keller et al. (2015)[34]; Lyte, Varcoe, and Bailey (1998)[35]; and Stilling, Dinan, and Cryan (2014)[36] |
| Rodent study | Bifidobacterium spp., Lactobacillus spp. | Decreased anxiety symptoms/behavior | Bercik et al. (2011)[37], Bravo et al. (2011)[13], and Messaoudi et al. (2011)[38][39] |
| Rodent study | Bifidobacterium spp., Lactobacillus spp. | Decreased depressive symptoms/behavior | Arseneault-Bréard et al. (2012)[40] and Bravo et al. (2011)[13] |
| Human study (Cross-sectional finding) | Alistipes, Bacteroidales, Enterobacteriaceae | Positive association with depression | Jiang et al. (2015)[41] and Naseribafrouei et al. (2014)[42] |
| Human study (Cross-sectional finding) | Faecalibacterium, Lachnospiraceae | Negative association with depression | Jiang et al. (2015)[41] and Naseribafrouei et al. (2014)[42] |
| Human study (Longitudinal finding) | Bifidobacterium spp., Lactobacillus spp., Lactobacillus helveticus | Decreased anxiety symptoms | Messaoudi et al. (2011)[38][39], Mohammadi et al., 2015)[43], and Rao et al. (2009)[44] |
| Human study (Longitudinal finding) | Bifidobacterium spp., Lactobacillus spp., Lactobacillus helveticus | Decreased depressive symptoms | Benton, Williams, and Brown (2007)[45], Messaoudi et al. (2011)[38][39], and Mohammadi et al., 2015)[43] |
| Human study (Longitudinal finding) | Bifidobacterium longum, Lactobacillus helveticus | Decreased anger/hostility | Messaoudi et al. (2011)[38][39] |
| Human study (Longitudinal finding) | Bifadobacterium spp., Lactobacillus spp., Lactococcus lactis | Decreased cognitive reactivity to negative stimuli, mediated by reduction in rumination and aggressive thoughts | Steenbergen, Sellaro, van Hemert, Bosch, & Colzato (2015)[46] |
| Human study (Longitudinal finding) | Bifidobacterium animalis subsp. Lactis, Lactobacillus bulgaricus, Lactococcus lactis subsp. Lactis, Streptococcus thermophiles | Decreased activity in emotional and sensory brain regions in response to negative stimuli | Tillisch et al. (2013)[47] |
| Human study (Longitudinal finding) | Bimuno-galacto-oligosaccharides | Decreased attentional bias toward negative stimuli | Schmidt et al. (2015)[48] |
Probiotic and prebiotic meta-analysis
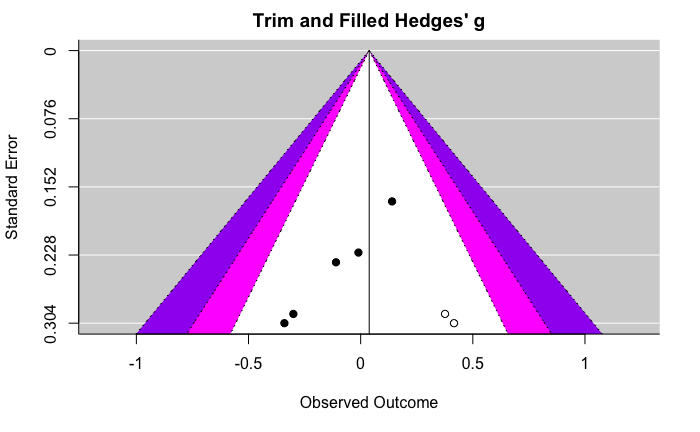
[edit | edit source]The following are funnel plots and forest plots displaying the effect of either probiotics or prebiotics on either depression or anxiety. These figures were adapted from the Liu et al. 2019 probiotic and prebiotic meta-analysis [32]. For information about the specific studies included in this meta-analysis, see the table below.
