Bleaching
 From Britannica 11th Edition (1911)
From Britannica 11th Edition (1911) Bleaching, the process of whitening or depriving objects of colour, an operation incessantly in activity in nature by the influence of light, air and moisture. The art of bleaching, of which we have here to treat, consists in inducing the rapid operation of whitening agencies, and as an industry it is mostly directed to cotton, linen, silk, wool and other textile fibres, but it is also applied to the whitening of paper-pulp, bees’-wax and some oils and other substances. The term bleaching is derived from the A.-S. blaecan, to bleach, or to fade, from which also comes the cognate German word bleichen, to whiten or render pale. Bleachers, down to the end of the 18th century, were known in England as “whitsters,” a name obviously derived from the nature of their calling.
The operation of bleaching must from its very nature be of the same antiquity as the work of washing textures of linen, cotton or other vegetable fibres. Clothing repeatedly washed, and exposed in the open air to dry, gradually assumes a whiter and whiter hue, and our ancestors cannot have failed to notice and take advantage of this fact. Scarcely anything is known with certainty of the art of bleaching as practised by the nations of antiquity. Egypt in early ages was the great centre of textile manufactures, and her white and coloured linens were in high repute among contemporary nations. As a uniformly well-bleached basis is necessary for the production of a satisfactory dye on cloth, it may be assumed that the Egyptians were fairly proficient in bleaching, and that still more so were the Phoenicians with their brilliant and famous purple dyes. We learn, from Pliny, that different plants, and likewise the ashes of plants, which no doubt contained alkali, were employed as detergents. He mentions particularly the Struthium as much used for bleaching in Greece, a plant which has been identified by some with Gypsophila Struthium. But as it does not appear from John Sibthorp’s Flora Graeca, edited by Sir James Smith, that this species is a native of Greece, Dr Sibthorp’s conjecture that the Struthium of the ancients was the Saponaria officinalis, a plant common in Greece, is certainly more probable.
In modern times, down to the middle of the 18th century, the Dutch possessed almost a monopoly of the bleaching trade although we find mention of bleach-works at Southwark near London as early as the middle of the 17th century. It was customary to send all the brown linen, then largely manufactured in Scotland, to Holland to be bleached. It was sent away in the month of March, and not returned till the end of October, being thus out of the hands of the merchant more than half a year.
The Dutch mode of bleaching, which was mostly conducted in the neighbourhood of Haarlem, was to steep the linen first in a waste lye, and then for about a week in a potash lye poured over it boiling hot. The cloth being taken out of this lye and washed, was next put into wooden vessels containing buttermilk, in which it lay under a pressure for five or six days. After this it was spread upon the grass, and kept wet for several months, exposed to the sunshine of summer.
In 1728 James Adair from Belfast proposed to the Scottish Board of Manufactures to establish a bleachfield in Galloway; this proposal the board approved of, and in the same year resolved to devote £2000 as premiums for the establishment of bleachfields throughout the country. In 1732 a method of bleaching with kelp, introduced by R. Holden, also from Ireland, was submitted to the board; and with their assistance Holden established a bleachfield for prosecuting his process at Pitkerro, near Dundee.
The bleaching process, as at that time performed, was very tedious, occupying a complete summer. It consisted in steeping the cloth in alkaline lyes for several days, washing it clean, and spreading it upon the grass for some weeks. The steeping in alkaline lyes, called bucking, and the bleaching on the grass, called crofting, were repeated alternately for five or six times. The cloth was then steeped for some days in sour milk, washed clean and crofted. These processes were repeated, diminishing every time the strength of the alkaline lye, till the linen had acquired the requisite whiteness.
For the first improvement in this tedious process, which was faithfully copied from the Dutch bleachfields, manufacturers were indebted to Dr Francis Home of Edinburgh, to whom the Board of Trustees paid £100 for his experiments in bleaching. He proposed to substitute water acidulated with sulphuric acid for the sour milk previously employed, a suggestion made in consequence of the new mode of preparing sulphuric acid, contrived some time before by Dr John Roebuck, which reduced the price of that acid to less than one-third of what it had formerly been. When this change was first adopted by the bleachers, there was the same outcry against its corrosive effects as arose when chlorine was substituted for crofting. A great advantage was found to result from the use of sulphuric acid, which was that a souring with sulphuric acid required at the longest only twenty-four hours, and often not more than twelve; whereas, when sour milk was employed, six weeks, or even two months, were requisite, according to the state of the weather. In consequence of this improvement, the process of bleaching was shortened from eight months to four, which enabled the merchant to dispose of his goods so much the sooner, and consequently to trade with less capital.
No further modification of consequence was introduced in the art till the year 1787, when a most important change was initiated by the use of chlorine (q.v.), an element which had been discovered by C.W. Scheele in Sweden about thirteen years before. The discovery that this gas possesses the property of destroying vegetable colours, led Berthollet to suspect that it might be introduced with advantage into the art of bleaching, and that it would enable practical bleachers greatly to shorten their processes. In a paper on chlorine or oxygenated muriatic acid, read before the Academy of Sciences at Paris in April 1785, and published in the Journal de Physique for May of the same year (vol. xxvi. p. 325), he mentions that he had tried the effect of the gas in bleaching cloth, and found that it answered perfectly. This idea is still further developed in a paper on the same substance, published in the Journal de Physique for 1786. In 1786 he exhibited the experiment to James Watt, who, immediately upon his return to England, commenced a practical examination of the subject, and was accordingly the person who first introduced the new method of bleaching into Great Britain. We find from Watt’s own testimony that chlorine was practically employed in the bleachfield of his father-in-law, Mr Macgregor, in the neighbourhood of Glasgow, in March 1787. Shortly thereafter the method was introduced at Aberdeen by Messrs Gordon, Barron & Co., on information received from De Saussure through Professor Patrick Copland of Aberdeen. Thomas Henry of Manchester was the first to bleach with chlorine in the Lancashire district, and to his independent investigations several of the early improvements in the application of the material were due.
In these early experiments, the bleacher had to make his own chlorine and the goods were bleached either by exposing them in chambers to the action of the gas or by steeping them in its aqueous solution. If we consider the inconveniences which must have arisen in working with such a pungent substance as free chlorine, with its detrimental effect on the health of the work-people, it will be readily understood that the process did not at first meet with any great amount of success. The first important improvement was the introduction in 1792 of eau de Javel, which was prepared at the Javel works near Paris by absorbing chlorine in a solution of potash (1 part) in water (8 parts) until effervescence began. The greatest impetus to the bleaching industry was, however, given by the introduction in 1799 of chloride of lime, or bleaching-powder, by Charles Tennant of Glasgow, whereby the bleacher was supplied with a reagent in solid form which contained up to one-third of its weight of available chlorine. Latterly frequent attempts have been made to replace bleaching-powder by hypochlorite of soda, which is prepared by the bleacher as required, by the electrolytic decomposition of a solution of common salt in specially constructed cells, but up to the present this mode of procedure has met with only a limited success (see Alkali Manufacture).
Bleaching of Cotton.
Cotton is bleached in the raw state, as yarn and in the piece. In the raw state, and as yarn, the only impurities present are those which are naturally contained in the fibres and which include cotton wax, fatty acids, pectic substances, colouring matters, albuminoids and mineral matter, amounting in all to some 5% of the weight of the material. Both in the raw state and in the manufactured condition cotton also contains small black particles which adhere firmly to the material and are technically known as “motes.” These consist of fragments of the cotton seed husk, which cannot be completely removed by mechanical means. The bleaching of cotton pieces is more complicated, since the bleacher is called upon to remove the sizing materials with which the manufacturer strengthens the warp before weaving (see below).
In principle, the bleaching of cotton is a comparatively simple process in which three main operations are involved, viz. (1) boiling with an alkali; (2) bleaching the organic colouring matters by means of a hypochlorite or some other oxidizing agent; (3) souring, i.e. treating with weak hydrochloric or sulphuric acid. For loose cotton and yarn these three operations are sufficient, but for piece goods a larger number of operations is usually necessary in order to obtain a satisfactory result.
Loose Cotton.—The bleaching of loose or raw cotton previous to spinning is only carried out to a very limited extent, and consists essentially in first steeping the material in a warm solution of soda for some hours, after which it is washed and treated with a solution of bleaching powder or sodium hypochlorite. It is then again washed, soured with weak sulphuric or hydrochloric acid, and ultimately washed free from acid. Careful treatment is necessary in order to avoid any undue matting of the fibres, while any drastic treatment, such as heating with caustic soda and soap, as used for other cotton materials, cannot be employed, since the natural wax would thereby be removed, and this would detract from the spinning qualities of the fibre. In case the cotton is not intended to be spun, but is to serve for cotton wool or for the manufacture of gun cotton, more drastic treatment can be employed, and is, in fact, desirable. Thus, cotton waste is first extracted with petroleum spirit or some other suitable solvent, in order to remove any mineral oil or grease which may be present. It is then boiled with dilute caustic soda and resin soap, washed, bleached white with bleaching-powder, washed, soured and finally washed free from acid. In these operations, a certain amount of matting is unavoidable, and it is consequently necessary to open out the material after drying, in scutchers.
Cotton Yarn.—Cotton yarn is bleached in the form of cops, hanks or warps. In principle the processes employed are the same in each case, but the machinery necessarily differs. Most yarn is bleached in the hank, and it will suffice to give an account of this process only. The sequence of operations is the same as in the bleaching of cotton waste, and these can be conducted for small lots in an ordinary rectangular wooden vat as used in dyeing, in which the yarn is suspended in the liquor from poles which rest with their ends on the two longer sides of the vat. For bleaching yarn in bulk, however, this mode of procedure would involve so much manual labour that the process would become too expensive. It is, therefore, mainly with the object of economy that machinery has been introduced, by means of which large quantities can be dealt with at a time.
The first operation, viz. that of boiling in alkali, is carried out in a “kier,” a large, egg-ended, upright cylindrical vessel, constructed of boiler-plate and capable of treating from one to three tons of yarn at a time. In construction, the kiers used for yarn bleaching are similar in construction to those used for pieces (see below). The yarn to be bleached is evenly packed in the kier, and is then boiled by means of steam with the alkaline lye (3-4% of soda ash or 2% caustic soda on the weight of the cotton being usually employed) for periods varying from six to twelve hours. It is essential that a thorough circulation of the liquor should be maintained during the boiling, and this is effected either by means of a steam injector, or in other ways. As a rule low pressure kiers (working up to 10 lb pressure) are employed for yarn bleaching, though some bleachers prefer to use high pressure kiers for the purpose.
When the boiling has continued for the requisite time (6-8 hours), the steam is shut off, and the kier liquor blown off, when the yarn is washed in the kier by filling the latter with water and then running off, this operation being repeated two or three times. The hanks are now transferred to a stone cistern provided with a false bottom, from beneath which a pipe connects the cistern with a well situated below the floor line. The well contains a solution of bleaching-powder, usually of 2° Tw. strength, and this is drawn up by means of a centrifugal brass pump and showered over the top of the goods through a perforated wooden tray, passing then by gravitation through the goods back into the well. The circulation is maintained for one and a half to two hours, when the yarn will be found to be white. The bleaching-powder solution is now allowed to drain off, and water is circulated through the cistern to wash out what bleaching powder remains in the goods. The souring is next carried out either in the same or in a separate cistern by circulating hydrochloric or sulphuric acid of 2° Tw. for about half an hour. This is also allowed to drain, and the yarn is thoroughly washed to remove all acid, when it is taken out and wrung or hydroextracted. At this stage the yarn may be dyed in light or bright shades without further treatment, but if it is to be sold as white yarn, it is blued. The blueing may either be effected by dyeing or tinting with a colouring matter like Victoria blue 4R or acid violet, or by treatment in wash stocks with a suspension of ultramarine in weak soap until the colour is uniformly distributed throughout the material. The yarn is now straightened out and dried.
The bleaching of cotton yarn is a very straightforward process, and it is very seldom that either complications or faults arise, providing that reasonable care and supervision are exercised.
The raison d’être of the various operations is comparatively simple. The effect of boiling with alkali is to remove the pectic acid, the fatty acids, part of the cotton wax and the bulk of the colouring matter, while the albuminoids are destroyed and the motes swelled up. If soap be used along with the alkali, the whole of the wax is removed by emulsification. In the operation of bleaching proper, the calcium hypochlorite of the chloride of lime through coming into contact with the carbonic acid of the atmosphere suffers decomposition according to the equation, Ca(OCl)2 + CO2 + H2O → CaCO3 + 2HOCl, and the hypochlorous acid thus liberated destroys the colouring matter still remaining from the first operation, by oxidation. At the same time the motes which were swelled up by the alkali are broken up into small fragments and are thus removed. In the operation of souring, the lime which has been deposited on the fibres during the treatment with bleaching powder is dissolved, while at the same time any other metallic oxides (iron, copper, &c.) are removed.
Cotton Pieces.—By far the largest bulk of cotton is bleached in the piece, as it can be more conveniently and more economically dealt with in this form than in any other. Though similar in principle to yarn bleaching, the process of piece bleaching is somewhat more complex because the pieces contain in addition to the natural impurities of the cotton a considerable amount of foreign matter in the form of size which has been incorporated with the warp before weaving, with the object of strengthening it. This size consists essentially of starch (farina), with additions of tallow, zinc chloride, and occasionally other substances such as paraffin wax, magnesium chloride, soap, &c., all of which must be removed if a perfect bleach is to result. Besides, mineral oil stains from the machinery of the weaving-shed are of common occurrence in piece goods.
Cotton pieces are bleached either for whites, for prints or for dyed goods. The processes employed for these different classes vary but slightly and only in detail. The most drastic bleach is that required for goods which are subsequently to be printed. For dyed goods, the main object is not so much to obtain a perfect white as to remove any impurities which might interfere with the dyeing, while avoiding the formation of any oxycellulose. In bleaching for whites (“market bleaching”) it is essential that the white should be as perfect as possible, and such goods are consequently invariably blued after bleaching.
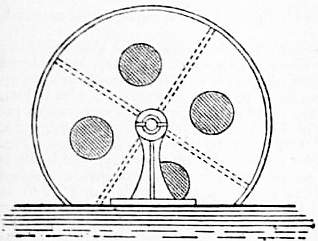 |
| Fig. 1.—Section of a Dash-wheel. |
For small lots (1-20 pieces) the bleaching can be conducted on very simple machinery. Thus many small piece dyers conduct the whole of their bleaching on the jigger, a simple form of dyeing machine on which most cotton piece goods are dyed (see Dyeing). For muslins, laces and other very light fabrics, which will not stand rough handling, the operations are conducted mainly by hand, washing being effected in the dash-wheel (fig. 1), which consists of a cylindrical box, revolving on its axis. It has four divisions, as shown by the dotted lines, and an opening into each division. A number of pieces are put into each, abundance of water is admitted behind, and the knocking of the pieces as they alternately dash from one side of the division to the other during the revolution of the wheel effects the washing. The process lasts from four to six minutes.
For velveteens, corduroys, heavy drills, pocketings and other fabrics in which creasing has to be avoided as much as possible, the so-called “open bleach” is resorted to, which differs from the ordinary process chiefly in that the goods are treated throughout at full width.
The great bulk of cotton pieces is bleached in rope form, i.e. stitched together end to end and laterally collapsed, so that they will pass through a ring of 4 to 5 in. in diameter.
The first operation which the goods undergo on arriving in the grey-room of the bleachworks is that of stamping with tar or some other indelible material in order that they may be identified after passing through the whole process. They are then stitched together end to end by means of special sewing machines, the stitch being of such a nature (chain stitch) that the thread can be ripped out at one pull at the end of the operations.
Singeing.—In the condition in which the pieces leave the loom and come into the hands of the bleacher, the surface of the fabric is seen to be covered with a nap of projecting fibres which gives it a downy appearance. For some classes of goods this is not a disadvantage, but in the majority of cases, especially for prints where a clean surface is essential, the nap is removed before bleaching. This is usually effected by running the pieces at full width over a couple of arched copper plates heated to a full red heat by direct fire. An arrangement of the kind is shown in fig. 2, in which the singe-plates, a and b, are mounted over the flues of a coal fire. The plate b is most highly heated, a being at the end of the flue farthest removed from the fire. The cloth enters over a rail A, and in passing over the plate a is thoroughly dried and prepared for the singeing it receives when it comes to the highly-heated plate b. A block d, carrying two rails in the space between the plates, can be raised or lowered so as to increase or lessen the pressure of the cloth against the plates, or, if necessary, to lift it quite free of contact with them.
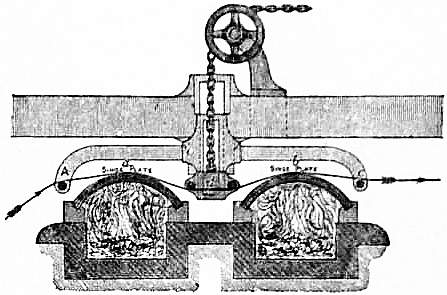 |
| Fig. 2.—Section of Singe-stove. |
The pieces on leaving the singeing machine are passed either through a water trough or through a steam box with the object of extinguishing sparks, and are then plaited down. The speed at which the pieces travel over the singe plates is necessarily considerable and varies with different classes of material.1
In lieu of plates, a cast-iron cylinder is sometimes employed (“roller singeing”), the heating being effected by causing the flame of the fire to be drawn through the roller, which is carried on two small rollers at each end and revolves slowly in the reverse direction to that followed by the piece, thus exposing continuously a freshly heated surface and avoiding uneven cooling.
