Epoxide
 From Nwe
From Nwe 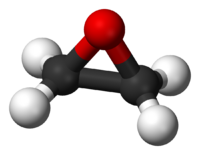
An epoxide is a cyclic ether with only three ring atoms. The simplest epoxide is ethylene oxide, also known as oxirane, which is regarded as the "parent" compound. Thus, members of the class of epoxides are also called oxiranes. Epoxides are more reactive than ordinary ethers.
A polymer containing unreacted epoxide units is called a polyepoxide or an epoxy. Epoxy resins are used as adhesives and structural materials. Polymerization of an epoxide gives a polyether. For example, polymerization of ethylene oxide generates polyethylene glycol, also known as polyethylene oxide, which is commercially the most important form of polyether.
Nomenclature
Simple epoxides are named from the parent compound oxirane (ethylene oxide), such as in chloromethyloxirane. When epoxide is considered a functional group in a larger compound, it is referred to with the epoxy prefix. An example is the compound 1,2-epoxycycloheptane, which can also be called cycloheptene epoxide.
A polymer containing unreacted epoxide units is called a polyepoxide or an epoxy.
Synthesis
Epoxides are usually produced by one of the reactions given below.
Olefin peroxidation
Olefin peroxidation, also known as the Prilezhaev reaction,[1] involves oxidation of an alkene with a peroxide, usually a peroxyacid like meta-chloroperoxybenzoic acid (m-CPBA) or with a dioxirane such as dimethyldioxirane (DMDO). An example is the epoxidation of styrene with perbenzoic acid to styrene oxide:[2]
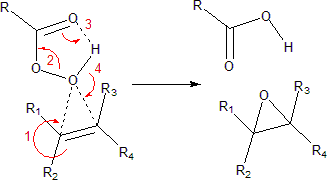
The reaction proceeds via what is commonly known as the Butterfly Mechanism.[3] It is easiest to consider the oxygen as an electrophile and the alkene as a nucleophile, although they both operate in that capacity, and the reaction is thought to be concerted. (The numbers in the mechanism below are for simplification.)
Related processes include some catalytic enantioselective reactions, such as the:
- Sharpless epoxidation
- Jacobsen epoxidation
- Shi epoxidation
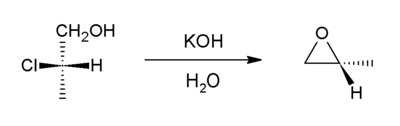
Intramolecular SN2 substitution
This method is a variant of the Williamson ether synthesis. In this case, the alkoxide ion and the halide are right next to each other in the same molecule (such compounds are generically called halohydrins), which makes this a simple ring closure reaction. For example, with 2-chloropropanol:[4]
Johnson-Corey-Chaykovsky reaction
In the Johnson-Corey-Chaykovsky reaction, epoxides are generated from carbonyl groups and sulfonium ylides.
Reactions
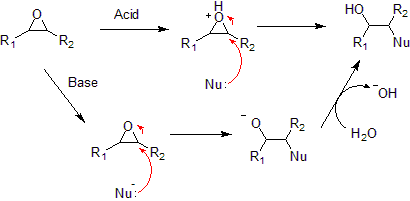
The three-membered ring of epoxide is approximately an equilateral triangle, that is, its bond angles are about 60°, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers, especially towards nucleophiles. Typical epoxide reactions are noted below.
- Nucleophilic addition to an epoxide can be catalyzed by a base or an acid.
-
- Under acidic conditions, the nucleophile attacks the carbon that will form the most stable carbocation, that is, the most substituted carbon (similar to a halonium ion). Under basic conditions, the nucleophile attacks the least substituted carbon, in accordance with standard SN2 nuclephilic addition reaction process.
- Hydrolysis of an epoxide in presence of an acid catalyst generates a glycol. The hydrolysis process of epoxides can be considered to be the nucleophilic addition of water to the epoxide under acidic conditions.
- Reduction of an epoxide with lithium aluminum hydride and water generates an alcohol. This reduction process can be considered to be the nucleophilic addition of hydride (H-) to the epoxide under basic conditions.
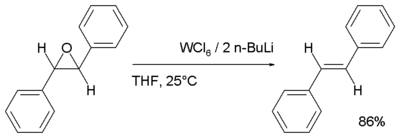
- Reduction with tungsten hexachloride and n-butyllithium generates the alkene. This reaction in effect is a de-epoxidation:[5]
See also
- Alcohol
- Epoxy
- Ether
- Ethylene glycol
- Ethylene oxide
- Polyethylene glycol
Notes
- ↑ Jerry March, Advanced Organic Chemistry: Reactions, Mechanisms and Structure, 3rd edition. (John Wiley & Sons, 1985, ISBN 0471854727).
- ↑ Harold Hibbert and Pauline Burt, Styrene Oxide, Org. Synth. Coll. Vol. 1: 494. Retrieved September 22, 2008.
- ↑ Bartlett, Rec. Chem. Prog 11:47.
- ↑ B. Koppenhoefer and V. Schurig, (R)-Alkyloxiranes of High Enantiomeric Purity from (S)-2-Chloroalkanoic Acids via (S)-2-Chloro-1-Alkanols: (R)-Methyloxirane, Org. Synth. Coll. Vol. 8: 434. Retrieved September 22, 2008.
- ↑ K. Barry Sharpless, Martha A. Umbreit, Marjorie T. Nieh, and Thomas C. Flood, Lower valent tungsten halides. New class of reagents for deoxygenation of organic molecules. J. Am. Chem. Soc. 94(18): 6538-6540.
References
ISBN links support NWE through referral fees
- March, Jerry. Advanced Organic Chemistry: Reactions, Mechanisms and Structure, 3rd ed. John Wiley & Sons, 1985. ISBN 0471854727.
- McMurry, John. Organic Chemistry, 6th ed. Belmont, CA: Thomson, 2004. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. Organic Chemistry, 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1992. ISBN 0136436692.
- Solomons, T.W. Graham, and Craig B. Fryhle. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley, 2004. ISBN 0471417998.
- Streitwieser, Andrew, and Clayton H. Heathcock. Introduction to Organic Chemistry. New York: Macmillan, 1976. ISBN 0024180106.
External links
All links retrieved August 19, 2017.
- Oxidation of Alcohols.
- Alcohols as Acids, Ethers and Thiols.
- Material Safety Data Sheet: Ethylene Oxide Andersen Sterilizers, Haw River, NC.
- Ethylene Oxide National Institute for Occupational Safety and Health.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
↧ Download as ZWI file | Last modified: 02/04/2023 05:44:48 | 31 views
☰ Source: https://www.newworldencyclopedia.org/entry/Epoxide | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: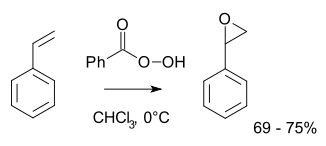
 KSF
KSF