Effect of prebiotics on depression
|
|---|
  |
Effect of prebiotics on anxiety
|
|---|
  |
Effect of probiotics on depression
|
|---|
 |
Effect of probiotics on anxiety
|
|---|
  |
| Prebiotic or probiotic | Na | % Femalea | Mean agea | Sample | Prebiotic compound/probiotic microbe(s) | Length of treatment | Clinical outcome | Clinical measure(s) | Citation |
|---|---|---|---|---|---|---|---|---|---|
| Prebiotic | 79 | 75.95 | 41.67 | Medical (IBS patients) | scFOS | 4 weeks | anx, dep | HADS-A, HADS-D | Azpiroz et al. (2017)b |
| Prebiotic | 72 | 70.83 | 36.68 | Clinical (MDD patients) | GOS | 8 weeks | dep | BDI-II | Kazemi et al. (2019)d |
| Prebiotic | 45 | 51.11 | 23.69 | Community | B-GOS, FOS | 3 weeks | anx | STAI | Schmidt et al. (2015)b |
| Prebiotic | 44 | 63.64 | 54 | Medical (IBS patients) | B-GOS | 4 weeks | anx, dep | HADS-A, HADS-D | Silk et al. (2009) |
| Prebiotic | 142 | 51 | 32 | Community | FOS-enriched inulin | 2 weeks | anx, dep | HADS-A, HADS-D | Smith (2005)c |
| Prebiotic | 47 | 59.57 | 23 | Community | FOS-enriched inulin | 4 hours | dep | SSM | Smith et al. (2015)c |
| Probiotic | 40 | 85 | 37.2 | Clinical (MDD patients) | Bifidobacterium bifidium, Lactobacillus acidophilus, Lactobacillus casei | 8 weeks | dep | BDI-I | Akkasheh et al. (2016)d |
| Probiotic | 36 | 44.4 | 65 | Community | Lactobacillus helveticus | 12 weeks | dep | GDS-SF | Chung et al. (2014)b |
| Probiotic | 30 | 83.3 | 45 | Community | Bifidobacterium lactis, Lactobacillus acidophilus, Lactobacillus bulgari, Lactococcus lactis subspecies lactis, Lactobacillus plantarum, Lactobacillus reuteri, Streptococcus thermophiles, Streptococcus thermophilus | 3 weeks | anx | HAM-A | Colica et al. (2017) |
| Probiotic | 40 | 0.65 | 40.95 | Medical (IBS patients) | Lactobacillus paracasei | 4 weeks | anx, dep | HADS-A, HADS-D | Cremon et al. (2018)c |
| Probiotic | 40 | 34.98 | 70 | Clinical (MDD patients) | FOS, Bifidobacterium breve, Bifidobacterium longum, Lactobacillus acidofilus, Lactobacillus bulgarigus, Lactobacillus casaei, Lactobacillus rhamnosus, Streptococus thermophilus | 6 weeks | dep | HAM-D | Ghorbani et al. (2018)d,e |
| Probiotic | 47 | 44.68 | 22.85 | Community (medical students) | Lactobacillus casei Shirota | 8 weeks | anx, dep | HADS-A, HADS-D | Kato-Kataoka et al. (2016)b |
| Probiotic | 74 | 68.92 | 36.08 | Clinical (MDD patients) | Bifidobacterium longum, Lactobacillus helveticus | 8 weeks | dep | BDI-II | Kazemi et al. (2019)d |
| Probiotic | 29 | 0 | 24.59 | Community | Lactobacillus rhamnosus | 4 weeks | anx, dep | BAI, BDI (version unspecified), STAI | Kelly et al. (2017)b, c |
| Probiotic | 16 | 0 | 20.69 | Community | Lactobacillus paracasei | 8 days | anx | STAI | Kitaoka et al. (2009)b |
| Probiotic | 60 | 83.33 | 34.1 | Medical (MS patients) | Bifidobacterium bifidum, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus fermentum | 12 weeks | dep | BDI-I | Kouchaki et al. (2017) |
| Probiotic | 391 | 74.68 | 47.9 | Medical (IBS patients) | Lactobacillus acidophilus | 12 weeks | anx, dep | HADS-A, HADS-D | Lyra et al. (2016) |
| Probiotic | 40 | 85 | 42.12 | Clinical and Medical (patients with MDD and IBS) | Bacillus coagulans | 90 days | dep | CES-D, HAM-D, MADRS | Majeed et al. (2018)d |
| Probiotic | 136 | 70.59 | NR | Community (college students) | Lactobacillus casei, Lactobacillus delbrueckii subspecies bulgaricus, Streptococcus salivarius subspecies thermophiles | 6 weeks | anx | STAI | Marcos et al. (2004) |
| Probiotic | 55 | 74.55 | 42.82 | Community | Bifidobacterium longum, Lactobacillus helveticus | 30 days | anx, dep | HADS-A, HADS-D | Messaoudi et al. (2011)b |
| Probiotic | 224 | 69.2 | 53.92 | Community | Bifidobacterium longum, Lactobacillus gasseri | 12 weeks | dep | GHQ-28 depression subscale | Nishihira et al. (2014) |
| Probiotic | 238 | 61.04 | 72.3 | Community (elderly) | Lactobacillus reuteri | 12 weeks | anx, dep | HADS-A, HADS-D | Östlund-Lagerström et al. (2016) |
| Probiotic | 44 | 54 | 43.25 | Medical (IBS patients) | Bifidobacterium longum | 6 weeks | anx, dep | HADS-A, HADS–D, STAI | Pinto-Sanchez et al. (2017)f |
| Probiotic | 65 | 0 | 50.3 | Community (smokers) | Lactobacillus casei Shirota | 3 weeks | anx | STAI | Reale et al. (2012) |
| Probiotic | 31 | 90.32 | 52.71 | Medical (fibromyalgia patients) | Bifidobacterium bifidus, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus rhamnosus | 8 weeks | anx, dep | BDI-I, STAI | Roman et al. (2018) |
| Probiotic | 79 | 78.48 | 35.45 | Clinical | Bifidobacterium longum, Lactobacillus helveticus | 8 weeks | anx, dep | DASS, MADRS, QIDS-SR16 | Romijn et al. (2017)d |
| Probiotic | 125 | 61.6 | 36.01 | Medical (obese subjects) | FOS, inulin, Lactobacillus rhamnosus | 24 weeks | anx, dep | BDI-I, STAI | Sanchez et al. (2017)e, g |
| Probiotic | 29 | 0 | 19.99 | Community (college athletes) | Lactobacillus gasseri | 4 weeks | anx, dep | POMS | Sashihara et al. (2013) |
| Probiotic | 278 | 50.36 | 70.9 | Community | Lactobacillus pentosus | 20 weeks | anx, dep | POMS | Shinkai et al. (2013) |
| Probiotic | 74 | 43 | 70.27 | Medical (IBS patients) | Bifidobacterium lactis, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus paracasei, Streptococcus thermophilus | 8 weeks | anx, dep | HADS-A, HADS-D | Simrén et al. (2010)b |
| Probiotic | 381 | 100 | 33.6 | Community (pregnant women) | Lactobacillus rhamnosus | 45 weeks | anx, dep | EPDS, STAI | Slykerman et al. (2017) |
| Probiotic | 40 | 60 | 19.95 | Community | Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus, Lactobacillus brevis, Lactobacillus casei, Lactobacillus salivarius, Lactococcus lactis | 4 weeks | anx, dep | BAI, BDI-II | Steenbergen et al. (2015)b |
| Probiotic | 23 | 100 | 30 | Community | Bifidobacterium animalis, Lactobacillus bulgaricus, Lactococcus lactis, Streptococcus thermophiles | 4 weeks | anx, dep | HADS-A, HADS-D | Tillisch et al. (2013)b |
| Probiotic | 46 | 100 | 42.78 | Medical (rheumatoid arthritis patients) | Lactobacillus casei | 8 weeks | anx | STAI Form Y | Vaghef-Mehrabany et al. (2014) |
| Probiotic | 20 | 50 | 58.05 | Medical (laryngeal cancer patients) | Clostridium butyricum | 2 weeks | anx | HAM-A | Yang et al. (2016)b, h |
Note: anx = anxiety; BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory; B-GOS = Bimuno®-galactooligosaccharide; CES-D = Center for Epidemiologic Studies – Depression Scale; DASS = Depression Anxiety and Stress Scale; dep = depression; EPDS = Edinburgh Postnatal Depression Scale; FOS = fructooligosaccharide; GDS-SF = Geriatric Depression Scale – Short Form; GHQ = General Health Questionnaire; GOS = galactooligosaccharide; HADS-A = Hospital Anxiety and Depression Scale – Anxiety subscale; HADS-D = Hospital Anxiety and Depression Scale – Depression subscale; HAM-A = Hamilton Rating Scale for Anxiety; IBS = irritable bowel syndrome; MADRS = Montgomery-Åsberg Depression Rating Scale; MDD = major depressive disorder; MS = multiple sclerosis; NR = not reported; POMS = Profile of Mood States; QIDS- SR16 = Quick Inventory of Depressive Symptomatology – 16-item short-form; scFOS = short-chain fructooligosaccharide; SSM = study-specific measure; STAI = State-Trait Anxiety Inventory.
Note: In cases where data for multiple outcome measures are available in a given study, the mean of the effects for these measures was incorporated into the relevant meta-analysis.
(a) The number, mean age, and % female of participants included in relevant analyses, rather than for the entire study sample, are presented and were incorporated in moderator analyses whenever available and applicable.(b) Prospective subjects with psychopathology were screened out. These studies were categorized as having non-clinical samples for the purpose of moderator analyses. A cross-over design was employed.(c) Subjects were selected for elevated depression (no studies selected for elevated anxiety).(d) Subjects were administered a compound consisting of both prebiotics and probiotics.(e) Prospective subjects with minimal and severe depression and anxiety symptoms were screened out. Separate effects were reported by sex.(f) Outlier excluded from meta-analysis.
References
[edit | edit source]- ↑ Smith, Peter Andrey (2015-06-23). "Can the Bacteria in Your Gut Explain Your Mood?". The New York Times. ISSN 0362-4331. Retrieved 2017-11-10.
- ↑ Schmidt, Charles. "Mental Health May Depend on Creatures in the Gut". Scientific American. doi:10.1038/scientificamerican0315-S12. https://www.scientificamerican.com/article/mental-health-may-depend-on-creatures-in-the-gut/.
- ↑ Gregoire, Carolyn (2016-11-10). "How 'Psychobiotics' Use Gut Bacteria To Treat Mental Illness". Huffington Post. Retrieved 2017-11-10.
- ↑ Rogers, G B; Keating, D J; Young, R L; Wong, M-L; Licinio, J; Wesselingh, S (2016-04-19). "From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways". Molecular Psychiatry 21 (6): 738–748. doi:10.1038/mp.2016.50. ISSN 1476-5578. https://www.nature.com/articles/mp201650.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Kramer, Peter; Bressan, Paola (2015-07-14). "Humans as Superorganisms". Perspectives on Psychological Science. 10 (4): 464–481. doi:10.1177/1745691615583131.
- ↑ 6.0 6.1 6.2 Lloyd-Price, Jason; Abu-Ali, Galeb; Huttenhower, Curtis (2016-04-27). "The healthy human microbiome". Genome Medicine. 8: 51. ISSN 1756-994X. doi:10.1186/s13073-016-0307-y.
- ↑ Rodríguez, Juan Miguel; Murphy, Kiera; Stanton, Catherine; Ross, R. Paul; Kober, Olivia I.; Juge, Nathalie; Avershina, Ekaterina; Rudi, Knut et al. (2015). "The composition of the gut microbiota throughout life, with an emphasis on early life". Microbial Ecology in Health & Disease 26 (0). doi:10.3402/mehd.v26.26050. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4315782/.
- ↑ 8.0 8.1 Dantzer, Robert; O'Connor, Jason C.; Freund, Gregory G.; Johnson, Rodney W.; Kelley, Keith W. (2008-01-01). "From inflammation to sickness and depression: when the immune system subjugates the brain". Nature Reviews Neuroscience 9 (1): 46–56. doi:10.1038/nrn2297. ISSN 1471-0048. http://www.nature.com/doifinder/10.1038/nrn2297.
- ↑ Li, Wei; Wu, Xiaoli; Hu, Xu; Wang, Tao; Liang, Shan; Duan, Yunfeng; Jin, Feng; Qin, Bin (2017-11). "Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features". Science China Life Sciences 60 (11): 1223–1233. doi:10.1007/s11427-016-9001-4. ISSN 1674-7305. http://link.springer.com/10.1007/s11427-016-9001-4.
- ↑ Liśkiewicz, Paweł; Pełka-Wysiecka, Justyna; Kaczmarczyk, Mariusz; Łoniewski, Igor; Wroński, Michał; Bąba-Kubiś, Agata; Skonieczna-Żydecka, Karolina; Marlicz, Wojciech et al. (2019-02-01). "Fecal Microbiota Analysis in Patients Going through a Depressive Episode during Treatment in a Psychiatric Hospital Setting". Journal of Clinical Medicine 8 (2): 164. doi:10.3390/jcm8020164. ISSN 2077-0383. PMID 30717162. PMC PMC6407012. http://www.mdpi.com/2077-0383/8/2/164.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 Liu, Richard T. (2017-10). "The microbiome as a novel paradigm in studying stress and mental health.". American Psychologist 72 (7): 655–667. doi:10.1037/amp0000058. ISSN 1935-990X. PMID 29016169. PMC PMC5637404. http://doi.apa.org/getdoi.cfm?doi=10.1037/amp0000058.
- ↑ 12.0 12.1 12.2 12.3 12.4 Forsythe, Paul; Bienestock, John (2008-01-01). Probiotics in Neurology and Psychiatry (in en). American Society of Microbiology. pp. 285–298. doi:10.1128/9781555815462.ch22. http://www.asmscience.org/content/book/10.1128/9781555815462.ch22.
- ↑ 13.0 13.1 13.2 Bravo, Javier A.; Forsythe, Paul; Chew, Marianne V.; Escaravage, Emily; Savignac, Hélène M.; Dinan, Timothy G.; Bienenstock, John; Cryan, John F. (2011-09-20). "Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve". Proceedings of the National Academy of Sciences of the United States of America 108 (38): 16050–16055. doi:10.1073/pnas.1102999108. ISSN 1091-6490. PMID 21876150. PMC PMC3179073. https://www.ncbi.nlm.nih.gov/pubmed/21876150.
- ↑ Bercik, P.; Park, A. J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P. A. et al. (2011-12). "The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication". Neurogastroenterology & Motility 23 (12): 1132–1139. doi:10.1111/j.1365-2982.2011.01796.x. PMID 21988661. PMC PMC3413724. http://doi.wiley.com/10.1111/j.1365-2982.2011.01796.x.
- ↑ Hegde, Manjunath; Wood, Thomas K.; Jayaraman, Arul (September 2009). "The neuroendocrine hormone norepinephrine increases Pseudomonas aeruginosa PA14 virulence through the las quorum-sensing pathway". Applied Microbiology and Biotechnology 84 (4): 763–776. doi:10.1007/s00253-009-2045-1. ISSN 1432-0614. PMID 19517106. https://www.ncbi.nlm.nih.gov/pubmed/19517106.
- ↑ "Microbial endocrinology: the interplay between the microbiota and the endocrine system (PDF Download Available)". ResearchGate. Retrieved 2017-11-09.
- ↑ Logan, Alan C.; Jacka, Felice N.; Craig, Jeffrey M.; Prescott, Susan L. (2016-05-31). "The Microbiome and Mental Health: Looking Back, Moving Forward with Lessons from Allergic Diseases". Clinical Psychopharmacology and Neuroscience. 14 (2): 131–147. ISSN 1738-1088. doi:10.9758/cpn.2016.14.2.131.
- ↑ Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. (2014-08-01). "Correlation between the human fecal microbiota and depression". Neurogastroenterology & Motility. 26 (8): 1155–1162. ISSN 1365-2982. doi:10.1111/nmo.12378.
- ↑ Rosenblat, Joshua D.; Cha, Danielle S.; Mansur, Rodrigo B.; McIntyre, Roger S.. "Inflamed moods: A review of the interactions between inflammation and mood disorders". Progress in Neuro-Psychopharmacology and Biological Psychiatry 53: 23–34. doi:10.1016/j.pnpbp.2014.01.013. http://linkinghub.elsevier.com/retrieve/pii/S0278584614000141.
- ↑ Anderson, George; Maes, Michael (2015-02-01). "Bipolar Disorder: Role of Immune-Inflammatory Cytokines, Oxidative and Nitrosative Stress and Tryptophan Catabolites". Current Psychiatry Reports 17 (2): 8. doi:10.1007/s11920-014-0541-1. ISSN 1523-3812. https://link.springer.com/article/10.1007/s11920-014-0541-1.
- ↑ Anderson, George; Maes, Michael (2015-02-01). "Bipolar Disorder: Role of Immune-Inflammatory Cytokines, Oxidative and Nitrosative Stress and Tryptophan Catabolites". Current Psychiatry Reports 17 (2): 8. doi:10.1007/s11920-014-0541-1. ISSN 1523-3812. https://link.springer.com/article/10.1007/s11920-014-0541-1.
- ↑ Severance, Emily G.; Alaedini, Armin; Yang, Shuojia; Halling, Meredith; Gressitt, Kristin L.; Stallings, Cassie R.; Origoni, Andrea E.; Vaughan, Crystal et al.. "Gastrointestinal inflammation and associated immune activation in schizophrenia". Schizophrenia Research 138 (1): 48–53. doi:10.1016/j.schres.2012.02.025. http://linkinghub.elsevier.com/retrieve/pii/S0920996412001478.
- ↑ Severance, Emily G.; Gressitt, Kristin L.; Buka, Stephen L.; Cannon, Tyrone D.; Yolken, Robert H.. "Maternal complement C1q and increased odds for psychosis in adult offspring". Schizophrenia Research 159 (1): 14–19. doi:10.1016/j.schres.2014.07.053. http://linkinghub.elsevier.com/retrieve/pii/S0920996414004137.
- ↑ Severance, Emily G.; Gressitt, Kristin L.; Halling, Meredith; Stallings, Cassie R.; Origoni, Andrea E.; Vaughan, Crystal; Khushalani, Sunil; Alaedini, Armin et al.. "Complement C1q formation of immune complexes with milk caseins and wheat glutens in schizophrenia". Neurobiology of Disease 48 (3): 447–453. doi:10.1016/j.nbd.2012.07.005. http://linkinghub.elsevier.com/retrieve/pii/S096999611200246X.
- ↑ Vitetta, Luis; Bambling, Matthew; Alford, Hollie (2014-12-01). "The gastrointestinal tract microbiome, probiotics, and mood". Inflammopharmacology 22 (6): 333–339. doi:10.1007/s10787-014-0216-x. ISSN 0925-4692. https://link.springer.com/article/10.1007/s10787-014-0216-x.
- ↑ Vuong, Helen E.; Hsiao, Elaine Y. "Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder". Biological Psychiatry. 81 (5): 411–423. doi:10.1016/j.biopsych.2016.08.024.
- ↑ Finegold, Sydney M.; Dowd, Scot E.; Gontcharova, Viktoria; Liu, Chengxu; Henley, Kathleen E.; Wolcott, Randall D.; Youn, Eunseog; Summanen, Paula H. et al.. "Pyrosequencing study of fecal microflora of autistic and control children". Anaerobe 16 (4): 444–453. doi:10.1016/j.anaerobe.2010.06.008. http://linkinghub.elsevier.com/retrieve/pii/S1075996410001010.
- ↑ Parracho, Helena MRT; Bingham, Max O; Gibson, Glenn R; McCartney, Anne L (2005). "Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children". Journal of Medical Microbiology 54 (10): 987–991. doi:10.1099/jmm.0.46101-0. http://jmm.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.46101-0.
- ↑ Song, Yuli; Liu, Chengxu; Finegold, Sydney M. (2004-11-01). "Real-Time PCR Quantitation of Clostridia in Feces of Autistic Children". Applied and Environmental Microbiology 70 (11): 6459–6465. doi:10.1128/aem.70.11.6459-6465.2004. ISSN 0099-2240. PMID 15528506. http://aem.asm.org/content/70/11/6459.
- ↑ Ibrahim, Samar H.; Voigt, Robert G.; Katusic, Slavica K.; Weaver, Amy L.; Barbaresi, William J. (2009-08-01). "Incidence of Gastrointestinal Symptoms in Children With Autism: A Population-Based Study". Pediatrics 124 (2): 680–686. doi:10.1542/peds.2008-2933. ISSN 0031-4005. PMID 19651585. http://pediatrics.aappublications.org/content/124/2/680.
- ↑ Borgo, Francesca; Riva, Alessandra; Benetti, Alberto; Casiraghi, Maria Cristina; Bertelli, Sara; Garbossa, Stefania; Anselmetti, Simona; Scarone, Silvio et al. (2017-06-21). "Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests". PLOS ONE 12 (6): e0179739. doi:10.1371/journal.pone.0179739. ISSN 1932-6203. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0179739.
- ↑ 32.00 32.01 32.02 32.03 32.04 32.05 32.06 32.07 32.08 32.09 Liu, Richard T.; Walsh, Rachel F.L.; Sheehan, Ana E. (2019-07). "Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials". Neuroscience & Biobehavioral Reviews 102: 13–23. doi:10.1016/j.neubiorev.2019.03.023. PMID 31004628. PMC PMC6584030. https://linkinghub.elsevier.com/retrieve/pii/S0149763419300533.
- ↑ Deans, Emily (2016-06-27). "Microbiome and mental health in the modern environment". Journal of Physiological Anthropology. 36: 1. ISSN 1880-6805. doi:10.1186/s40101-016-0101-y.
- ↑ Bruce-Keller, Annadora J.; Salbaum, J. Michael; Luo, Meng; Blanchard, Eugene; Taylor, Christopher M.; Welsh, David A.; Berthoud, Hans-Rudolf (2015-04-01). "Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity". Biological Psychiatry 77 (7): 607–615. doi:10.1016/j.biopsych.2014.07.012. ISSN 1873-2402. PMID 25173628. PMC PMC4297748. https://www.ncbi.nlm.nih.gov/pubmed/25173628.
- ↑ Lyte, M.; Varcoe, J. J.; Bailey, M. T. (August 1998). "Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation". Physiology & Behavior 65 (1): 63–68. ISSN 0031-9384. PMID 9811366. https://www.ncbi.nlm.nih.gov/pubmed/9811366.
- ↑ Stilling, R. M.; Dinan, T. G.; Cryan, J. F. (January 2014). "Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis". Genes, Brain, and Behavior 13 (1): 69–86. doi:10.1111/gbb.12109. ISSN 1601-183X. PMID 24286462. https://www.ncbi.nlm.nih.gov/pubmed/24286462.
- ↑ Bercik, Premysl; Denou, Emmanuel; Collins, Josh; Jackson, Wendy; Lu, Jun; Jury, Jennifer; Deng, Yikang; Blennerhassett, Patricia et al. (August 2011). "The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice". Gastroenterology 141 (2): 599–609, 609.e1–3. doi:10.1053/j.gastro.2011.04.052. ISSN 1528-0012. PMID 21683077. https://www.ncbi.nlm.nih.gov/pubmed/21683077.
- ↑ 38.0 38.1 38.2 38.3 Messaoudi, Michaël; Lalonde, Robert; Violle, Nicolas; Javelot, Hervé; Desor, Didier; Nejdi, Amine; Bisson, Jean-François; Rougeot, Catherine et al. (March 2011). "Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects". The British Journal of Nutrition 105 (5): 755–764. doi:10.1017/S0007114510004319. ISSN 1475-2662. PMID 20974015. https://www.ncbi.nlm.nih.gov/pubmed/20974015.
- ↑ 39.0 39.1 39.2 39.3 Messaoudi, Michaël; Violle, Nicolas; Bisson, Jean-François; Desor, Didier; Javelot, Hervé; Rougeot, Catherine (July 2011). "Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers". Gut Microbes 2 (4): 256–261. doi:10.4161/gmic.2.4.16108. ISSN 1949-0984. PMID 21983070. https://www.ncbi.nlm.nih.gov/pubmed/21983070.
- ↑ Arseneault-Bréard, Jessica; Rondeau, Isabelle; Gilbert, Kim; Girard, Stéphanie-Anne; Tompkins, Thomas A.; Godbout, Roger; Rousseau, Guy (June 2012). "Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model". The British Journal of Nutrition 107 (12): 1793–1799. doi:10.1017/S0007114511005137. ISSN 1475-2662. PMID 21933458. https://www.ncbi.nlm.nih.gov/pubmed/21933458.
- ↑ 41.0 41.1 Jiang, Haiyin; Ling, Zongxin; Zhang, Yonghua; Mao, Hongjin; Ma, Zhanping; Yin, Yan; Wang, Weihong; Tang, Wenxin et al. (August 2015). "Altered fecal microbiota composition in patients with major depressive disorder". Brain, Behavior, and Immunity 48: 186–194. doi:10.1016/j.bbi.2015.03.016. ISSN 1090-2139. PMID 25882912. https://www.ncbi.nlm.nih.gov/pubmed/25882912.
- ↑ 42.0 42.1 Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. (August 2014). "Correlation between the human fecal microbiota and depression". Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society 26 (8): 1155–1162. doi:10.1111/nmo.12378. ISSN 1365-2982. PMID 24888394. https://www.ncbi.nlm.nih.gov/pubmed/24888394.
- ↑ 43.0 43.1 Mohammadi, Ali Akbar; Jazayeri, Shima; Khosravi-Darani, Kianoush; Solati, Zahra; Mohammadpour, Nakisa; Asemi, Zatollah; Adab, Zohre; Djalali, Mahmoud et al. (November 2016). "The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers". Nutritional Neuroscience 19 (9): 387–395. doi:10.1179/1476830515Y.0000000023. ISSN 1476-8305. PMID 25879690. https://www.ncbi.nlm.nih.gov/pubmed/25879690.
- ↑ Rao, A. Venket; Bested, Alison C.; Beaulne, Tracey M.; Katzman, Martin A.; Iorio, Christina; Berardi, John M.; Logan, Alan C. (2009-03-19). "A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome". Gut Pathogens 1 (1): 6. doi:10.1186/1757-4749-1-6. ISSN 1757-4749. PMID 19338686. PMC PMC2664325. https://www.ncbi.nlm.nih.gov/pubmed/19338686.
- ↑ Benton, D.; Williams, C.; Brown, A. (March 2007). "Impact of consuming a milk drink containing a probiotic on mood and cognition". European Journal of Clinical Nutrition 61 (3): 355–361. doi:10.1038/sj.ejcn.1602546. ISSN 0954-3007. PMID 17151594. https://www.ncbi.nlm.nih.gov/pubmed/17151594.
- ↑ Steenbergen, Laura; Sellaro, Roberta; van Hemert, Saskia; Bosch, Jos A.; Colzato, Lorenza S. (August 2015). "A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood". Brain, Behavior, and Immunity 48: 258–264. doi:10.1016/j.bbi.2015.04.003. ISSN 1090-2139. PMID 25862297. https://www.ncbi.nlm.nih.gov/pubmed/25862297.
- ↑ Tillisch, Kirsten; Labus, Jennifer; Kilpatrick, Lisa; Jiang, Zhiguo; Stains, Jean; Ebrat, Bahar; Guyonnet, Denis; Legrain-Raspaud, Sophie et al. (June 2013). "Consumption of fermented milk product with probiotic modulates brain activity". Gastroenterology 144 (7): 1394–1401, 1401.e1–4. doi:10.1053/j.gastro.2013.02.043. ISSN 1528-0012. PMID 23474283. PMC PMC3839572. https://www.ncbi.nlm.nih.gov/pubmed/23474283.
- ↑ Schmidt, Charles (2015-02-26). "Mental health: thinking from the gut". Nature 518 (7540): S12–15. doi:10.1038/518S13a. ISSN 1476-4687. PMID 25715275. https://www.ncbi.nlm.nih.gov/pubmed/25715275.
 KSF
KSF