Cellulose
 From Nwe
From Nwe 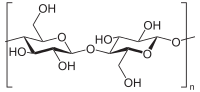
Cellulose (C6H10O5)n is a long-chain polymeric polysaccharide carbohydrate of beta-glucose, and is by far the most abundant organic (carbon-containing) compound on Earth.
Cellulose forms the primary structural component of green plants. The primary cell wall of green plants is made primarily of cellulose; the secondary wall contains cellulose with variable amounts of lignin. Lignin and cellulose, considered together, are termed lignocellulose, which (as wood) is the most common biopolymer on Earth. While humans cannot digest cellulose, many even-toed ungulates and termites can digest cellulose through a mutually beneficial symbiotic relationship with particular microorganisms that can break down the cellulose to usable form.
Like the polysaccharides starch and glycogen, cellulose is also a polymer of glucose, but the repeating monosaccharide unit is β-glucose. Because of the stability of its β-glycosidic linkages, cellulose is an excellent structural material that can withstand harsh environmental conditions.
In addition to its role as part of the natural environment, cellulose and its derivatives provide direct benefit to human beings, being used for clothing, paper, and dietary fiber, as well as in the production of plastics, rayon, and adhesives. Cellulose was used for the production of some of the first synthetic polymers.
Chemistry
Cellulose monomers (β-glucose) are linked together through 1→4 glycosidic bonds by condensation. Cellulose is a straight chain polymer: unlike starch, no coiling occurs, and the molecule adopts an extended rod-like conformation. In microfibrils, the multiple hydroxyl groups on the glucose residues hydrogen bond with each other, holding the chains firmly together and contributing to their high tensile strength. This strength is important in cell walls, where they are meshed into a carbohydrate matrix, helping keep plant cells rigid.
Given a cellulose material, the portion that does not dissolve in a 17.5 percent solution of sodium hydroxide at 20 °C is α cellulose, which is true cellulose; the portion that dissolves and then precipitates upon acidification is β cellulose; and the proportion that dissolves but does not precipitate is γ cellulose.
Cellulose can be assayed using a method described by Updegraff in 1969, where the fiber is dissolved in acetic and nitric acid, and allowed to react with anthrone in sulfuric acid. The resulting colored compound is assayed spectrophotometrically at a wavelength of approximately 635 nm.
History and applications
Cellulose occurs naturally in almost pure form in cotton fiber. In combination with lignin and hemicellulose, it is found in all plant material. Cellulose is the most abundant form of living terrestrial biomass (Crawford 1981).
Some animals, particularly ruminants and termites, can digest cellulose with the help of symbiotic micro-organisms. Cellulose is not digestible by humans, and is often referred to as 'dietary fiber' or 'roughage', acting as a hydrophilic bulking agent for feces.
Cellulose is the major constituent of paper; further processing can be performed to make cellophane and rayon, and more recently Modal, a textile derived from beechwood cellulose. Cellulose is used within the laboratory as a solid-state substrate for thin layer chromatography, and cotton linters is used in the manufacture of nitrocellulose, historically used in smokeless gunpowder.
Viscose is a very important fiber made out of cellulose and has been used for textiles since the beginning of the twentieth century.
The hydroxyl groups of cellulose can be partially or fully reacted with various chemicals to provide derivatives with useful properties. Cellulose esters and cellulose ethers are the most important commercial materials. In principle, though not always in current industrial practice, cellulosic polymers are renewable resources.
Among the esters are cellulose acetate and triacetate, which are film- and fiber-forming materials that find a variety of uses. Cellulose acetate, which is one of the cheapest raw materials produced, is used in making tools, eyeglass frames, electrical insulation, and packaging material, among other products. The inorganic ester nitrocellulose was initially used as an explosive and was an early film forming material. Cellulose nitrate was the first successful plastic.
Ether derivatives include
- Ethylcellulose, a water-insoluble commercial thermoplastic used in coatings, inks, flashlight cases, binders, fire extinguishers, and controlled-release drug tablets, and is the lightest of the cellulosics (and among the most expensive);
- Hydroxypropyl cellulose;
- Carboxymethyl cellulose;
- Hydroxypropyl methyl cellulose, E464, used as a viscosity modifier, gelling agent, foaming agent and binding agent;
- Hydroxyethyl methyl cellulose, used in production of cellulose films.
References
ISBN links support NWE through referral fees
- Crawford, R. L. 1981. Lignin biodegradation and transformation. New York: John Wiley and Sons. ISBN 0471057436.
- Ozturk, H. B., S. Okubayashi, and T. Bechtold. 2006. Splitting tendency of cellulosic fibers—Part 1. The effect of shear force on mechanical stability of swollen lyocell fibers. Cellulose 13(4):393-402.
- Updegraff, D. M. 1969. Semimicro determination of cellulose in biological materials. Analytical Biochemistry 32:420–424.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- Cellulose history
The history of this article since it was imported to New World Encyclopedia:
- History of "Cellulose"
Note: Some restrictions may apply to use of individual images which are separately licensed.
↧ Download as ZWI file | Last modified: 02/04/2023 03:36:17 | 131 views
☰ Source: https://www.newworldencyclopedia.org/entry/Cellulose | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: KSF
KSF