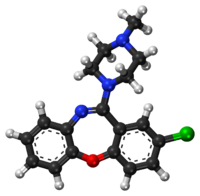
Loxapine
 From Mdwiki
From Mdwiki 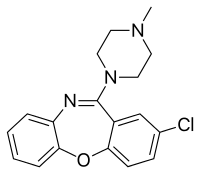 | |
 | |
| Names | |
|---|---|
| Trade names | Loxitane, Adasuve, others |
| |
| Clinical data | |
| Drug class | Typical antipsychotic[1] |
| Main uses | Psychosis[1] |
| Side effects | Movement disorders, sleepiness, dry mouth, blurry vision, low blood pressure with standing, fast heart rate[1] |
| Routes of use | By mouth, powder for inhalation |
| Typical dose | 60 to 100 mg per day[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682311 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 96.8%[2] |
| Metabolism | Extensive hepatic; active metabolites include amoxapine and 8-hydroxyloxapine. Inhibits P-gp and is a substrate of CYP1A2, CYP3A4 and CYP2D6[2] |
| Elimination half-life | 4 hours (oral); 7.61 hours (inhalation)[2] |
| Excretion | Majority are excreted within 24 hours, main route through urine (conjugated metabolites), small amounts through the feces (unconjugated metabolites) |
| Chemical and physical data | |
| Formula | C18H18ClN3O |
| Molar mass | 327.81 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 109 to 110 °C (228 to 230 °F) |
| |
| |
Loxapine, sold under the brand names Loxitane among others, is a typical antipsychotic used to treat psychosis including schizophrenia and for agitation.[1][3] Benefit in people with intellectual disability is unclear.[1] It is taken by mouth or inhaled.[1][3]
Common side effects include movement disorders, sleepiness, dry mouth, blurry vision, low blood pressure with standing, and fast heart rate.[1] Other side effects may include milk production, seizures, sunburns, neuroleptic malignant syndrome, and increased risk of death in those with dementia.[1] It is believed to work by blocking dopamine D2 and 5-HT2A receptors.[3]
Loxapine was approved for medical use in the United States in 1975.[1] In the United States 60 pills of 50 mg costs about 38 USD as of 2021.[4]
Medical uses[edit | edit source]
The US Food and Drug Administration (FDA) has approved loxapine inhalation powder for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults.[5]
A brief review of loxapine found no conclusive evidence that it was particularly effective in patients with paranoid schizophrenia.[6] A subsequent systematic review considered that the limited evidence did not indicate a clear difference in its effects from other antipsychotics.[7]
Dosage[edit | edit source]
Loxapine can be taken by mouth as a capsule or a liquid oral concentrate.[8] It is also available as an intramuscular injection and as a powder for inhalation.[5][8]
It is generally started at 10 mg twice per day and is typically increased to 60 to 100 mg per day.[1]
Side effects[edit | edit source]
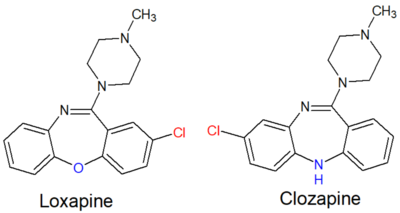
Loxapine can cause side effects that are generally similar to that of other medications in the typical antipsychotic class of medications. These include, e.g., gastrointestinal problems (like constipation and abdominal pain), cardiovascular problems (like tachycardia), moderate likelihood of drowsiness (relative to other antipsychotics),[9] and movement problems (i.e. extrapyramidal symptoms [EPS]).[10] At lower dosages its propensity for causing EPS appears to be similar to that of atypical antipsychotics.[11] Although it is structurally similar to clozapine, it does not have the same risk of agranulocytosis (which, even with clozapine, is less than 1%); however, mild and temporary fluctuations in blood leukocyte levels can occur.[12][13] Abuse of loxapine has been reported.[14]