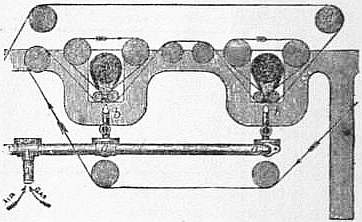 |
| Fig. 3.—Gas Singeing Apparatus. |
For figured pieces which have an uneven surface, it is obvious that plate or roller singeing would only affect the portions which project most, leaving the rest untouched. For such goods, “gas singeing” is employed, which consists in running the pieces over a non-luminous gas flame, the breadth of which slightly exceeds that of the piece, or in drawing the flame right through the piece.2 The construction of an ordinary gas singeing apparatus is seen in section in fig. 3. Coal gas mixed with air is sent under pressure through pipe a into the burners b, b, where the mixture burns with an intense heat. The cloth travels in the direction of the arrows, and in passing over the small nap rollers c comes into contact with the flame four times in succession before leaving the machine.
Gas singeing is also used for plain goods, and being cleaner and under better control has largely replaced plate singeing.
At this stage the goods which have been browned on the surface by singeing are ready for the bleaching operations. A great many innovations have been introduced in recent years in the bleaching of calico, but although it is generally admitted that in point of view of time and economy many of these processes offer considerable advantages, the old process, in which a lime boil precedes the other operations, is still the one which is most largely employed by bleachers in England. In this, the sequence of operations is the following—
Grey Washing.—This operation (which is sometimes omitted) simply consists in running the pieces through an ordinary washing machine (as shown in fig. 5) through water in order to wet them out. On leaving the machine they are piled in a heap and left over night, when fermentation sets in, which results in the starch being to a large extent hydrolysed and rendered soluble in water.
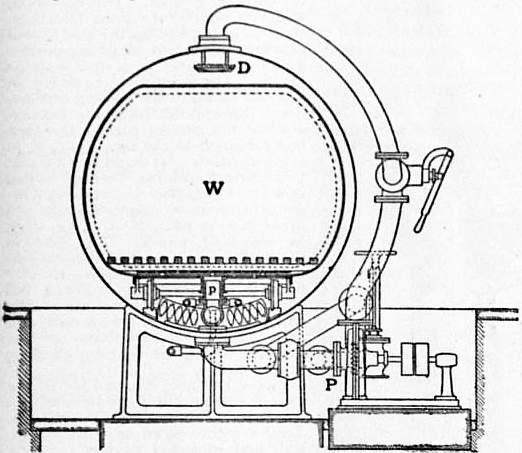
Lime Boil.—In this operation, which is also known as bowking (Ger. beuchen), the pieces are first run through milk of lime contained in an ordinary washing machine and of such a strength that they take up about 4% of their weight of lime (CaO). They are then run over winches and guided through smooth porcelain rings (“pot-eyes”) into the kier, where they are evenly packed by boys who enter the vessel through the manhole at the top. It is of the greatest importance that the goods should be evenly packed, for, if channels or loosely-packed places are left, the liquor circulating through the kier, when boiling is subsequently in progress, will follow the line of least resistance, and the result is an uneven treatment. Of the numerous forms of kier in use, the injector kier is the one most generally adopted. This consists of an egg-ended cylindrical vessel constructed of stout boiler plate and shown in sectional elevation in fig. 4. The kier is from 10 to 12 ft. in height and from 6 to 7 ft. in diameter, and stands on three iron legs riveted to the sides, but not shown in the figure. The bottom exit pipe E is covered with a shield-shaped false bottom of boiler plate, or (and this is more usual) the whole bottom of the kier is covered with large rounded stones from the river bed, the object in either case being simply to provide space for the accumulation of liquor and to prevent the pipe E being blocked. The cloth is evenly packed up to within about 3 to 4 ft. of the manholes M, when lime water is run in through the liquor pipe until the level of the liquid reaches within about 2 ft. of the top of the goods. The manholes are now closed, and steam is turned on at the injector J by opening the valve v. The effect of this is to suck the liquor through E, and to force it up through pipe P into the top of the kier, where it dashes against the umbrella-shaped shield U and is distributed over the pieces, through which it percolates, until on arriving at E it is again carried to the top of the kier, a continuous circulation being thus effected. As the circulation proceeds, the steam condensing in the liquor rapidly heats the latter to the boil, and as soon as, in the opinion of the foreman, all air has been expelled, the blow-through tap is closed and the boiling is continued for periods varying from six to twelve hours under 20-60 ℔ pressure. Steam is now turned off, and by opening the valve V the liquor, which is of a dark-brown colour, is forced out by the pressure of the steam it contains.
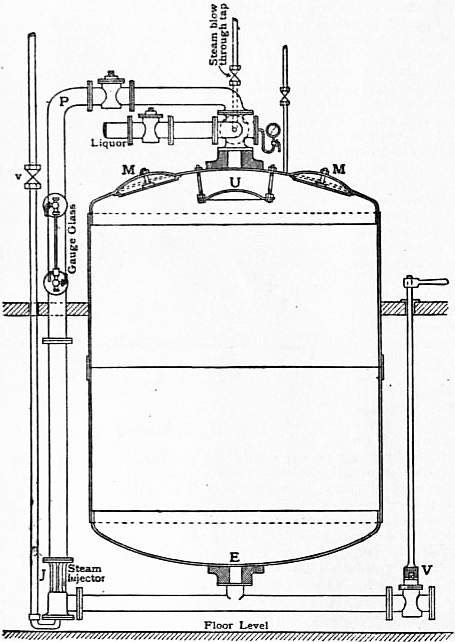 |
| Fig 4.—High Pressure Blow-through Kier. |
The pieces are now run through a continuous washing machine, which is provided with a plentiful supply of water. The machine, which is shown in fig. 5, consists essentially of a wooden vat, over which there is a pair of heavy wooden (sycamore) bowls or squeezers. The pieces enter the machine at each end, as indicated by the arrows, and pass rapidly through the bowls down to the bottom of the vat over a loose roller, thence between the first pair of guide pegs through the bowls again, and travel thus in a spiral direction until they arrive at the middle of the machine, when they leave at the side opposite to that on which they entered. The same type of machine is used for liming, chemicking, and souring.
The next operation is the “grey sour,” in which the goods are run through a washing machine containing hydrochloric acid of 2° Tw. strength, with the object of dissolving out the lime which the goods retain in considerable quantity after the lime boil. The goods are then well washed, and are now boiled again in the ash bowking kier, which is similar in construction to the lime kier, with soda ash (3%) and a solution of rosin (1½%) in caustic soda (1¼%) for eight to ten hours. For white bleaching the rosin soap is omitted, soda ash alone being employed.
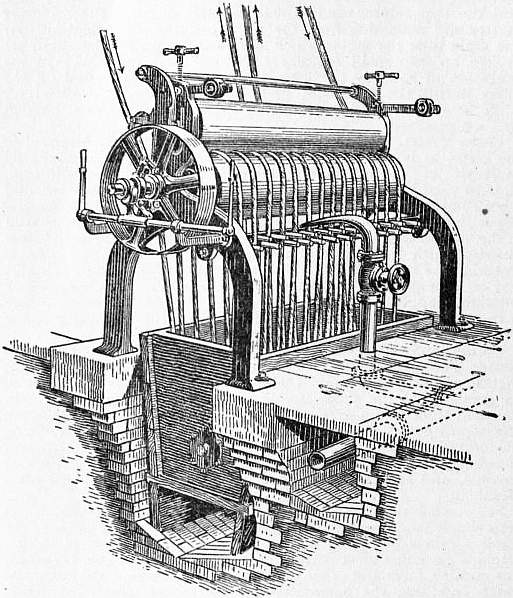 |
| Fig 5.—Roller Washing Machine. |
The pieces are now washed free from alkali and the bleaching proper or “chemicking” follows. This operation may be effected in various ways, but the most efficient is to run the goods in a washing machine through bleaching powder solution at ½°-1° Tw., and allow them to lie loosely piled over night, or in some cases for a longer period. They are now washed, run through dilute sulphuric or hydrochloric acid at 2° Tw. (“white sour”) and washed again. Should the white not appear satisfactory at this stage (and this is usually the case with very heavy or dense materials), they are boiled again in soda ash, chemicked with bleaching powder at 1⁄8° Tw. or even weaker, soured and washed. It is of the utmost importance that the final washing should be as thorough as possible, in order to completely remove the acid, for if only small quantities of the latter are left in the goods, they are liable to become tender in the subsequent drying, through formation of hydrocellulose.
The modern processes of bleaching cotton pieces differ from the one described above, chiefly in that the lime boil is entirely dispensed with, its place being taken by a treatment in the kier with caustic soda (or a mixture of caustic soda and soda ash) and resin soap. The best known and probably the most widely practised of these processes is one which was worked out by the late M. Horace Koechlin in conjunction with Sir William Mather, and this differs from the old process not only in the sequence of the operations but also in the construction of the kier. This consists of a horizontal egg-ended cylinder, and is shown in transverse and longitudinal sections in figs. 6 and 7. One of the ends E constitutes a door which can be raised or lowered by means of the power-driven chain C. The goods to be bleached are packed in wagons W outside the kier, and when filled these are pushed home into the kier, so that the pipes p fit with their flanges on to the fixed pipes at the bottom of the kier. The heating is effected by means of steam pipes at the lowest extremity of the kier, while the circulation of the liquor is brought about by means of the centrifugal pump P, which draws the liquor through the pipes p from beneath the false bottoms of the wagons and showers it over distributors D on to the goods. By this mode of working a considerable economy is effected in point of time, as the kier can be worked almost continuously; for as soon as one lot of goods has been boiled, the wagons are run out and two freshly-packed wagons take their place. The following is the sequence of operations:—The goods are first steeped over night in dilute sulphuric acid, after which they are washed and run through old kier liquor from a previous operation. They are then packed evenly in the wagons which are pushed into the kier, and, the door having been closed, they are boiled for about eight hours at 7-15 lb pressure with a liquor containing soda ash, caustic soda, resin soap and a small quantity of sulphite of soda. The rest of the operations (chemicking, souring and washing) are the same as in the old process.
 |
| Fig. 6.—The Mather Kier, cross section. |
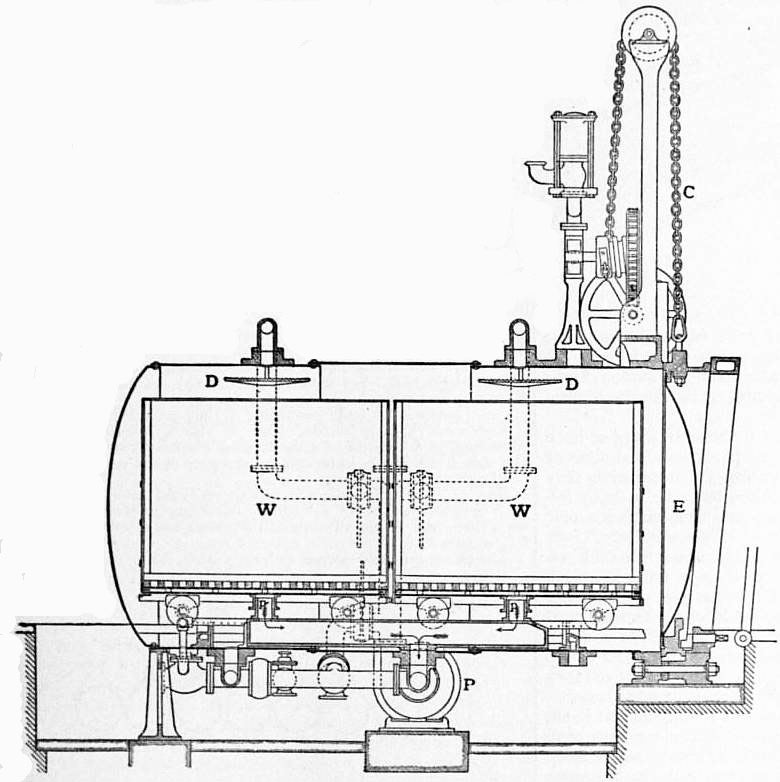 |
| Fig. 7.—The Mather Kier, longitudinal section. |
A somewhat different principle is involved in the Thies-Herzig process. In this the kier is vertical, and the circulation of the liquor is effected by means of a centrifugal or other form of pump, while the heating of the liquor is brought about outside the kier in a separate vessel between the pump and the kier by means of indirect steam. The sequence of operations is similar to that adopted in the Mather-Koechlin process, differing chiefly from the latter in the first operation, which consists in running the goods, after singeing, through very dilute boiling sulphuric or hydrochloric acid, containing in either case a small proportion of hydrofluoric acid, and then running them through a steam box, the whole operation lasting from twenty to sixty seconds.
Bleached by any of the above processes, the cloth is next passed over a mechanical contrivance known as a “scutcher,” which opens it out from the rope form to its full breadth, and is then dried on a continuous drying machine. Fig. 8 shows the appearance and construction of an improved form of the horizontal drying machine, which is in more common use for piece goods than the vertical form. The machine consists essentially of a series of copper or tinned iron cylinders, which are geared together so as to run at a uniform speed. Steam at 10-15 ℔ pressure is admitted through the journalled bearings at one side of the machine, and the condensed water is forced out continuously through the bearings at the other side. The pieces pass in the direction of the arrow (fig. 9) over a scrimp rail or expanding roller round the first cylinder, then in a zigzag direction over all succeeding cylinders, and ultimately leave the machine dry, being mechanically plaited down at the other end.
If the bleaching process has been properly conducted, the pieces should not only show a uniform pure white colour, but their strength should remain unimpaired. Careful experiments conducted by the late Mr. Charles O’Neill showed in fact that carefully bleached cotton may actually be stronger than in the unbleached condition, and this result has since been corroborated by others. Excessive blueing, which is frequently resorted to in order to cover the defects of imperfect bleaching, can readily be detected by washing a sample of the material in water, or, better still, in water containing a little ammonia, and then comparing with the original. The formation of oxycellulose during the bleaching process may either take place in boiling under pressure with lime or caustic soda in consequence of the presence of air in the kier, or through excessive action of bleaching powder, which may either result from the latter not being properly dissolved or being used too strong. Its detection may be effected by dyeing a sample of the bleached cotton in a cold, very dilute solution of methylene blue for about ten minutes, when any portions of the fabric in which the cellulose has been converted into oxycellulose will assume a darker colour than the rest. The depth of the colour is at the same time an indication of the extent to which such conversion has taken place. Most bleached cotton contains some oxycellulose, but as long as the formation has not proceeded far enough to cause tendering, its presence is of no importance in white goods. If, on the other hand, the cotton has to be subsequently dyed with direct cotton colours (see Dyeing), the presence of oxycellulose may result in uneven dyeing. Tendering of the pieces, due to insufficient washing after the final souring operation, is a common defect in bleached goods. As a rule the free acid can be detected by extracting the tendered material with distilled water and adding to the extract a drop of methyl orange solution, when the latter will turn pink if free acid be present. Other defects which may occur in bleached goods are iron stains, mineral oil stains, and defects due to the addition of paraffin wax in the size.
Bleaching of Linen.
The bleaching of linen is a much more complicated and tedious process than the bleaching of cotton. This is due in part to the fact that in linen the impurities amount to 20% or more of the weight of the fibre, whereas in cotton they do not usually exceed 5%. Furthermore these impurities, which include colouring matter, intracellular substances and a peculiar wax known as “flax wax,” are more difficult to attack than those which are present in cotton, and the difficulty is still further enhanced in the case of piece goods owing to their dense or impervious character.
Till towards the end of the 18th century the bleaching of linen both in the north of Ireland and in Scotland was accomplished by bowking in cows’ dung and souring with sour milk, the pieces being exposed to light on the grass between these operations for prolonged periods. Subsequently potash and later on soda was substituted for the cows’ dung, while sour milk was replaced by sulphuric acid. This “natural bleach” is still in use in Holland, a higher price being paid for linen bleached in this way than for the same material bleached with the aid of bleaching powder. In the year 1744 Dr. James Ferguson of Belfast received a premium of £300 from the Irish Linen Board for the application of lime in the bleaching of linen. Notwithstanding this reward, the use of lime in the bleaching of linen was for a long time afterwards forbidden in Ireland under statutory penalties, and so late as 1815 Mr Barklie, a respectable linen bleacher of Linen Vale, near Keady, was “prosecuted for using lime in the whitening of linens in his bleachyard.”
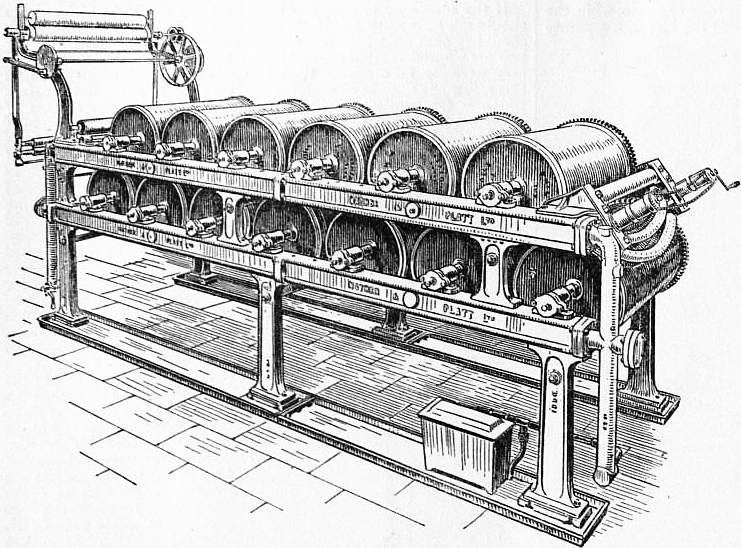 |
| Fig. 8.—Mather & Platt’s Horizontal Drying Machine. |
The methods at present employed for the bleaching of linen are, except in one or two unimportant particulars, the same as were used in the middle of the 19th century. In principle they resemble those used in cotton bleaching, but require to be frequently repeated, while an additional operation, which is a relic of the old-fashioned process, viz. that of “grassing” or “crofting,” is still essential for the production of the finest whites. Considerably more care has to be exercised in linen bleaching than is the case with cotton, and the process consequently necessitates a greater amount of manual labour. The practical result of this is that whereas cotton pieces can be bleached and finished in less than a week, linen pieces require at least six weeks. Many attempts have naturally been made to shorten and cheapen the process, but without success. The use of stronger reagents and more drastic treatment, which would at first suggest itself, incurs the risk of injury to the fibre, not so much in respect to actual tendering as to the destruction of its characteristic gloss, while if too drastic a treatment is employed at the beginning the colouring matter is liable to become set in the fibre, and it is then almost impossible to remove it. Among the many modern improvements which have been suggested, mention may be made of the use of hypochlorite of soda in place of bleaching powder, the use of oil in the first treatment in alkali (Cross & Parkes), while de Keukelaere suggests the use of sodium sulphide for this purpose. With the object of dispensing with the operation of grassing, which besides necessitating much manual labour is subject to the influences of the atmospheric conditions, Siemens & Halske of Berlin have suggested exposure of the goods in a chamber to the action of electrolytically prepared ozone. Jardin seeks to achieve the same object by steeping the linen in dilute nitric acid.
Since the qualities of linen which are submitted to the bleacher vary considerably, and the mode of treatment has to be varied accordingly, it is not possible to give more than a bare outline of linen bleaching.
Linen is bleached in the yarn and in the piece. Whenever one of the operations is repeated, the strength of the reagent is successively diminished. In yarn-bleaching the sequence of the operations is about as follows:—(1) Boil in kier with soda ash. (2) Reel in bleaching powder. This operation, which is peculiar to linen bleaching, consists in suspending the hanks from a square roller into bleaching powder solution contained in a shallow stone trough. The roller revolves slowly, so that the hanks, while passing continuously through the bleaching powder, are for the greater part of the time being exposed to the air. (3) Sour in sulphuric acid. (4) Scald in soda ash. (The term “scalding” means boiling in a kier.) (5) Reel in bleaching powder. (6) Sour in sulphuric acid. (7) Scald in soda ash. (8) Dip, i.e. steep in bleaching powder. (9) Sour in sulphuric acid. (10) Scald in soda ash. (11) Dip in bleaching powder. (12) Sour in sulphuric acid. For a full white, two more operations are usually required, viz. (13) scald in soda ash, and (14) dip in bleaching powder. Washing intervenes between all these operations.
Pieces are not stamped as in the case of cotton, but thread-marked by hand with cotton dyed Turkey red. They are then sewn together end to end, and subjected to the following operations:—
Boil with lime in kier.
The pieces are now separated and made up into bundles (except in the case of very light linens, which may pass through the whole of the operations in rope form) and soured with sulphuric acid.
First lye boil with soda ash and caustic soda.
Second lye boil. For some classes of goods no less than six lye boils may be required.
Grass between lye boils (according to their number).
Rub with rubbing boards. This is also a speciality in linen bleaching, and consists of a mechanical treatment with soft soap, the object of which is to remove black stains in the yarn.
Bleach with hypochlorite of soda.
Scald. The two latter treatments are repeated three to five times, each series constituting a “turn.” Grassing intervenes between each turn, and in some instances the pieces are rubbed before the last soda boil.
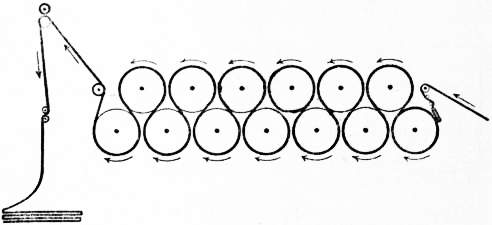 |
| Fig. 9.—Diagram showing the Horizontal Drying Machine threaded with Cloth. |
The pieces are next steeped in large vessels (kiers) in weak hypochlorite of soda, and then in weak sulphuric acid, these treatments being repeated several times.
Ultimately the goods are mill-washed, blued with smalt and dried.
Bleaching of other Vegetable Textile Fabrics.
Hemp may be bleached by a process similar to that used for linen, but this is seldom done owing to the expense entailed. China grass is bleached like cotton. Jute contains in its raw state a considerable amount of colouring matter and intracellular substance. Since the individual fibres are very short, the complete removal of the latter would be attended by a disintegration of the material. Although it is possible to bleach jute white, this is seldom if ever carried out on a large scale owing to the great expense involved. A half-bleach on jute is obtained by steeping the goods alternately in bleaching powder (or hypochlorite of soda) and sulphuric acid, washing intervening. For a cream these treatments are repeated.
Bleaching of Straw.
In the Luton district, straw is bleached principally in the form of plait, in which form it is imported. The bleaching is effected by steeping the straw for periods varying from twelve hours to several days in fairly strong alkaline peroxide of hydrogen. The number of baths depends upon the quality of straw and the degree of whiteness required. Good whites are thus obtained, and no further process would be necessary if the hats had not subsequently to be “blocked” or pressed at a high temperature which brings about a deterioration of the colour. After bleaching with peroxide and drying, the straw consequently undergoes a further process of sulphuring, i.e. exposure to gaseous sulphurous acid. Panama hats are bleached after making up, but in this case only peroxide of hydrogen is used and a very lengthy treatment entailing sometimes fourteen days’ steeping is required.
Bleaching of Wool.
In the condition in which it is delivered to the manufacturers wool is generally a very impure article, even if it has been washed on the sheep’s back before shearing. The impurities which it contains consist in the main of the natural grease (in reality a kind of wax) exuded from the skin of the sheep and technically known as the “yolk,” the dried-up perspiration from the body of the sheep; technically called “suint,” and dust, dirt, burrs, &c., which mechanically adhere to the sticky surfaces of the fibres. In this condition wool is quite unfit for any manufacturing purposes and must be cleansed before any mechanical operations can be commenced. Formerly the washing was effected in stale urine, which owed its detergent properties mainly to the presence of ammonium carbonate. The stale urine or lant was diluted with four to five times its bulk of water, and in this liquor, heated to 40°-50° C., the washing was effected.
At the present day this method has been entirely abandoned, the washing or “scouring” being effected with soap, assisted by ammonia, potash, soda or silicate of soda. The finest qualities of wool are washed with soft soap and potash, while for inferior qualities, cheaper detergents are employed. The operation is in principle perfectly simple, the wool being submerged in the warm soap solution, where it is moved about with forks and then taken out and allowed to drain. A second treatment in weaker soap serves to complete the process. In dealing with large quantities, wool-washing machines are employed, which consist essentially of long cast-iron troughs which contain the soap solution. The wool to be washed is fed in at one end of the machine and is slowly propelled to the other end by means of a system of mechanically-driven forks or rakes. As it passes from the machine, it is squeezed through a pair of rollers. Three such machines are usually required for efficient washing, the first containing the strongest and the third the weakest soap.
The washing of wool is in the main a mechanical process, in which the water dissolves out the suint while the soap emulsifies the yolk and thus removes it from the fibre. The attendant earthy impurities pass mechanically into the surrounding liquid and are swilled away.
In some works the wool is washed first with water alone, the aqueous extract thus obtained being evaporated to dryness and the residue calcined. A very good quality of potash is thus obtained as a by-product. In many works in Yorkshire and elsewhere, the dirty soap liquors obtained in wool-washing are not allowed to run to waste, but are run into tanks and there treated with sulphuric acid. The effect of this treatment is to decompose the soap, and the fatty acids along with the wool-grease rise as a magma to the surface. The purified product is known in the trade as “Yorkshire grease.”
Attempts have been made from time to time to extract the natural grease from wool by means of organic solvents, such as carbon bisulphide, carbon tetrachloride, petroleum spirit, &c., but have not met with much success.
Worsted yarn spun on the English system, as well as woollen yarn and fabrics made from them, contain oil which has been incorporated with the wool to facilitate the spinning. This oil must be got rid of previous to bleaching, and this is effected by scouring in warm soap with or without the assistance of alkalis.
The actual bleaching of wool may be effected in two ways, viz. by treating the material either with sulphurous acid or with hydrogen peroxide. Sulphurous acid may either be applied in the gaseous form or in solution as bisulphite of soda. In working by the first method, which is technically known as “stoving,” the scoured yarn is wetted in very weak soap containing a small amount of blue colouring matter, wrung or hydro-extracted and then suspended in a chamber or stove. Sulphur contained in a vessel on the floor of the chamber is now lighted, and the door having been closed, is allowed to burn itself out. The goods are left thus exposed to the sulphur dioxide overnight, when they are taken out and washed in water. For piece goods a somewhat different arrangement is employed, the pieces passing through a slit into a chamber supplied with sulphur dioxide, then slowly up and down over a large number of rollers and ultimately emerging again at the same slit. Wool may also be bleached by steeping in a fairly strong solution of bisulphite of soda and then washing well in water. Wool bleached with sulphurous acid or bisulphite is readily affected by alkalis, the natural yellow colour returning on washing with soap or soda. A more permanent bleach is obtained by steeping the wool in hydrogen peroxide (of 12 volumes strength), let down with about three times its bulk of water and rendered slightly alkaline with ammonia or silicate of soda. Black or brown wools cannot be bleached white, but when treated with peroxide they assume a golden colour, a change which is frequently desired in human hair.
Bleaching of Silk.
In raw silk, the fibre proper is uniformly coated with a proteid substance known as silk-gum, silk-glue or sericine which amounts to 19-25% of the weight of the material, and it is only after the removal of this coating that the characteristic properties of the fibre become apparent. This is effected by the process of “discharging” or “boiling-off,” which consists in suspending the hanks of raw silk over poles or sticks in a vat containing a strong hot soap solution (30% of soap on the weight of the silk). The liquor is kept just below boiling point for two or three hours, the hanks being turned from time to time. During the process, the sericine at first swells up considerably, the fibres becoming slippery, but as the operation proceeds it passes into solution. It is important that only soft water should be used for boiling-off since calcareous impurities are liable to mar the lustre of the silk.
The silk is now rinsed in weak soda solution and wrung. In this condition it is suitable for being dyed, but if it is to be bleached, the hanks are tied up loosely with smooth tape, put into coarse linen bags to prevent the silk becoming entangled, and boiled again in soap solution which is half as strong as that used in the first operation. The hanks are now taken out, rinsed in a weak soda solution, washed in water and wrung.
The actual bleaching of silk is usually effected by stoving as in the case of wool, with this difference, that the operation is repeated several times and blueing or tinting with other colours is effected after bleaching. Silk may also be bleached with peroxide of hydrogen, but this method is only used for certain qualities of spun silk and for tussore.
Ornamental feathers are best bleached by steeping in peroxide of hydrogen, rendered slightly alkaline by the addition of ammonia. The same treatment is applied to the bleaching of ivory. If peroxide of hydrogen could be prepared at a moderate cost, it would doubtless find a much more extensive application in bleaching, since it combines efficiency with safety, and gives good results with both vegetable and animal substances.
1 Besides being used for cotton goods, plate singeing is also employed for certain classes of worsted goods (alpacas, bunting, &c.), and for most union goods (cotton warp and worsted weft).
2 A machine working on this principle has been constructed by F. Binder, and the makers of the machine (Messrs Mather & Platt, Ltd.) claim that it does better service than the machines constructed on the older principle.
↧ Download as ZWI file | Last modified: 11/17/2022 15:23:08 | 43 views
☰ Source: https://oldpedia.org/article/britannica11/Bleaching | License: Public domain in the USA. Project Gutenberg License
 ZWI signed:
ZWI signed: KSF
KSF