Silver Sulfide
 From Handwiki
From Handwiki 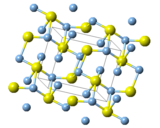
| |

| |
| Names | |
|---|---|
| IUPAC name
Silver(I) sulfide
| |
| Other names
Silver sulfide
Argentous sulfide | |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
Ag2S |
| Molar mass | 247.80 g·mol−1 |
| Appearance | Grayish-blackish crystal |
| Odor | Odorless |
| Density | 7.234 g/cm3 (25 °C)[1][2] 7.12 g/cm3 (117 °C)[3] |
| Melting point | 836 °C (1,537 °F; 1,109 K)[1] |
Solubility in water
|
6.21·10−15 g/L (25 °C) |
Solubility product (Ksp)
|
6.31·10−50 |
| Solubility | Soluble in aq. HCN, aq. citric acid with KNO3 Insoluble in acids, alkalies, aqueous ammoniums[4] |
| Structure | |
Crystal structure
|
Cubic, cI8 (α-form) Monoclinic, mP12 (β-form) Cubic, cF12 (γ-form)[3][5] |
Space group
|
Im3m, No. 229 (α-form)[5] P21/n, No. 14 (β-form) Fm3m, No. 225 (γ-form)[3] |
Point group
|
2/m (α-form)[5] 4/m 3 2/m (β-form, γ-form)[3] |
Lattice constant
|
a = 4.23 Å, b = 6.91 Å, c = 7.87 Å (α-form)[5] α = 90°, β = 99.583°, γ = 90°
|
| Thermochemistry | |
Heat capacity (C)
|
76.57 J/mol·K[6] |
Std molar
entropy (S |
143.93 J/mol·K[6] |
Std enthalpy of
formation (ΔfH⦵298) |
−32.59 kJ/mol[6] |
Gibbs free energy (ΔfG˚)
|
−40.71 kJ/mol[6] |
| Hazards | |
| Main hazards | May cause irritation |
| GHS pictograms |  [2] [2]
|
| GHS Signal word | Warning |
GHS hazard statements
|
H315, H319, H335[2] |
GHS precautionary statements
|
P261, P305+351+338[2] |
| NFPA 704 (fire diamond) | [7] 
0
0
0 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
- SizeSet
Silver sulfide is an inorganic compound with the formula Ag2S. A dense black solid, it is the only sulfide of silver. It is useful as a photosensitizer in photography. It constitutes the tarnish that forms over time on silverware and other silver objects. Silver sulfide is insoluble in most solvents, but is degraded by strong acids. Silver sulfide is a network solid made up of silver (electronegativity of 1.98) and sulfur (electronegativity of 2.58) where the bonds have low ionic character (approximately 10%).
Formation
Silver sulfide naturally occurs as the tarnish on silverware. When combined with silver, hydrogen sulfide gas creates a layer of black silver sulfide patina on the silver, protecting the inner silver from further conversion to silver sulfide.[8] Silver whiskers can form when silver sulfide forms on the surface of silver electrical contacts operating in an atmosphere rich in hydrogen sulfide and high humidity.[9] Such atmospheres can exist in sewage treatment and paper mills.[10][11]
Structure and properties
Three forms are known: monoclinic acanthite (β-form), stable below 179 °C, body centered cubic so-called argentite (α-form), stable above 180 °C, and a high temperature face-centred cubic (γ-form) stable above 586 °C.[5] The higher temperature forms are electrical conductors. It is found in nature as relatively low temperature mineral acanthite. Acanthite is an important ore of silver. The acanthite, monoclinic, form features two kinds of silver centers, one with two and the other with three near neighbour sulfur atoms.[12] Argentite refers to a cubic form, which, due to instability in "normal" temperatures, is found in form of the pseudomorphosis of acanthite after argentite.
History
In 1833 Michael Faraday noticed that the resistance of silver sulfide decreased dramatically as temperature increased. This constituted the first report of a semiconducting material.[13]
Silver sulfide is a component of classical qualitative inorganic analysis.[14]
References
- ↑ 1.0 1.1 Lide, David R., ed (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ↑ 2.0 2.1 2.2 2.3 Sigma-Aldrich Co., Silver sulfide. Retrieved on 2014-07-13.
- ↑ 3.0 3.1 3.2 3.3 Tonkov, E. Yu (1992). High Pressure Phase Transformations: A Handbook. 1. Gordon and Breach Science Publishers. p. 13. ISBN 978-2-88124-761-3. https://books.google.com/books?id=uo8wVw9Gq7wC&pg=PA13.
- ↑ Comey, Arthur Messinger; Hahn, Dorothy A. (February 1921). A Dictionary of Chemical Solubilities: Inorganic (2nd ed.). New York: The MacMillan Company. p. 835. https://archive.org/details/in.ernet.dli.2015.171090.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Silver sulfide (Ag2S) crystal structure". Non-Tetrahedrally Bonded Elements and Binary Compounds I. Landolt-Börnstein - Group III Condensed Matter. 41C. Springer Berlin Heidelberg. 1998. pp. 1–4. doi:10.1007/10681727_86. ISBN 978-3-540-31360-1. https://link.springer.com/static-content/lookinside/996/chp%3A10.1007%2F10681727_86/000.png.
- ↑ 6.0 6.1 6.2 6.3 Pradyot, Patnaik (2003). Handbook of Inorganic Chemicals. The McGraw-Hill Companies, Inc.. p. 845. ISBN 978-0-07-049439-8.
- ↑ "MSDS of Silver Sulfide". Salt Lake Metals. http://www.saltlakemetals.com/MSDS_Silver_Sulfide.htm.
- ↑ Zumdahl, Steven S.; DeCoste, Donald J. (2013). Chemical Principles (7th ed.). Cengage Learning. p. 505. ISBN 978-1-111-58065-0. https://books.google.com/books?id=hsuV9JTGaP8C&pg=PA505.
- ↑ "Degradation of Power Contacts in Industrial Atmosphere: Silver Corrosion and Whiskers". 2002. https://nepp.nasa.gov/whisker/reference/tech_papers/chudnovsky2002-paper-silver-corrosion-whiskers.pdf.
- ↑ Dutta, Paritam K.; Rabaey, Korneel; Yuan, Zhiguo; Rozendal, René A.; Keller, Jürg (2010). "Electrochemical sulfide removal and recovery from paper mill anaerobic treatment effluent". Water Research 44 (8): 2563–2571. doi:10.1016/j.watres.2010.01.008. ISSN 0043-1354. PMID 20163816. Bibcode: 2010WatRe..44.2563D.
- ↑ "Control of Hydrogen Sulfide Generation | Water & Wastes Digest" (in en). 5 March 2012. https://www.wwdmag.com/corrosion/control-hydrogen-sulfide-generation.
- ↑ Frueh, A. J. (1958). The crystallography of silver sulfide, Ag2S. Zeitschrift für Kristallographie-Crystalline Materials, 110(1-6), 136-144.
- ↑ "1833 - First Semiconductor Effect is Recorded". http://www.computerhistory.org/semiconductor/timeline/1833-first.html.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
External links
- Tarnishing of Silver: A Short Review V&A Conservation Journal
- Images of silver whiskers NASA
 |
Categories: [Sulfides] [Silver compounds] [Semiconductors]
↧ Download as ZWI file | Last modified: 09/07/2024 02:34:45 | 15 views
☰ Source: https://handwiki.org/wiki/Chemistry:Silver_sulfide | License: CC BY-SA 3.0

 KSF
KSF