The inhaled formulation of loxapine carries a low risk for a type of airway adverse reaction called bronchospasm that is not thought to occur when loxapine is taken by mouth.[5]
Pharmacology[edit | edit source]
| Site | LOX | AMX |
|---|---|---|
| 5-HT1A | 2,460 | ND |
| 5-HT1B | 388 | ND |
| 5-HT1D | 3,470 | ND |
| 5-HT1E | 1,400 | ND |
| 5-HT2A | 6.6 | 0.5 |
| 5-HT2C | 13 | 2 (rat) |
| 5-HT3 | 190 | ND |
| 5-HT5A | 780 | ND |
| 5-HT6 | 31 | 50 |
| 5-HT7 | 88 | 40 (rat) |
| α1A | 31 | ND |
| α1B | 53 | ND |
| α2A | 151 | ND |
| α2B | 108 | ND |
| α2C | 80 | ND |
| β1 | >10,000 | ND |
| β2 | >10,000 | ND |
| M1 | 120 | ND |
| M2 | 445 | ND |
| M3 | 211 | ND |
| M4 | 1,270 | ND |
| M5 | 166 | ND |
| D1 | 54 | ND |
| D2 | 11 | 21 |
| D3 | 19 | 21 |
| D4 | 8.4 | 21 |
| D5 | 75 | ND |
| H1 | 2.2–4.9 | 7.9–25 |
| H2 | 208 | ND |
| H3 | 55,000 | >100,000 |
| H4 | 5,050–8,710 | 6,310 |
| SERT | >10,000 | 58 |
| NET | 5,700 | 16 |
| DAT | >10,000 | 58 |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||
Mechanism of action[edit | edit source]
Loxapine is a "mid-potency" typical antipsychotic.[13] However, unlike most other typical antipsychotics, it has significant potency at the 5HT2A receptor (6.6 nM), which is similar to atypical antipsychotics like clozapine (5.35 nM). The higher likelihood of EPS with loxapine, compared to clozapine, may be due to its high potency for the D2 receptor.[13]
Several researchers have argued that loxapine may behave as an atypical antipsychotic.[17] Loxapine may be metabolized by N-demethylation to amoxapine, a tricyclic antidepressant.[18]
Pharmacokinetics[edit | edit source]
Loxapine is metabolized to amoxapine, as well as its 8-hydroxy metabolite (8-hydroxyloxapine).[2] Amoxapine is further metabolized to its 8-hydroxy metabolite (8-hydroxyamoxapine), which is also found in the blood of people taking loxapine.[19] At steady-state after taking loxapine by mouth, the relative amounts of loxapine and its metabolites in the blood is as follows: 8-hydroxyloxapine > 8-hydroxyamoxapine > loxapine.[19]
The pharmacokinetics of loxapine change depending on how it is given. Intramuscular injections of loxapine lead to higher blood levels and area under the curve of loxapine than when it is taken by mouth.[19]
Chemistry[edit | edit source]
Loxapine is a dibenzoxazepine and is structurally related to clozapine.
See also[edit | edit source]
References[edit | edit source]
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Loxapine Monograph for Professionals". Drugs.com. Archived from the original on 27 January 2021. Retrieved 24 November 2021.
- ↑ 2.0 2.1 2.2 2.3 Truven Health Analytics, Inc. DrugPoint System (Internet) [cited 2013 Sep 21]. Greenwood Village, CO: Thomsen Healthcare; 2013.
- ↑ 3.0 3.1 3.2 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 407. ISBN 978-0857114105.
- ↑ "Loxapine Prices, Coupons & Savings Tips". GoodRx. Archived from the original on 11 December 2021. Retrieved 24 November 2021.
- ↑ 5.0 5.1 5.2 "ADASUVE Package Insert" (PDF). Galen US Inc. Archived (PDF) from the original on 2018-08-15. Retrieved 2021-10-26.
- ↑ "Clozapine and loxapine for schizophrenia". Drug and Therapeutics Bulletin. 29 (11): 41–2. May 1991. PMID 1747161.
- ↑ Chakrabarti A, Bagnall A, Chue P, et al. (2007). Chakrabarti A (ed.). "Loxapine for schizophrenia". Cochrane Database of Systematic Reviews (4): CD001943. doi:10.1002/14651858.CD001943.pub2. PMC 7017975. PMID 17943763.
- ↑ 8.0 8.1 "LOXITANE Package Insert" (PDF). Watson Laboratories, Inc. Archived (PDF) from the original on 2021-04-08. Retrieved 2021-10-26.
- ↑ Taylor D, Paton C, Kapur S, Taylor D, South London and Maudsley NHS Trust. The Maudsley prescribing guidelines in psychiatry [Internet]. Chichester, West Sussex: John Wiley & Sons; 2012 [cited 2013 Sep 21]. Available from: http://site.ebrary.com/lib/uqat/Doc?id=10531429 Archived 2018-09-16 at the Wayback Machine
- ↑ Chakrabarti, Abhijit; Bagnall, Anne-Marie; Chue, Pierre; Fenton, Mark; Palanisamy, Vikram; Wong, Winson; Xia, Jun (17 October 2007). "Loxapine for schizophrenia". Cochrane Database of Systematic Reviews (4): CD001943. doi:10.1002/14651858.CD001943.pub2. PMC 7017975. PMID 17943763.
- ↑ Nordstrom K. Inhaled loxapine for rapid treatment of agitation in schizophrenia and bipolar disorder: an update. Neuropsychiatry [Internet]. 2012 Jun [cited 2013 Sep 21];2(3):253–60. Available from: Nordstrom, Kimberly (2012). "Archived copy". Neuropsychiatry. 2 (3): 253–260. doi:10.2217/npy.12.23. S2CID 39718567. Archived from the original on 2021-11-01. Retrieved 2021-10-26.
{{cite journal}}: CS1 maint: archived copy as title (link) - ↑ DePaulo, J. Raymond; Ayd, Frank J. (March 1982). "Loxapine: Fifteen years' clinical expenence". Psychosomatics. 23 (3): 261–271. doi:10.1016/S0033-3182(82)73416-4. PMID 7041162.
- ↑ 13.0 13.1 13.2 Singh, AN; Barlas, C; Singh, S; Franks, P; Mishra, RK (January 1996). "A neurochemical basis for the antipsychotic activity of loxapine: interactions with dopamine D1, D2, D4 and serotonin 5-HT2 receptor subtypes". Journal of Psychiatry & Neuroscience. 21 (1): 29–35. PMC 1188731. PMID 8580115.
- ↑ Sperry L, Hudson B, Chan CH (March 1984). "Loxapine abuse". The New England Journal of Medicine. 310 (9): 598. doi:10.1056/NEJM198403013100920. PMID 6694719.
- ↑ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 9 May 2021. Retrieved 14 August 2017.
- ↑ Appl H, Holzammer T, Dove S, Haen E, Strasser A, Seifert R (2012). "Interactions of recombinant human histamine H1R, H2R, H3R, and H4R receptors with 34 antidepressants and antipsychotics". Naunyn Schmiedebergs Arch. Pharmacol. 385 (2): 145–70. doi:10.1007/s00210-011-0704-0. PMID 22033803. S2CID 14274150.
- ↑ Glazer WM (1999). "Does loxapine have "atypical" properties? Clinical evidence". The Journal of Clinical Psychiatry. 60 (Suppl 10): 42–6. PMID 10340686.
- ↑ Cheung SW, Tang SW, Remington G (March 1991). "Simultaneous quantitation of loxapine, amoxapine and their 7- and 8-hydroxy metabolites in plasma by high-performance liquid chromatography". Journal of Chromatography. 564 (1): 213–21. doi:10.1016/0378-4347(91)80083-O. PMID 1860915.
- ↑ 19.0 19.1 19.2 Simpson, George M.; Cooper, Thomas B.; Lee, J. Hillary; Young, Michael A. (1978). "Clinical and plasma level characteristics of intramuscular and oral loxapine". Psychopharmacology. 56 (2): 225–232. doi:10.1007/BF00431855. PMID 417377. S2CID 21795809.
External links[edit | edit source]
| Identifiers: |
|
|---|
- Product monograph Archived 2021-11-01 at the Wayback Machine from Medscape (free registration required).
Categories: [Chloroarenes] [Dibenzoxazepines] [Piperazines] [Typical antipsychotics] [RTT]
↧ Download as ZWI file | Last modified: 03/05/2023 22:04:35 | 19 views
☰ Source: https://mdwiki.org/wiki/Loxapine | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF