Postsynaptic Density
 From Handwiki
From Handwiki | Postsynaptic density | |
|---|---|
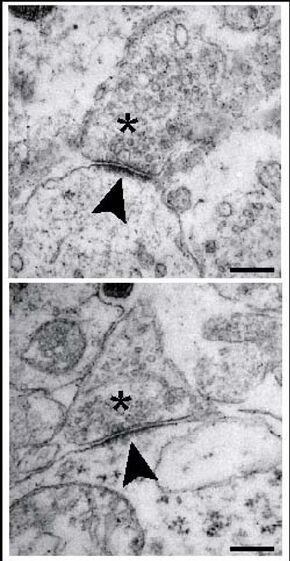 Ultra-structural analysis of synapses in the brainstem of wild-type (WT)mice at embryonic day 18.5. Synapses of WT neurons in the pre-Bötzinger-complex area exhibit presynaptic vesicles (asterisks), a synaptic cleft and a distinct postsynaptic density (arrowheads). Scale bar, 250 nm. From Heupel et al., 2008 | |
| Details | |
| System | Nervous system |
| Identifiers | |
| Latin | densitas postsynaptica |
| Anatomical terms of neuroanatomy [edit on Wikidata] | |
The postsynaptic density (PSD) is a protein dense specialization attached to the postsynaptic membrane. PSDs were originally identified by electron microscopy as an electron-dense region at the membrane of a postsynaptic neuron. The PSD is in close apposition to the presynaptic active zone and ensures that receptors are in close proximity to presynaptic neurotransmitter release sites.[1] PSDs vary in size and composition among brain regions, and have been studied in great detail at glutamatergic synapses. Hundreds of proteins have been identified in the postsynaptic density, including glutamate receptors, scaffold proteins, and many signaling molecules.
Structure
The structure and composition of the PSD have been the focus of numerous molecular studies of synaptic plasticity, a cellular model of learning and memory. PSDs are sized on the order of 250 to 500 nanometres in diameter and 25 to 50 nanometres in thickness, depending on the activity state of the synapse. During synaptic plasticity, the total size of the PSD is increasing along with an increase in synaptic size and strength after inducing long-term potentiation at single synapses.[2]
Composition
Many proteins in the PSD are involved in the regulation of synaptic function. These include
- postsynaptic density-95 (PSD95)[3]
- neuroligin (a cellular adhesion molecule)
- NMDA receptors, AMPA receptors
- calcium/calmodulin-dependent protein kinase II
- actin
As protein detection technologies have increased in sensitivity, such as with improvements in mass spectrometry techniques, more numerous proteins have been attributed to the PSD. Current estimates are greater than several hundred proteins are found at PSDs among brain regions and during different states of development and synaptic activity. PSDs also contain cell adhesion molecules and a diverse set of other signaling proteins. Many of the PSD proteins contain PDZ domains.[3]
Function
The PSD has been proposed to concentrate and organize neurotransmitter receptors in the synaptic cleft.[1] The PSD also serves as a signaling apparatus. For instance kinases and phosphatases in the PSD are activated and released from the PSD to change the activity of proteins located in the spine or are transported to the nucleus to affect protein synthesis. Some of the features of the PSD are similar to the neuromuscular junction and other cellular junctions, as the PSD has been modeled as a specialized cellular junction that allows for rapid, asymmetrical signaling.
References
- ↑ 1.0 1.1 Sweatt, J. D. (2008-01-01), Byrne, John H., ed., "4.20 - The NMDA Receptor" (in en), Learning and Memory: A Comprehensive Reference (Oxford: Academic Press): pp. 409–426, doi:10.1016/b978-012370509-9.00020-6, ISBN 978-0-12-370509-9, http://www.sciencedirect.com/science/article/pii/B9780123705099000206, retrieved 2020-12-23
- ↑ Meyer, D.; Bonhoeffer T.; Scheuss V. (2014). "Balance and Stability of Synaptic Structures during Synaptic Plasticity". Neuron 82 (2): 430–443. doi:10.1016/j.neuron.2014.02.031. PMID 24742464.
- ↑ 3.0 3.1 Sell, Gabrielle L.; Barrow, Stephanie L.; McAllister, A. Kimberley (2020-01-01), Rubenstein, John; Rakic, Pasko; Chen, Bin et al., eds., "Chapter 1 - Molecular composition of developing glutamatergic synapses" (in en), Synapse Development and Maturation (Academic Press): pp. 3–32, doi:10.1016/b978-0-12-823672-7.00001-6, ISBN 978-0-12-823672-7, http://www.sciencedirect.com/science/article/pii/B9780128236727000016, retrieved 2020-12-23
General review
- Kennedy MB (June 1997). "The postsynaptic density at glutamatergic synapses". Trends in Neurosciences 20 (6): 264–268. doi:10.1016/S0166-2236(96)01033-8. PMID 9185308.
- Ziff EB (December 1997). "Enlightening the postsynaptic density". Neuron 19 (6): 1163–74. doi:10.1016/S0896-6273(00)80409-2. PMID 9427241.
- Kennedy MB (October 2000). "Signal-processing machines at the postsynaptic density". Science 290 (5492): 750–4. doi:10.1126/science.290.5492.750. PMID 11052931. Bibcode: 2000Sci...290..750K.
Structure and composition
- "Proteins of the Postsynaptic Density". J. Cell Biol. 63 (2 Pt 1): 456–65. November 1974. doi:10.1083/jcb.63.2.456. PMID 4419608.
- "The structure of postsynaptic densities isolated from dog cerebral cortex: I. overall morphology and protein composition". J. Cell Biol. 74 (1): 181–203. July 1977. doi:10.1083/jcb.74.1.181. PMID 194906.
- "The structure of postsynaptic densities isolated from dog cerebral cortex: II. characterization and arrangement of some of the major proteins within the structure". J. Cell Biol. 74 (1): 204–25. July 1977. doi:10.1083/jcb.74.1.204. PMID 406264.
- "Identification of proteins in the postsynaptic density fraction by mass spectrometry". J. Neurosci. 20 (11): 4069–80. June 2000. doi:10.1523/JNEUROSCI.20-11-04069.2000. PMID 10818142. PMC 6772646. https://authors.library.caltech.edu/30310/1/4069.full.pdf.
- "Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry". J. Biol. Chem. 279 (20): 21003–11. May 2004. doi:10.1074/jbc.M400103200. PMID 15020595.
- "Identification and verification of novel rodent postsynaptic density proteins". Mol. Cell. Proteomics 3 (9): 857–71. September 2004. doi:10.1074/mcp.M400045-MCP200. PMID 15169875.
- "An architectural framework that may lie at the core of the postsynaptic density". Science 311 (5760): 531–5. January 2006. doi:10.1126/science.1118995. PMID 16439662. Bibcode: 2006Sci...311..531B.
- "Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome". J. Neurochem. 97 (Suppl 1): 16–23. April 2006. doi:10.1111/j.1471-4159.2005.03507.x. PMID 16635246.
- Alié Alexandre; Manuel Michaël (February 2010). "The backbone of the post-synaptic density originated in a unicellular ancestor of choanoflagellates and metazoans". BMC Evol. Biol. 10: 34. doi:10.1186/1471-2148-10-34. PMID 20128896.
External links
- Postsynaptic Density- Cell Centered Database
 |
Categories: [Neurohistology]
↧ Download as ZWI file | Last modified: 08/24/2025 02:51:38 | 13 views
☰ Source: https://handwiki.org/wiki/Biology:Postsynaptic_density | License: CC BY-SA 3.0

 KSF
KSF