Rhenium Trioxide Chloride
 From Handwiki
From Handwiki 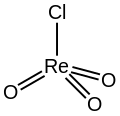
| |
| Names | |
|---|---|
| Other names
Rhenium oxychloride
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
ClO3Re |
| Molar mass | 269.65 g·mol−1 |
| Appearance | colorless liquid |
| Density | 4.665 g/cm3 (-100 °C) |
| Melting point | 4.5 °C (40.1 °F; 277.6 K) |
| Boiling point | 113 °C (235 °F; 386 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
- SizeSet
Rhenium trioxide chloride is an inorganic compound with the formula ReO
3Cl. It is a colorless, distillable, diamagnetic liquid. It is a rhenium oxychloride.[1] The material is used as a reagent in the preparation of rhenium compounds.
Synthesis and reactions
Rhenium trioxide chloride can be prepared by chlorination of rhenium trioxide:[2]
- 2 ReO
3 + Cl
2 → 2 ReO
3Cl
With Lewis bases (L), rhenium trioxide chloride reacts to form adducts with the formula ReO
3ClL
2.[3]
The compound hydrolyzes readily to give perrhenic acid.
Structure
The compound adopts a tetrahedral structure with Re-O and Re-Cl bond distances of 1.71 and 2.22 Å.[4] In contrast rhenium trioxide fluoride (ReO
3F) is polymeric with octahedral Re centers.
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1052. ISBN 978-0-08-037941-8.
- ↑ O. Glemser; R. Sauer (1963). "Rhenium (VII) Oxychloride". in G. Brauer. Handbook of Preparative Inorganic Chemistry, 2nd Ed.. 2pages=1480. NY, NY: Academic Press.
- ↑ Noh, Wontae; Girolami, Gregory S. (2007). "Rhenium Oxohalides: Synthesis and Crystal Structures of ReO3Cl(THF)2, ReOCl4(THF), Re2O3Cl6(THF)2, and Re2O3Cl6(H2O)2". Dalton Transactions (6): 674–679. doi:10.1039/b616495a. PMID 17268601.
- ↑ Spandl, Johann; Supeł, Joanna; Drews, Thomas; Seppelt, Konrad (2006). "MnO3Cl, Isolation and Crystal Structure". Zeitschrift für Anorganische und Allgemeine Chemie 632 (14): 2222–2225. doi:10.1002/zaac.200600186.
 |
Categories: [Rhenium compounds] [Chlorides] [Transition metal oxides]
↧ Download as ZWI file | Last modified: 02/19/2024 17:18:12 | 10 views
☰ Source: https://handwiki.org/wiki/Chemistry:Rhenium_trioxide_chloride | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF