Sulfenyl Chloride
 From Handwiki
From Handwiki 
In organosulfur chemistry, a sulfenyl chloride is a functional group with the connectivity R–S–Cl, where R is alkyl[1] or aryl. Sulfenyl chlorides are reactive compounds that behave as sources of RS+
. They are used in the formation of RS–N and RS–O bonds. According to IUPAC nomenclature they are named as alkyl thiohypochlorites, i.e. esters of thiohypochlorous acid.
Preparation
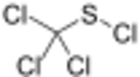
Sulfenyl chlorides are typically prepared by chlorination of disulfides:[2][3]
- [math]\ce{ R2S2 + Cl2 -> 2 R-SCl }[/math]
This reaction is sometimes called the Zincke disulfide reaction, in recognition of Theodor Zincke.[4][5] Typically, sulfenyl halides are stabilized by electronegative substituents. This trend is illustrated by the stability of CCl
3SCl obtained by chlorination of carbon disulfide.
Some thioethers (R–S–R’) with electron-withdrawing substituents undergo chlorinolysis of a C–S bond to afford the sulfenyl chloride.[6][7]
Reactions
Perchloromethyl mercaptan (CCl
3SCl) reacts with N–H bonds in the presence of base to give the sulfenamides:
- [math]\ce{ CCl3SCl + R2NH -> CCl3SNR2 + HCl }[/math]
This method is used in the production of the fungicides Captan and Folpet.
Sulfenyl chlorides add across alkenes, for example ethylene:[8]
- [math]\ce{ CH2=CH2 + R-SCl -> R-SC2H4Cl }[/math]
They undergo chlorination to the trichlorides:[3]
- [math]\ce{ R-SCl + Cl2 -> [R-SCl2]Cl }[/math]
Sulfenyl chlorides react with water and alcohols to give sulfenyl esters (R–S–O–R′):[9]
- [math]\ce{ R-SCl + H2O -> R-SOH + HCl }[/math]
- [math]\ce{ R-SCl + R'-OH -> R-SO-R' + HCl }[/math]
Route to sulfinyl halides
Sulfenyl chlorides can be converted to sulfinyl chlorides (RS(O)Cl). In one approach, the sulfinyl chloride is generated in two steps starting with reaction of a thiol (–SH) with sulfuryl chloride (SO
2Cl
2). In some cases the sulfenyl chloride results instead, as happens with 2,2,2-trifluoro-1,1-diphenylethanethiol. A trifluoroperacetic acid oxidation then provides a general approach to formation of sulfinyl chlorides from sulfenyl chlorides:[10]
Cl.png)
Related compounds
Sulfenyl fluorides and bromides are also known.[11] Simple sulfenyl iodides are unknown because they are unstable with respect to the disulfide and iodine:
- [math]\ce{ 2 R-SI -> (R-S)2 + I2 }[/math]
Sulfenyl iodides can be isolated as stable compounds if they bear alkyl steric protecting groups as part of a cavity-shaped framework, illustrating the technique of kinetic stabilization of a reactive functionality, as in the case of sulfenic acids.[12]
A related class of compounds are the alkylsulfur trichlorides, as exemplified by methylsulfur trichloride, CH
3SCl
3.[13]
The corresponding selenenyl halides, R–SeCl, are more commonly encountered in the laboratory. Sulfenyl chlorides are used in the production of agents used in the vulcanization of rubber.
References
- ↑ Drabowicz, J.; Kiełbasiński, P.; Łyżwa, P.; Zając, A.; Mikołajczyk, M. (2008). Kambe, N.. ed. Alkanesulfenyl Halides. Science of Synthesis. 39. pp. 544–550. ISBN 9781588905307.
- ↑ Hubacher, Max H. (1943). "o-Nitrophenylsulfur chloride". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV2P0455.; Collective Volume, 2, pp. 455
- ↑ 3.0 3.1 Douglass, Irwin B.; Norton, Richard V. (1973). "Methanesulfinyl Chloride". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV5P0709.; Collective Volume, 5, pp. 709–715
- ↑ "Über eine neue Reihe aromatischer Schwefelverbindungen" (in de). Chemische Berichte 44 (1): 769–771. 1911. doi:10.1002/cber.191104401109. https://zenodo.org/record/1426425.
- ↑ "Über o-Nitrophenylschwefelchlorid und Umwandlungsprodukte" (in de). Justus Liebig's Annalen der Chemie 391 (1): 57–88. 1912. doi:10.1002/jlac.19123910106. https://zenodo.org/record/1427597.
- ↑ F. B. Wells, C. F. H. Allen (1935). "2,4-Dinitroaniline". Organic Syntheses 15: 22. doi:10.15227/orgsyn.015.0022.
- ↑ Norman Kharasch, Robert B. Langford (1964). "2,4-Dinitrobenzenesulfenyl Chloride". Organic Syntheses 44: 47. doi:10.15227/orgsyn.044.0047.
- ↑ Brintzinger, H.; Langheck, M., "Synthesen mit Alkylschwefelchloriden (X. Mitteil. über organische Schwefelchloride)", Chemische Berichte 1954, volume 87, 325-330. doi:10.1002/cber.19540870306
- ↑ Petrovic, Goran; Saicic, Radomir N.; Cekovic, Zivorad (2005). "Phenylsulfenylation of Nonactivated Carbon Atom by Photolysiis of Alkyl Benzenesulfenated: Preparation of 2-Phenylthio-5-heptanol". Organic Syntheses 81: 244. doi:10.15227/orgsyn.081.0244.
- ↑ Page, P. C. B.; Wilkes, R. D.; Reynolds, D. (1995). "Alkyl Chalcogenides: Sulfur-based Functional Groups". in Ley, Steven V.. Synthesis: Carbon with One Heteroatom Attached by a Single Bond. Comprehensive Organic Functional Group Transformations. Elsevier. pp. 113–276. ISBN 9780080423234. https://books.google.com/books?id=BPcxrmIgLKMC&pg=PA173.
- ↑ Reno, Daniel S.; Pariza, Richard J. (1998). "Phenyl Vinyl Sulfide". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV9P0662.; Collective Volume, 9, pp. 662
- ↑ Sase, S.; Aoki, Y.; Abe, N.; Goto, K. (2009). "Stable Sulfenyl Iodide Bearing a Primary Alkyl Steric Protection Group with a Cavity-shaped Framework". Chemistry Letters 38 (12): 1188–1189. doi:10.1246/cl.2009.1188.
- ↑ Braverman, S.; Cherkinsky, M.; Levinger, S. (2008). "Alkylsulfur Trihalides". Sci. Synth. 39: 187–188. ISBN 9781588905307.
 |
Categories: [Organosulfur compounds] [Functional groups]
↧ Download as ZWI file | Last modified: 08/18/2024 16:42:15 | 3 views
☰ Source: https://handwiki.org/wiki/Chemistry:Sulfenyl_chloride | License: CC BY-SA 3.0

 KSF
KSF