Dodecahedral Molecular Geometry
 From Handwiki
From Handwiki | Dodecahedral molecular geometry | |
|---|---|
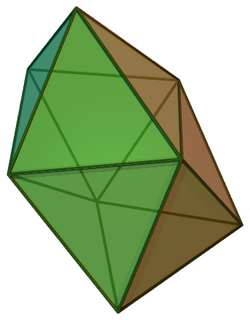 | |
| Examples | Mo(CN)4−8 |
| Point group | D2d |
| Coordination number | 8 |
| μ (Polarity) | 0 |
In chemistry, the dodecahedral molecular geometry describes the shape of compounds where eight atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a snub disphenoid (also known as a trigonal dodecahedron). This shape has D2d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the square antiprism and the bicapped trigonal prism.[1][2]
One example of the dodecahedral molecular geometry is the Mo(CN)4−8 ion.[2]
References
 |
Categories: [Stereochemistry] [Molecular geometry]
↧ Download as ZWI file | Last modified: 12/30/2023 18:27:57 | 16 views
☰ Source: https://handwiki.org/wiki/Physics:Dodecahedral_molecular_geometry | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF