Indigo
 From Britannica 11th Edition (1911)
From Britannica 11th Edition (1911) Indigo (earlier indico, from Lat. indicum, the Indian substance or dye; the Sans. name was niti, from nila, dark blue, and this through Arab. al-nil, annil, gives “aniline”) one of the most important and valuable of all dyestuffs. Until comparatively recently it was obtained exclusively from the aqueous extract of certain plants, principally of the genus Indigofera which belongs to the natural order Leguminosae. Small quantities are also obtained from Lonchocarpus eyanescens (west coast of Africa), Polygonum tintorium (China) and the woad plant Isatis tinctoria. The latter is of historical interest, since up to the middle of the 17th century it was the only blue dyestuff used by dyers in England and on the adjoining continent; at the present time woad is still cultivated in Europe, but serves merely as a ferment in the setting of the fermentation indigo vat or so-called “woad vat” used in wool dyeing.
The bulk of the natural indigo which is brought into the market comes from India, while smaller quantities are imported from Java, Guatemala and other places. The plant from which indigo is made in Bengal is the Indigofera sumatrana, which is reared from seed sown about the end of April or the beginning of March. By the middle of June the plant has attained a height of from 3 to 5 ft., and it is at this period that the first manufacturing begins, a second crop being obtained in August. The indigo is contained in the leaf of the plant in the form of a colourless glucoside, known as indican, C14H17O6N·3H2O. This substance is soluble in water and by the joint action of an enzyme, contained in the leaf, and atmospheric oxygen it yields indigotine, the colouring matter of indigo. It is on these facts that the manufacturing of indigo from the plant is based.
The plant is cut early in the morning and transported to the factory in bullock carts. Here it is steeped in water in steeping vats having a capacity of about 1000 cub. ft. for periods varying, according to circumstances, from nine to fourteen hours, when the liquid—the colour of which varies from a bright orange to an olive green—is run into the beating vats which lie at a lower level. The beating, the object of which is to bring the liquor as freely as possible into contact with the air, was formerly done by striking the surface with bamboo sticks, but is now effected either by means of a paddle wheel or by forcing a current of air from a steam blower or a compressor through the liquid. When the beating is finished, the precipitated indigo is allowed to settle, the supernatant liquid being drawn off and run to waste. The indigo mud thus obtained, which is known as mal, is strained, boiled for a short period for the purpose of sterilizing, formed into bars, cut into blocks of about 3 in. cube and dried.1 The actual amount of colouring matter yielded by the leaf is but small, averaging, according to Ch. Rawson, 0.5%, but the yield from the whole plant is considerably less, since the stalks and twigs contain practically no colour.
Since the introduction on a large scale of synthetic indigo efforts have been made in India and in Java to place the cultivation of the plant and the manufacture of the natural product on a more scientific basis. But although many important improvements have been achieved from the agricultural as well as from the manufacturing point of view, resulting no doubt in the retention of a portion of the industry, the synthetic product has gained the upper hand and is likely to retain it.
Natural indigoes vary considerably in composition, containing in some qualities as much as 90% and in others as little as 20% of colouring matter. The blue colouring matter which indigo contains is known as indigotine, but there are usually also present in small quantities other colouring matters such as indigo red or indirubrine, a yellow colour known as kaempferol, indigo green and indigo brown, as well as indigo gluten and more or less mineral matter.
The bulk of the indigo which now comes into the European market is prepared synthetically from coal tar. The following figures indicate the values of the imports into England of natural and synthetic indigo, and are taken from the official Board of Trade returns:—
| Natural Indigo. | Synthetic Indigo. | |
| 1899 | £986,090 | .. |
| 1900 | 542,089 | .. |
| 1901 | 788,820 | .. |
| 1902 | 498,043 | £143,613 |
| 1903 | 262,775 | 110,970 |
| 1904 | 316,070 | 83,397 |
| 1905 | 116,902 | 121,269 |
| 1906 | 111,455 | 147,325 |
| 1907 | 151,297 | 158,481 |
| 1908 | 136,882 | 134,052 |
During the period 1899-1908, the average price of indigo had declined from a fraction under 3s. to about 2s. 2½d. per ℔. At first sight it might appear that the use of indigo in England was rapidly declining, but this does not necessarily follow when it is borne in mind that London was formerly the distributing centre of natural indigo for the continent and America.
Chemistry.—Our knowledge of the chemistry of indigo is largely derived from the classical researches of A. von Baeyer and his collaborators. In 1841 Erdmann and Laurent observed that on oxidation indigo yielded isatin; and in 1848 Fritzsche obtained aniline by distilling the dyestuff with potash. In 1870 A. v. Baeyer and Knop succeeded in preparing indigotine by heating isatin with phosphorus trichloride, acetyl chloride and phosphorus. In the same year, C. Engler and A. Emmerling obtained small quantities of the dyestuff by heating nitroacetophenone with soda-lime and zinc dust, while in 1875 M. v. Nencki prepared it by the oxidation of indol by ozone. Indol had been previously obtained from albuminoids by means of the pancreas ferment. It was not, however, until 1880 that v. Baeyer, who had been at work on the subject since 1865, was able to obtain indigotine from more or less easily accessible coal tar derivatives of known constitution. The most important of these synthetic processes due to the researches of v. Baeyer was the production of the dyestuff from ortho-nitrophenylpropiolic acid (see Propiolic Acid), which yields indigotine on being treated with caustic soda and a reducing agent such as grape sugar or xanthate of soda. Although used in small quantities in calico printing, it never attained any commercial importance as a means of producing indigo, the cost of production being far too high.
Many synthetic processes of preparing indigotine have since been
devised, but the one which stands out pre-eminently from a technical
point of view and the one which ultimately led to the commercial
success of the synthetic product is that of Heumann who showed in
1890 that indigotine can be prepared by melting phenylglycocoll
(phenylglycine), C6H5·NH·CH2·COOH, with caustic alkalis. The
yield was at first very unsatisfactory. It was subsequently found,
however, that by starting with phenylglycocoll-ortho-carboxylic
acid, the yield was sufficiently good to render the process a practical
success. The starting-point for the manufacture of synthetic
indigo is naphthalene, C10H8, which is oxidized, by heating with
concentrated sulphuric acid in the presence of a little mercury, to
phthalic anhydride, C6H4(CO)2O, which is then converted into
ortho-aminobenzoic acid, C6H4(NH2)(CO2H), by treatment with an
alkaline hypochlorite. This acid is then condensed with monochloracetic
acid to form phenylglycocoll-ortho-carboxylic acid,
C6H4(NH·CH2·CO2H)(CO2H), which on being melted with caustic
alkali yields indoxylic acid,
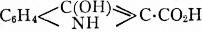 , and this readily
loses carbon dioxide and passes over into indoxyl,
, and this readily
loses carbon dioxide and passes over into indoxyl,
 .
By alkaline oxidation indoxyl is converted into indigotine.
.
By alkaline oxidation indoxyl is converted into indigotine.
The patent literature of processes for bringing about the conversion of the phenylglycine or its carboxylic acid into indoxylic acid, indoxyl and indigotine is enormous; a circumstance due to the fact that the efficiency of this operation controls the price of the synthetic dyestuff. Caustic soda has been practically given up, being replaced partly or wholly by caustic potash; in addition, alkaline earths, sodamide, nitrides, alkali carbides, &c., have been used. In 1906, Meister, Lucius and Brüning patented the addition of lead and sodium to a mixture of caustic potash and soda; the Basler Chemische Fabrik use a mixture of caustic potash and soda at 210°-260°; Léon Lilienfeld added slaked lime or magnesia to the fused alkali, with a subsequent heating in a current of ammonia at 150°-300°, and in 1908 patented a process wherein the melt is heated under greatly reduced pressure; this gave a yield of 80-90%.
Synthetic indigo comes into the market chiefly in the form of a 20% paste but is also sold in the solid state in the form of a powder.
Indigotine, C16H10N2O2, is a derivative of indol and its constitution is

It can be prepared in an almost pure state by extracting good qualities of Bengal or Java indigo or synthetic indigo with boiling nitrobenzene, from which it crystallizes on cooling in dark blue crystals having a metallic sheen. When heated in an open vessel it readily volatilizes, yielding a violet vapour which condenses on cooling in the form of crystals. Indigotine is also soluble in boiling aniline oil, quinoline, glacial acetic acid and chloroform, but is insoluble in water, dilute acids and alkalis and ordinary solvents like alcohol, ether, &c. By nitric acid and many other oxidizing agents it is readily converted into isatin, C8H5NO2. Heated with concentrated sulphuric acid it yields a disulphonic acid, C16H8N2O2(SO3H)2, the sodium salt of which finds application as an acid colour in wool dyeing under the name of Indigo carmine.2 By the action of reducing agents, indigotine is converted into indigo white, C16H12N2O2, which is readily soluble in alkalis or milk of lime with a yellow colour. On exposing the alkaline solution to the air the indigo white is rapidly oxidized back to indigotine, and on these two reactions the application of indigo in dyeing and printing is based. (See Dyeing and Textile Printing.)
Various halogen (chlorine and bromine) substitutive derivatives of
indigotine have been introduced which, while not differing essentially
from ordinary indigo in their properties, produce for the most part
redder shades in dyeing. They are claimed to be faster and brighter
colours. It has been shown by Friedländer (Ber., 1909, 42, p. 765)
that the reddish violet colouring matter obtained from the colour-yielding
glands of the mollusc Murex brandaris, by means of which
the famous Tyrian purple of the ancients was dyed, is a dibromindigo,
C16H8Br2N2O2. A new departure in the synthetic dyestuffs belonging
to the indigo group was inaugurated by the discovery in 1906
by P. Friedländer of thioindigo red, a derivative of thionaphthen,
which is formed from phenylthioglycol-ortho-carboxylic acid,
 .
This substance, on boiling with alkali and
then with dilute acid yields thioindoxyl,
.
This substance, on boiling with alkali and
then with dilute acid yields thioindoxyl,
 , which
is converted by alkaline oxidation into thioindigotin, having the
constitution
, which
is converted by alkaline oxidation into thioindigotin, having the
constitution
 . The new dyestuff is
therefore analogous to indigotine, from which it differs by having
the imino groups replaced by sulphur atoms. Thioindigo red can
be readily crystallized from boiling benzene, and forms reddish
brown crystals possessing a metallic reflex. Thioindigo scarlet,
. The new dyestuff is
therefore analogous to indigotine, from which it differs by having
the imino groups replaced by sulphur atoms. Thioindigo red can
be readily crystallized from boiling benzene, and forms reddish
brown crystals possessing a metallic reflex. Thioindigo scarlet,
 , is also obtained synthetically.
Both products come into the market in the form of pastes and are
used in dyeing like indigo (see Dyeing).
, is also obtained synthetically.
Both products come into the market in the form of pastes and are
used in dyeing like indigo (see Dyeing).
1 For a full account of the manufacture of indigo in northern Behar see Ch. Rawson, Journ. Soc. Dyers and Colourists (July 1899).
2 Although bright shades of blue are produced with this derivative, they are not fast.
↧ Download as ZWI file | Last modified: 11/17/2022 15:24:30 | 33 views
☰ Source: https://oldpedia.org/article/britannica11/Indigo | License: Public domain in the USA. Project Gutenberg License
 ZWI signed:
ZWI signed: KSF
KSF