Pyridine-4-Carbaldehyde
 From Handwiki
From Handwiki 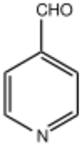
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Pyridine-4-carbaldehyde | |
| Other names
4-formylpyridine, 4-pyridinaldehyde, isonicotinaldehyde
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEMBL |
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C6H5NO |
| Molar mass | 107.112 g·mol−1 |
| Appearance | colorless liquid |
| Melting point | 4 °C (39 °F; 277 K) |
| Boiling point | 198 °C (388 °F; 471 K) |
| Acidity (pKa) | 4.72 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
GHS hazard statements
|
H315, H317, H319, H335 |
GHS precautionary statements
|
P261, P264, P271, P272, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P333+313, P337+313, P362, P363, P403+233, P405, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
- SizeSet
Pyridine-4-carbaldehyde is an organic compound with the formula C5H4NCHO. It is one of three isomeric pyridinaldehydes. The other isomers are pyridine-2-carboxaldehyde and pyridine-3-carboxaldehyde. Pyridine-4-carboxaldehyde is a colorless liquid, although aged samples can appear yellow or even brown. It undergoes many reactions expected for aromatic aldehydes such as reductive amination and Schiff base formation.[1] It condenses with pyrrole to give tetrapyridylporphyrin.[2] The pKa has been experimentally determined by NMR spectroscopy to be 4.72.[3]
References
- ↑ Chougnet, Antoinette; Woggon, Wolf-D. (2013). "Enantioselective Nitroaldol (Henry) Reaction of p-Nitrobenzaldehyde and Nitromethane Using a Copper (II) Complex Derived from (R,R)-1,2-Diaminocyclohexane: (1S)-1-(4-Nitrophenyl)-2-nitroethane-1-ol". Organic Syntheses 90: 52. doi:10.15227/orgsyn.090.0052.
- ↑ Drain, Charles Michael; Lehn, Jean-Marie (1994). "Self-Assembly of Square Multiporphyrin Arrays by Metal Ion Coordination". Journal of the Chemical Society, Chemical Communications (19): 2313. doi:10.1039/c39940002313.
- ↑ Handloser, Carolyn S.; Chakrabarty, M. R.; Mosher, Melvyn W. (July 1973). "Experimental determination of pKa values by use of NMR chemical shift" (in en). Journal of Chemical Education 50 (7): 510. doi:10.1021/ed050p510. ISSN 0021-9584. https://pubs.acs.org/doi/abs/10.1021/ed050p510.
 |
Categories: [Pyridines]
↧ Download as ZWI file | Last modified: 10/29/2022 18:59:36 | 5 views
☰ Source: https://handwiki.org/wiki/Chemistry:Pyridine-4-carbaldehyde | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF