Lithium Triflate
 From Handwiki
From Handwiki 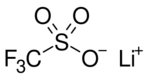
| |
| Names | |
|---|---|
| Preferred IUPAC name
Lithium trifluoromethanesulfonate | |
| Other names
LiOTf
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
CF3LiO3S |
| Molar mass | 156.00 g·mol−1 |
| Appearance | White solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
- SizeSet
Lithium triflate (lithium triflouromethanesulfonate or LiOTf) is a salt with the chemical formula LiCF3SO3. It is composed of the lithium cation (Li+) and triflate anion (CF3SO3−; TfO−). It is very hygroscopic. The salt is used in lithium-ion battery production.[1]
 Lithium triflate is hygroscopic and absorbs water from air humidity
Lithium triflate is hygroscopic and absorbs water from air humidity
References
- ↑ Lithium trifluoromethanesulfonate, Sigma Aldrich
 |
↧ Download as ZWI file | Last modified: 05/07/2025 21:16:17 | 10 views
☰ Source: https://handwiki.org/wiki/Chemistry:Lithium_triflate | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF