Heat
 From Britannica 11th Edition (1911)
From Britannica 11th Edition (1911) Heat (O. E. haétu, which like “hot,” Old Eng. hát, is from the Teutonic type haita, hit, to be hot; cf. Ger. hitze, heiss; Dutch, hitte, heet, &c.), a general term applied to that branch of physical science which deals with the effects produced by heat on material bodies, with the laws of transference of heat, and with the transformations of heat into other kinds of energy. The object of the present article is to give a brief sketch of the historical development of the science of heat, and to indicate the relation of the different branches of the subject, which are discussed in greater detail with reference to the latest progress in separate articles.
1. Meanings of the Term Heat.—The term heat is employed in ordinary language in a number of different senses. This makes it a convenient term to employ for the general title of the science, but the different meanings must be carefully distinguished in scientific reasoning. For the present purpose, omitting metaphorical significations, we may distinguish four principal uses of the term: (a) Sensation of heat; (b) Temperature, or degree of hotness; (c) Quantity of thermal energy; (d) Radiant heat, or energy of radiation.
(a) From the sense of heat, aided in the case of very hot bodies by the sense of sight, we obtain our first rough notions of heat as a physical entity, which alters the state of a body and its condition in respect of warmth, and is capable of passing from one body to another. By touching a body we can tell whether it is warmer or colder than the hand, and, by touching two similar bodies in succession, we can form a rough estimate, by the acuteness of the sensation experienced, of their difference in hotness or coldness over a limited range. If a hot iron is placed on a cold iron plate, we may observe that the plate is heated and the iron cooled until both attain appreciably the same degree of warmth; and we infer from similar cases that something which we call “heat” tends to pass from hot to cold bodies, and to attain finally a state of equable diffusion when all the bodies concerned are equally warm or cold. Ideas such as these derived entirely from the sense of heat, are, so to speak, embedded in the language of every nation from the earliest times.
(b) From the sense of heat, again, we naturally derive the idea of a continuous scale or order, expressed by such terms as summer heat, blood heat, fever heat, red heat, white heat, in which all bodies may be placed with regard to their degrees of hotness, and we speak of the temperature of a body as denoting its place in the scale, in contradistinction to the quantity of heat it may contain.
(c) The quantity of heat contained in a body obviously depends on the size of the body considered. Thus a large kettleful of boiling water will evidently contain more heat than a teacupful, though both may be at the same temperature. The temperature does not depend on the size of the body, but on the degree of concentration of the heat in it, i.e. on the quantity of heat per unit mass, other things being equal. We may regard it as axiomatic that a given body (say a pound of water) in a given state (say boiling under a given pressure) must always contain the same quantity of heat, and conversely that, if it contains a given quantity of heat, and if it is under conditions in other respects, it must be at a definite temperature, which will always be the same for the same given conditions.
(d) It is a matter of common observation that rays of the sun or of a fire falling on a body warm it, and it was in the first instance natural to suppose that heat itself somehow travelled across the intervening space from the sun or fire to the body warmed, in much the same way as heat may be carried by a current of hot air or water. But we now know that energy of radiation is not the same thing as heat, though it is converted into heat when the rays strike an absorbing substance. The term “radiant heat,” however, is generally retained, because radiation is commonly measured in terms of the heat it produces, and because the transference of energy by radiation and absorption is the most important agency in the diffusion of heat.
 |
| Fig. 1. Fig. 2. |
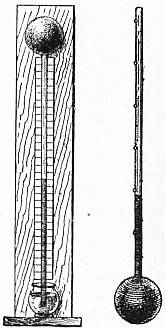
2. Evolution of the Thermometer.—The first step in the development of the science of heat was necessarily the invention of a thermometer, an instrument for indicating temperature and measuring its changes. The first requisite in the case of such an instrument is that it should always give, at least approximately the same indication at the same temperature. The air-thermoscope of Galileo, illustrated in fig. 1, which consisted of a glass bulb containing air, connected to a glass tube of small bore dipping into a coloured liquid, though very sensitive to variations of temperature, was not satisfactory as a measuring instrument, because it was also affected by variations of atmospheric pressure. The invention of the type of thermometer familiar at the present day, containing a liquid hermetically sealed in a glass bulb with a fine tube attached, is also generally attributed to Galileo at a slightly later date, about 1612. Alcohol was the liquid first employed, and the degrees, intended to represent thousandths of the volume of the bulb, were marked with small beads of enamel fused on the stem, as shown in fig. 2. In order to render the readings of such instruments comparable with each other, it was necessary to select a fixed point or standard temperature as the zero or starting-point of the graduations. Instead of making each degree a given fraction of the volume of the bulb, which would be difficult in practice, and would give different values for the degree with different liquids, it was soon found to be preferable to take two fixed points, and to divide the interval between them into the same number of degrees. It was natural in the first instance to take the temperature of the human body as one of the fixed points. In 1701 Sir Isaac Newton proposed a scale in which the freezing-point of water was taken as zero, and the temperature of the human body as 12°. About the same date (1714) Gabriel Daniel Fahrenheit proposed to take as zero the lowest temperature obtainable with a freezing mixture of ice and salt, and to divide the interval between this temperature and that of the human body into 12°. To obtain finer graduations the number was subsequently increased to 96°. The freezing-point of water was at that time supposed to be somewhat variable, because as a matter of fact it is possible to cool water several degrees below its freezing-point in the absence of ice. Fahrenheit showed, however, that as soon as ice began to form the temperature always rose to the same point, and that a mixture of ice or snow with pure water always gave the same temperature. At a later period he also showed that the temperature of boiling water varied with the barometric pressure, but that it was always the same at the same pressure, and might therefore be used as the second fixed point (as Edmund Halley and others had suggested) provided that a definite pressure, such as the average atmospheric pressure, were specified. The freezing and boiling-points on one of his thermometers, graduated as already explained, with the temperature of the body as 96°, came out in the neighbourhood of 32° and 212° respectively, giving an interval of 180° between these points. Shortly after Fahrenheit’s death (1736) the freezing and boiling-points of water were generally recognized as the most convenient fixed points to adopt, but different systems of subdivision were employed. Fahrenheit’s scale, with its small degrees and its zero below the freezing-point, possesses undoubted advantages for meteorological work, and is still retained in most English-speaking countries. But for general scientific purposes, the centigrade system, in which the freezing-point is marked 0° and the boiling-point 100°, is now almost universally employed, on account of its greater simplicity from an arithmetical point of view. For work of precision the fixed points have been more exactly defined (see Thermometry), but no change has been made in the fundamental principle of graduation.
3. Comparison of Scales based on Expansion.—Thermometers constructed in the manner already described will give strictly comparable readings, provided that the tubes be of uniform bore, and that the same liquid and glass be employed in their construction. But they possess one obvious defect from a theoretical point of view, namely, that the subdivision of the temperature scale depends on the expansion of the particular liquid selected as the standard. A liquid such as water, which, when continuously heated at a uniform rate from its freezing-point, first contracts and then expands, at a rapidly increasing rate, would obviously be unsuitable. But there is no a priori reason why other liquids should not behave to some extent in a similar way. As a matter of fact, it was soon observed that thermometers carefully constructed with different liquids, such as alcohol, oil and mercury, did not agree precisely in their indications at points of the scale intermediate between the fixed points, and diverged even more widely outside these limits. Another possible method, proposed in 1694 by Carlo Renaldeni (1615-1698), professor of mathematics and philosophy at Pisa, would be to determine the intermediate points of the scale by observing the temperatures of mixtures of ice-cold and boiling water in varying proportions. On this method, the temperature of 50° C. would be defined as that obtained by mixing equal weights of water at 0° C. and 100° C.; 20° C., that obtained by mixing 80 parts of water at 0° C. with 20 parts of water at 100° C. and so on. Each degree rise of temperature in a mass of water would then represent the addition of the same quantity of heat. The scale thus obtained would, as a matter of fact, agree very closely with that of a mercury thermometer, but the method would be very difficult to put in practice, and would still have the disadvantage of depending on the properties of a particular liquid, namely, water, which is known to behave in an anomalous manner in other respects. At a later date, the researches of Gay-Lussac (1802) and Regnault (1847) showed that the laws of the expansion of gases are much simpler than those of liquids. Whereas the expansion of alcohol between 0° C. and 100° C. is nearly seven times as great as that of mercury, all gases (excluding easily condensible vapours) expand equally, or so nearly equally that the differences between them cannot be detected without the most refined observations. This equality of expansion affords a strong a priori argument for selecting the scale given by the expansion of a gas as the standard scale of temperature, but there are still stronger theoretical grounds for this choice, which will be indicated in discussing the absolute scale (§ 21). Among liquids mercury is found to agree most nearly with the gas scale, and is generally employed in thermometers for scientific purposes on account of its high boiling-point and for other reasons. The differences of the mercurial scale from the gas scale having been carefully determined, the mercury thermometer can be used as a secondary standard to replace the gas thermometer within certain limits, as the gas thermometer would be very troublesome to employ directly in ordinary investigations. For certain purposes, and especially at temperatures beyond the range of mercury thermometers, electrical thermometers, also standardized by reference to the gas thermometer, have been very generally employed in recent years, while for still higher temperatures beyond the range of the gas thermometer, thermometers based on the recently established laws of radiation are the only instruments available. For a further discussion of the theory and practice of the measurement of temperature, the reader is referred to the article Thermometry.
4. Change of State.—Among the most important effects of heat is that of changing the state of a substance from solid to liquid, or from liquid to vapour. With very few exceptions, all substances, whether simple or compound, are known to be capable of existing in each of the three states under suitable conditions of temperature and pressure. The transition of any substance, from the state of liquid to that of solid or vapour under the ordinary atmospheric pressure, takes place at fixed temperatures, the freezing and boiling-points, which are very sharply defined for pure crystalline substances, and serve in fact as fixed points of the thermometric scale. A change of state cannot, however, be effected in any case without the addition or subtraction of a certain definite quantity of heat. If a piece of ice below the freezing-point is gradually heated at a uniform rate, its temperature may be observed to rise regularly till the freezing-point is reached. At this point it begins to melt, and its temperature ceases to rise. The melting takes a considerable time, during the whole of which heat is being continuously supplied without producing any rise of temperature, although if the same quantity of heat were supplied to an equal mass of water, the temperature of the water would be raised nearly 80° C. Heat thus absorbed in producing a change of state without rise of temperature is called “Latent Heat,” a term introduced by Joseph Black, who was one of the first to study the subject of change of state from the point of view of heat absorbed, and who in many cases actually adopted the comparatively rough method described above of estimating quantities of heat by observing the time required to produce a given change when the substance was receiving heat at a steady rate from its surroundings. For every change of state a definite quantity of heat is required, without which the change cannot take place. Heat must be added to melt a solid, or to vaporize a solid or a liquid, and conversely, heat must be subtracted to reverse the change, i.e. to condense a vapour or freeze a liquid. The quantity required for any given change depends on the nature of the substance and the change considered, and varies to some extent with the conditions (as to pressure, &c.) under which the change is made, but is always the same for the same change under the same conditions. A rough measurement of the latent heat of steam was made as early as 1764 by James Watt, who found that steam at 212° F., when passed from a kettle into a jar of cold water, was capable of raising nearly six times its weight of water to the boiling point. He gives the volume of the steam as about 1800 times that of an equal weight of water.
The phenomena which accompany change of state, and the physical laws by which such changes are governed, are discussed in a series of special articles dealing with particular cases. The articles on Fusion and Alloys deal with the change from the solid to the liquid state, and the analogous case of solution is discussed in the article on Solution. The articles on Condensation of Gases, Liquid Gases and Vaporization deal with the theory of the change of state from liquid to vapour, and with the important applications of liquid gases to other researches. The methods of measuring the latent heat of fusion or vaporization are described in the article Calorimetry, and need not be further discussed here except as an introduction to the history of the evolution of knowledge with regard to the nature of heat.
5. Calorimetry by Latent Heat.—In principle, the simplest and most direct method of measuring quantities of heat consists in observing the effects produced in melting a solid or vaporizing a liquid. It was, in fact, by the fusion of ice that quantities of heat were first measured. If a hot body is placed in a cavity in a block of ice at 0° C., and is covered by a closely fitting slab of ice, the quantity of ice melted will be directly proportional to the quantity of heat lost by the body in cooling to 0° C. None of the heat can possibly escape through the ice, and conversely no heat can possibly get in from outside. The body must cool exactly to 0° C., and every fraction of the heat it loses must melt an equivalent quantity of ice. Apart from heat lost in transferring the heated body to the ice block, the method is theoretically perfect. The only difficulty consists in the practical measurement of the quantity of ice melted. Black estimated this quantity by mopping out the cavity with a sponge before and after the operation. But there is a variable film of water adhering to the walls of the cavity, which gives trouble in accurate work. In 1780 Laplace and Lavoisier used a double-walled metallic vessel containing broken ice, which was in many respects more convenient than the block, but aggravated the difficulty of the film of water adhering to the ice. In spite of this practical difficulty, the quantity of heat required to melt unit weight of ice was for a long time taken as the unit of heat. This unit possesses the great advantage that it is independent of the scale of temperature adopted. At a much later date R. Bunsen (Phil. Mag., 1871), adopting a suggestion of Sir John Herschel’s, devised an ice-calorimeter suitable for measuring small quantities of heat, in which the difficulty of the water film was overcome by measuring the change in volume due to the melting of the ice. The volume of unit mass of ice is approximately 1.0920 times that of unit mass of water, so that the diminution of volume is 0.092 a cubic centimetre for each gramme of ice melted. The method requires careful attention to details of manipulation, which are more fully discussed in the article on Calorimetry.
For measuring large quantities of heat, such as those produced by the combustion of fuel in a boiler, the most convenient method is the evaporation of water, which is commonly employed by engineers for the purpose. The natural unit in this case is the quantity of heat required to evaporate unit mass of water at the boiling point under atmospheric pressure. In boilers working at a higher pressure, or supplied with water at a lower temperature, appropriate corrections are applied to deduce the quantity evaporated in terms of this unit.
For laboratory work on a small scale the converse method of condensation has been successfully applied by John Joly, in whose steam-calorimeter the quantity of heat required to raise the temperature of a body from the atmospheric temperature to that of steam condensing at atmospheric pressure is observed by weighing the mass of steam condensed on it. (See Calorimetry.)
6. Thermometric Calorimetry.—For the majority of purposes the most convenient and the most readily applicable method of measuring quantities of heat, is to observe the rise of temperature produced in a known mass of water contained in a suitable vessel or calorimeter. This method was employed from a very early date by Count Rumford and other investigators, and was brought to a high pitch of perfection by Regnault in his extensive calorimetric researches (Mémoires de l’Institut de Paris, 1847); but it is only within comparatively recent years that it has really been placed on a satisfactory basis by the accurate definition of the units involved. The theoretical objections to the method, as compared with latent heat calorimetry, are that some heat is necessarily lost by the calorimeter when its temperature is raised above that of the surroundings, and that some heat is used in heating the vessel containing the water. These are small corrections, which can be estimated with considerable accuracy in practice. A more serious difficulty, which has impaired the value of much careful work by this method, is that the quantity of heat required to raise the temperature of a given mass of water 1° C. depends on the temperature at which the water is taken, and also on the scale of the thermometer employed. It is for this reason, in many cases, impossible to say, at the present time, what was the precise value, within ½ or even 1% of the heat unit, in terms of which many of the older results, such as those of Regnault, were expressed. For many purposes this would not be a serious matter, but for work of scientific precision such a limitation of accuracy would constitute a very serious bar to progress. The unit generally adopted for scientific purposes is the quantity of heat required to raise 1 gram (or kilogram) of water 1° C., and is called the calorie (or kilo-calorie). English engineers usually state results in terms of the British Thermal Unit (B.Th.U.), which is the quantity of heat required to raise 1 ℔ of water 1° F.
7. Watt’s Indicator Diagram; Work of Expansion.—The rapid development of the steam-engine (q.v.) in England during the latter part of the 18th century had a marked effect on the progress of the science of heat. In the first steam-engines the working cylinder served both as boiler and condenser, a very wasteful method, as most of the heat was transferred directly from the fire to the condensing water without useful effect. The first improvement (about 1700) was to use a separate boiler, but the greater part of the steam supplied was still wasted in reheating the cylinder, which had been cooled by the injection of cold water to condense the steam after the previous stroke. In 1769 James Watt showed how to avoid this waste by using a separate condenser and keeping the cylinder as hot as possible. In his earlier engines the steam at full boiler pressure was allowed to raise the piston through nearly the whole of its stroke. Connexion with the boiler was then cut off, and the steam at full pressure was discharged into the condenser. Here again there was unnecessary waste, as the steam was still capable of doing useful work. He subsequently introduced “expansive working,” which effected still further economy. The connexion with the boiler was cut off when a fraction only, say ¼, of the stroke had been completed, the remainder of the stroke being effected by the expansion of the steam already in the cylinder with continually diminishing pressure. By the end of the stroke, when connexion was made to the condenser, the pressure was so reduced that there was comparatively little waste from this cause. Watt also devised an instrument called an indicator (see Steam Engine), in which a pencil, moved up and down vertically by the steam pressure, recorded the pressure in the cylinder at every point of the stroke on a sheet of paper moving horizontally in time with the stroke of the piston. The diagram thus obtained made it possible to study what was happening inside the cylinder, and to deduce the work done by the steam in each stroke. The method of the indicator diagram has since proved of great utility in physics in studying the properties of gases and vapours. The work done, or the useful effect obtained from an engine or any kind of machine, is measured by the product of the resistance overcome and the distance through which it is overcome. The result is generally expressed in terms of the equivalent weight raised through a certain height against the force of gravity.1 If, for instance, the pressure on a piston is 50 ℔ per sq. in., and the area of the piston is 100 sq. in., the force on the piston is 5000 ℔ weight. If the stroke of the piston is 1 ft., the work done per stroke is capable of raising a weight of 5000 ℔ through a height of 1 ft., or 50 ℔ through a height of 100 ft. and so on.
 |
| Fig. 3.—Watt’s Indicator Diagram. Patent of 1782. |
Fig. 3 represents an imaginary indicator diagram for a steam-engine, taken from one of Watt’s patents. Steam is admitted to the cylinder when the piston is at the beginning of its stroke, at S. ST represents the length of the stroke or the limit of horizontal movement of the paper on which the diagram is drawn. The indicating pencil rises to the point A, representing the absolute pressure of 60 ℔ per sq. in. As the piston moves outwards the pencil traces the horizontal line AB, the pressure remaining constant till the point B is reached, at which connexion to the boiler is cut off. The work done so far is represented by the area of the rectangle ABSF, namely AS × SF, multiplied by the area of the piston in sq. in. The result is in foot-pounds if the fraction of the stroke SF is taken in feet. After cut-off at B the steam expands under diminishing pressure, and the pencil falls gradually from B to C, following the steam pressure until the exhaust valve opens at the end of the stroke. The pressure then falls rapidly to that of the condenser, which for an ideal case may be taken as zero, following Watt. The work done during expansion is found by dividing the remainder of the stroke FT into a number of equal parts (say 8, Watt takes 20) and measuring the pressure at the points 1, 2, 3, 4, &c., corresponding to the middle of each. We thus obtain a number of small rectangles, the sum of which is evidently very nearly equal to the whole area BCTF under the expansion curve, or to the remainder of the stroke FT multiplied by the average or mean value of the pressure. The whole work done in the forward stroke is represented by the area ABCTSA, or by the average value of the pressure P over the whole stroke multiplied by the stroke L. This area must be multiplied by the area of the piston A in sq. in. as before, to get the work done per stroke in foot-pounds, which is PLA. If the engine repeats this cycle N times per minute, the work done per minute is PLAN foot-pounds, which is reduced to horse-power by dividing by 33,000. If the steam is ejected by the piston at atmospheric pressure (15 ℔ per sq. in.) instead of being condensed at zero pressure, the area CDST under the atmospheric line CD, representing work done against back-pressure on the return stroke must be subtracted. If the engine repeats the same cycle or series of operations continuously, the indicator diagram will be a closed curve, and the nett work done per cycle will be represented by the included area, whatever the form of the curve.
8. Thermal Efficiency.—The thermal efficiency of an engine is the ratio of the work done by the engine to the heat supplied to it. According to Watt’s observations, confirmed later by Clément and Désormes, the total heat required to produce 1 ℔ of saturated steam at any temperature from water at 0° C. was approximately 650 times the quantity of heat required to raise 1 ℔ of water 1° C. Since 1 ℔ of steam represented on this assumption a certain quantity of heat, the efficiency could be measured naturally in foot-pounds of work obtainable per ℔ of steam, or conversely in pounds of steam consumed per horse-power-hour.
In his patent of 1782 Watt gives the following example of the improvement in thermal efficiency obtained by expansive working. Taking the diagram already given, if the quantity of steam represented by AB, or 300 cub. in. at 60 ℔ pressure, were employed without expansion, the work realized, represented by the area ABSF, would be 6000/4 = 1500 foot-pounds. With expansion to 4 times its original volume, as shown in the diagram by the whole area ABCTSA, the mean pressure (as calculated by Watt, assuming Boyle’s law) would be 0.58 of the original pressure, and the work done would be 6000 × 0.58 = 3480 foot-pounds for the same quantity of steam, or the thermal efficiency would be 2.32 times greater. The advantage actually obtained would not be so great as this, on account of losses by condensation, back-pressure, &c., which are neglected in Watt’s calculation, but the margin would still be very considerable. Three hundred cub. in. of steam at 60 ℔ pressure would represent about .0245 of 1 ℔ of steam, or 28.7 B.Th.U., so that, neglecting all losses, the possible thermal efficiency attainable with steam at this pressure and four expansions (¼ cut-off) would be 3480/28.7, or 121 foot-pounds per B.Th.U. At a later date, about 1820, it was usual to include the efficiency of the boiler with that of the engine, and to reckon the efficiency or “duty” in foot-pounds per bushel or cwt. of coal. The best Cornish pumping-engines of that date achieved about 70 million foot-pounds per cwt., or consumed about 3.2 ℔ per horse-power-hour, which is roughly equivalent to 43 foot-pounds per B.Th.U. The efficiency gradually increased as higher pressures were used, with more complete expansion, but the conditions upon which the efficiency depended were not fully worked out till a much later date. Much additional knowledge with regard to the nature of heat, and the properties of gases and vapours, was required before the problem could be attacked theoretically.
9. Of the Nature of Heat.—In the early days of the science it was natural to ascribe the manifestations of heat to the action of a subtle imponderable fluid called “caloric,” with the power of penetrating, expanding and dissolving bodies, or dissipating them in vapour. The fluid was imponderable, because the most careful experiments failed to show that heat produced any increase in weight. The opposite property of levitation was often ascribed to heat, but it was shown by more cautious investigators that the apparent loss of weight due to heating was to be attributed to evaporation or to upward air currents. The fundamental idea of an imaginary fluid to represent heat was useful as helping the mind to a conception of something remaining invariable in quantity through many transformations, but in some respects the analogy was misleading, and tended greatly to retard the progress of science. The caloric theory was very simple in its application to the majority of calorimetric experiments, and gave a fair account of the elementary phenomena of change of state, but it encountered serious difficulties in explaining the production of heat by friction, or the changes of temperature accompanying the compression or expansion of a gas. The explanation which the calorists offered of the production of heat by friction or compression was that some of the latent caloric was squeezed or ground out of the bodies concerned and became “sensible.” In the case of heat developed by friction, they supposed that the abraded portions of the material were capable of holding a smaller quantity of heat, or had less “capacity for heat,” than the original material. From a logical point of view, this was a perfectly tenable hypothesis, and one difficult to refute. It was easy to account in this way for the heat produced in boring cannon and similar operations, where the amount of abraded material was large. To refute this explanation, Rumford (Phil. Trans., 1798) made his celebrated experiments with a blunt borer, in one of which he succeeded in boiling by friction 26.5 ℔ of cold water in 2½ hours, with the production of only 4145 grains of metallic powder. He then showed by experiment that the metallic powder required the same amount of heat to raise its temperature 1°, as an equal weight of the original metal, or that its “capacity for heat” (in this sense) was unaltered by reducing it to powder; and he argued that “in any case so small a quantity of powder could not possibly account for all the heat generated, that the supply of heat appeared to be inexhaustible, and that heat could not be a material substance, but must be something of the nature of motion.” Unfortunately Rumford’s argument was not quite conclusive. The supporters of the caloric theory appear, whether consciously or unconsciously, to have used the phrase “capacity for heat” in two entirely distinct senses without any clear definition of the difference. The phrase “capacity for heat” might very naturally denote the total quantity of heat contained in a body, which we have no means of measuring, but it was generally used to signify the quantity of heat required to raise the temperature of a body one degree, which is quite a different thing, and has no necessary relation to the total heat. In proving that the powder and the solid metal required the same quantity of heat to raise the temperature of equal masses of either one degree, Rumford did not prove that they contained equal quantities of heat, which was the real point at issue in this instance. The metal tin actually changes into powder below a certain temperature, and in so doing evolves a measurable quantity of heat. A mixture of the gases oxygen and hydrogen, in the proportions in which they combine to form water, evolves when burnt sufficient heat to raise more than thirty times its weight of water from the freezing to the boiling point; and the mixture of gases may, in this sense, be said to contain so much more heat than the water, although its capacity for heat in the ordinary sense is only about half that of the water produced. To complete the refutation of the calorists’ explanation of the heat produced by friction, it would have been necessary for Rumford to show that the powder when reconverted into the same state as the solid metal did not absorb a quantity of heat equivalent to that evolved in the grinding; in other words that the heat produced by friction was not simply that due to the change of state of the metal from solid to powder.
Shortly afterwards, in 1799, Davy2 described an experiment in which he melted ice by rubbing two blocks together. This experiment afforded a very direct refutation of the calorists’ view, because it was a well-known fact that ice required to have a quantity of heat added to it to convert it into water, so that the water produced by the friction contained more heat than the ice. In stating as the conclusion to be drawn from this experiment that “friction consequently does not diminish the capacity of bodies for heat,” Davy apparently uses the phrase capacity for heat in the sense of total heat contained in a body, because in a later section of the same essay he definitely gives the phrase this meaning, and uses the term “capability of temperature” to denote what we now term capacity for heat.
The delay in the overthrow of the caloric theory, and in the acceptance of the view that heat is a mode of motion, was no doubt partly due to some fundamental confusion of ideas in the use of the term “capacity for heat” and similar phrases. A still greater obstacle lay in the comparative vagueness of the motion or vibration theory. Davy speaks of heat as being “repulsive motion,” and distinguishes it from light, which is “projective motion”; though heat is certainly not a substance—according to Davy in the essay under discussion—and may not even be treated as an imponderable fluid, light as certainly is a material substance, and is capable of forming chemical compounds with ordinary matter, such as oxygen gas, which is not a simple substance, but a compound, termed phosoxygen, of light and oxygen. Accepting the conclusions of Davy and Rumford that heat is not a material substance but a mode of motion, there still remains the question, what definite conception is to be attached to a quantity of heat? What do we mean by a quantity of vibratory motion, how is the quantity of motion to be estimated, and why should it remain invariable in many transformations? The idea that heat was a “mode of motion” was applicable as a qualitative explanation of many of the effects of heat, but it lacked the quantitative precision of a scientific statement, and could not be applied to the calculation and prediction of definite results. The state of science at the time of Rumford’s and Davy’s experiments did not admit of a more exact generalization. The way was paved in the first instance by a more complete study of the laws of gases, to which Laplace, Dalton, Gay-Lussac, Dulong and many others contributed both on the experimental and theoretical side. Although the development proceeded simultaneously along many parallel lines, it is interesting and instructive to take the investigation of the properties of gases, and to endeavour to trace the steps by which the true theory was finally attained.
10. Thermal Properties of Gases.—The most characteristic property of a gaseous or elastic fluid, namely, the elasticity, or resistance to compression, was first investigated scientifically by Robert Boyle (1662), who showed that the pressure p of a given mass of gas varied inversely as the volume v, provided that the temperature remained constant. This is generally expressed by the formula pv = C, where C is a constant for any given temperature, and v is taken to represent the specific volume, or the volume of unit mass, of the gas at the given pressure and temperature. Boyle was well aware of the effect of heat in expanding a gas, but he was unable to investigate this properly as no thermometric scale had been defined at that date. According to Boyle’s law, when a mass of gas is compressed by a small amount at constant temperature, the percentage increase of pressure is equal to the percentage diminution of volume (if the compression is v/100, the increase of pressure is very nearly p/100). Adopting this law, Newton showed, by a most ingenious piece of reasoning (Principia, ii., sect. 8), that the velocity of sound in air should be equal to the velocity acquired by a body falling under gravity through a distance equal to half the height of the atmosphere, considered as being of uniform density equal to that at the surface of the earth. This gave the result 918 ft. per sec. (280 metres per sec.) for the velocity at the freezing point. Newton was aware that the actual velocity of sound was somewhat greater than this, but supposed that the difference might be due in some way to the size of the air particles, of which no account could be taken in the calculation. The first accurate measurement of the velocity of sound by the French Académie des Sciences in 1738 gave the value 332 metres per sec. as the velocity at 0° C. The true explanation of the discrepancy was not discovered till nearly 100 years later.
The law of expansion of gases with change of temperature was investigated by Dalton and Gay-Lussac (1802), who found that the volume of a gas under constant pressure increased by 1/267th part of its volume at 0° C. for each 1° C. rise in temperature. This value was generally assumed in all calculations for nearly 50 years. More exact researches, especially those of Regnault, at a later date, showed that the law was very nearly correct for all permanent gases, but that the value of the coefficient should be 1⁄173rd. According to this law the volume of a gas at any temperature t° C. should be proportional to 273 + t, i.e. to the temperature reckoned from a zero 273° below that of the Centigrade scale, which was called the absolute zero of the gas thermometer. If T = 273 + t, denotes the temperature measured from this zero, the law of expansion of a gas may be combined with Boyle’s law in the simple formula
pv = RT
which is generally taken as the expression of the gaseous laws. If equal volumes of different gases are taken at the same temperature and pressure, it follows that the constant R is the same for all gases. If equal masses are taken, the value of the constant R for different gases varies inversely as the molecular weight or as the density relative to hydrogen.
Dalton also investigated the laws of vapours, and of mixtures of gases and vapours. He found that condensible vapours approximately followed Boyle’s law when compressed, until the condensation pressure was reached, at which the vapour liquefied without further increase of pressure. He found that when a liquid was introduced into a closed space, and allowed to evaporate until the space was saturated with the vapour and evaporation ceased, the increase of pressure in the space was equal to the condensation pressure of the vapour, and did not depend on the volume of the space or the presence of any other gas or vapour provided that there was no solution or chemical action. He showed that the condensation or saturation-pressure of a vapour depended only on the temperature, and increased by nearly the same fraction of itself per degree rise of temperature, and that the pressures of different vapours were nearly the same at equal distances from their boiling points. The increase of pressure per degree C. at the boiling point was about 1⁄28th of 760 mm. or 27.2 mm., but increased in geometrical progression with rise of temperature. These results of Dalton’s were confirmed, and in part corrected, as regards increase of vapour-pressure, by Gay-Lussac, Dulong, Regnault and other investigators, but were found to be as close an approximation to the truth as could be obtained with such simple expressions. More accurate empirical expressions for the increase of vapour-pressure of a liquid with temperature were soon obtained by Thomas Young, J. P. L. A. Roche and others, but the explanation of the relation was not arrived at until a much later date (see Vaporization).
11. Specific Heats of Gases.—In order to estimate the quantities of heat concerned in experiments with gases, it was necessary in the first instance to measure their specific heats, which presented formidable difficulties. The earlier attempts by Lavoisier and others, employing the ordinary methods of calorimetry, gave very uncertain and discordant results, which were not regarded with any confidence even by the experimentalists themselves. Gay-Lussac (Mémoires d’Arcueil, 1807) devised an ingenious experiment, which, though misinterpreted at the time, is very interesting and instructive. With the object of comparing the specific heats of different gases, he took two equal globes A and B connected by a tube with a stop-cock. The globe B was exhausted, the other A being filled with gas. On opening the tap between the vessels, the gas flowed from A to B and the pressure was rapidly equalized. He observed that the fall of temperature in A was nearly equal to the rise of temperature in B, and that for the same initial pressure the change of temperature was very nearly the same for all the gases he tried, except hydrogen, which showed greater changes of temperature than other gases. He concluded from this experiment that equal volumes of gases had the same capacity for heat, except hydrogen, which he supposed to have a larger capacity, because it showed a greater effect. The method does not in reality afford any direct information with regard to the specific heats, and the conclusion with regard to hydrogen is evidently wrong. At a later date (Ann. de Chim., 1812, 81, p. 98) Gay-Lussac adopted A. Crawford’s method of mixture, allowing two equal streams of different gases, one heated and the other cooled about 20° C., to mix in a tube containing a thermometer. The resulting temperature was in all cases nearly the mean of the two, from which he concluded that equal volumes of all the gases tried, namely, hydrogen, carbon dioxide, air, oxygen and nitrogen, had the same thermal capacity. This was correct, except as regards carbon dioxide, but did not give any information as to the actual specific heats referred to water or any known substance. About the same time, F. Delaroche and J. E. Bérard (Ann. de chim., 1813, 85, p. 72) made direct determinations of the specific heats of air, oxygen, hydrogen, carbon monoxide, carbon dioxide, nitrous oxide and ethylene, by passing a stream of gas heated to nearly 100° C. through a spiral tube in a calorimeter containing water. Their work was a great advance on previous attempts, and gave the first trustworthy results. With the exception of hydrogen, which presents peculiar difficulties, they found that equal volumes of the permanent gases, air, oxygen and carbon monoxide, had nearly the same thermal capacity, but that the compound condensible gases, carbon dioxide, nitrous oxide and ethylene, had larger thermal capacities in the order given. They were unable to state whether the specific heats of the gases increased or diminished with temperature, but from experiments on air at pressures of 740 mm. and 1000 mm., they found the specific heats to be .269 and .245 respectively, and concluded that the specific heat diminished with increase of pressure. The difference they observed was really due to errors of experiment, but they regarded it as proving beyond doubt the truth of the calorists’ contention that the heat disengaged on the compression of a gas was due to the diminution of its thermal capacity.
Dalton and others had endeavoured to measure directly the rise of temperature produced by the compression of a gas. Dalton had observed a rise of 50° F. in a gas when suddenly compressed to half its volume, but no thermometers at that time were sufficiently sensitive to indicate more than a fraction of the change of temperature. Laplace was the first to see in this phenomenon the probable explanation of the discrepancy between Newton’s calculation of the velocity of sound and the observed value. The increase of pressure due to a sudden compression, in which no heat was allowed to escape, or as we now call it an “adiabatic” compression, would necessarily be greater than the increase of pressure in a slow isothermal compression, on account of the rise of temperature. As the rapid compressions and rarefactions occurring in the propagation of a sound wave were perfectly adiabatic, it was necessary to take account of the rise of temperature due to compression in calculating the velocity. To reconcile the observed and calculated values of the velocity, the increase of pressure in adiabatic compression must be 1.410 times greater than in isothermal compression. This is the ratio of the adiabatic elasticity of air to the isothermal elasticity. It was a long time, however, before Laplace saw his way to any direct experimental verification of the value of this ratio. At a later date (Ann. de chim., 1816, 3, p. 238) he stated that he had succeeded in proving that the ratio in question must be the same as the ratio of the specific heat of air at constant pressure to the specific heat at constant volume.
In the method of measuring the specific heat adopted by Delaroche and Bérard, the gas under experiment, while passing through a tube at practically constant pressure, contracts in cooling, as it gives up its heat to the calorimeter. Part of the heat surrendered to the calorimeter is due to the contraction of volume. If a gramme of gas at pressure p, volume v and temperature T abs. is heated 1° C. at constant pressure p, it absorbs a quantity of heat S = .238 calorie (according to Regnault) the specific heat at constant pressure. At the same time the gas expands by a fraction 1/T of v, which is the same as 1/273 of its volume at 0° C. If now the air is suddenly compressed by an amount v/T, it will be restored to its original volume, and its temperature will be raised by the liberation of a quantity of heat R′, the latent heat of expansion for an increase of volume v/T. If no heat has been allowed to escape, the air will now be in the same state as if a quantity of heat S had been communicated to it at its original volume v without expansion. The rise of temperature above the original temperature T will be S/s degrees, where s is the specific heat at constant volume, which is obviously equal to S − R′. Since p/T is the increase of pressure for 1° C. rise of temperature at constant volume, the increase of pressure for a rise of S/s degrees will be γp/T, where γ is the ratio S/s. But this is the rise of pressure produced by a sudden compression v/T, and is seen to be γ times the rise of pressure p/T produced by the same compression at constant temperature. The ratio of the adiabatic to the isothermal elasticity, required for calculating the velocity of sound, is therefore the same as the ratio of the specific heat at constant pressure to that at constant volume.
12. Experimental Verification of the Ratio of Specific Heats.—This was a most interesting and important theoretical relation to discover, but unfortunately it did not help much in the determination of the ratio required, because it was not practically possible at that time to measure the specific heat of air at constant volume in a closed vessel. Attempts had been made to do this, but they had signally failed, on account of the small heat capacity of the gas as compared with the containing vessel. Laplace endeavoured to extract some confirmation of his views from the values given by Delaroche and Bérard for the specific heat of air at 1000 and 740 mm. pressure. On the assumption that the quantities of heat contained in a given mass of air increased in direct proportion to its volume when heated at constant pressure, he deduced, by some rather obscure reasoning, that the ratio of the specific heats S and s should be about 1.5 to 1, which he regarded as a fairly satisfactory agreement with the value γ = 1.41 deduced from the velocity of sound.
The ratio of the specific heats could not be directly measured, but a few years later, Clément and Désormes (Journ. de Phys., Nov. 1819) succeeded in making a direct measurement of the ratio of the elasticities in a very simple manner. They took a large globe containing air at atmospheric pressure and temperature, and removed a small quantity of air. They then observed the defect of pressure p0 when the air had regained its original temperature. By suddenly opening the globe, and immediately closing it, the pressure was restored almost instantaneously to the atmospheric, the rise of pressure p0 corresponding to the sudden compression produced. The air, having been heated by the compression, was allowed to regain its original temperature, the tap remaining closed, and the final defect of pressure p1 was noted. The change of pressure for the same compression performed isothermally is then p0 − p1. The ratio p0/(p0 − p1) is the ratio of the adiabatic and isothermal elasticities, provided that p0 is small compared with the whole atmospheric pressure. In this way they found the ratio 1.354, which is not much smaller than the value 1.410 required to reconcile the observed and calculated values of the velocity of sound. Gay-Lussac and J. J. Welter (Ann. de chim., 1822) repeated the experiment with slight improvements, using expansion instead of compression, and found the ratio 1.375. The experiment has often been repeated since that time, and there is no doubt that the value of the ratio deduced from the velocity of sound is correct, the defect of the value obtained by direct experiment being due to the fact that the compression or expansion is not perfectly adiabatic. Gay-Lussac and Welter found the ratio practically constant for a range of pressure 144 to 1460 mm., and for a range of temperature from −20° to +40° C. The velocity of sound at Quito, at a pressure of 544 mm. was found to be the same as at Paris at 760 mm. at the same temperature. Assuming on this evidence the constancy of the ratio of the specific heats of air, Laplace (Mécanique céleste, v. 143) showed that, if the specific heat at constant pressure was independent of the temperature, the specific heat per unit volume at a pressure p must vary as p1/γ, according to the caloric theory. The specific heat per unit mass must then vary as p1/γ−1 which he found agreed precisely with the experiment of Delaroche and Bérard already cited. This was undoubtedly a strong confirmation of the caloric theory. Poisson by the same assumptions (Ann. de chim., 1823, 23, p. 337) obtained the same results, and also showed that the relation between the pressure and the volume of a gas in adiabatic compression or expansion must be of the form pvγ = constant.
P. L. Dulong (Ann. de chim., 1829, 41, p. 156), adopting a method due to E. F. F. Chladni, compared the velocities of sound in different gases by observing the pitch of the note given by the same tube when filled with the gases in question. He thus obtained the values of the ratios of the elasticities or of the specific heats for the gases employed. For oxygen, hydrogen and carbonic oxide, these ratios were the same as for air. But for carbonic acid, nitrous oxide and olefiant gas, the values were much smaller, showing that these gases experienced a smaller change of temperature in compression. On comparing his results with the values of the specific heats for the same gases found by Delaroche and Bérard, Dulong observed that the changes of temperature for the same compression were in the inverse ratio of the specific heats at constant volume, and deduced the important conclusion that “Equal volumes of all gases under the same conditions evolve on compression the same quantity of heat.” This is equivalent to the statement that the difference of the specific heats, or the latent heat of expansion R′ per 1°, is the same for all gases if equal volumes are taken. Assuming the ratio γ = 1.410, and taking Delaroche and Bérard’s value for the specific heat of air at constant pressure S = .267, we have s = S/1.41 = .189, and the difference of the specific heats per unit mass of air S − s = R′ = .078. Adopting Regnault’s value of the specific heat of air, namely, S = .238, we should have S − s = .069. This quantity represents the heat absorbed by unit mass of air in expanding at constant temperature T by a fraction 1/T of its volume v, or by 1⁄273rd of its volume 0° C.
If, instead of taking unit mass, we take a volume v0 = 22.30 litres at 0° C. and 760 mm. being the volume of the molecular weight of the gas in grammes, the quantity of heat evolved by a compression equal to v/T will be approximately 2 calories, and is the same for all gases. The work done in this compression is pv/T = R, and is also the same for all gases, namely, 8.3 joules. Dulong’s experimental result, therefore, shows that the heat evolved in the compression of a gas is proportional to the work done. This result had previously been deduced theoretically by Carnot (1824). At a later date it was assumed by Mayer, Clausius and others, on the evidence of these experiments, that the heat evolved was not merely proportional to the work done, but was equivalent to it. The further experimental evidence required to justify this assumption was first supplied by Joule.
| Latent heat of expansion R′ | = .069 calorie per gramme of air, per 1° C. |
| = 2.0 calories per gramme-molecule of any gas. | |
| Work done in expansion R | = .287 joule per gramme of air per 1° C. |
| = 8.3 joules per gramme-molecule of any gas. |
13. Carnot: On the Motive Power of Heat.—A practical and theoretical question of the greatest importance was first answered by Sadi Carnot about this time in his Reflections on the Motive Power of Heat (1824). How much motive power (defined by Carnot as weight lifted through a certain height) can be obtained from heat alone by means of an engine repeating a regular succession or “cycle” of operations continuously? Is the efficiency limited, and, if so, how is it limited? Are other agents preferable to steam for developing motive power from heat? In discussing this problem, we cannot do better than follow Carnot’s reasoning which, in its main features could hardly be improved at the present day.
Carnot points out that in order to obtain an answer to this question, it is necessary to consider the essential conditions of the process, apart from the mechanism of the engine and the working substance or agent employed. Work cannot be said to be produced from heat alone unless nothing but heat is supplied, and the working substance and all parts of the engine are at the end of the process in precisely the same state as at the beginning.3
Carnot’s Axiom.—Carnot here, and throughout his reasoning, makes a fundamental assumption, which he states as follows: “When a body has undergone any changes and after a certain number of transformations is brought back identically to its original state, considered relatively to density, temperature and mode of aggregation, it must contain the same quantity of heat as it contained originally.”4
Heat, according to Carnot, in the type of engine we are considering, can evidently be a cause of motive power only by virtue of changes of volume or form produced by alternate heating and cooling. This involves the existence of cold and hot bodies to act as boiler and condenser, or source and sink of heat, respectively. Wherever there exists a difference of temperature, it is possible to have the production of motive power from heat; and conversely, production of motive power, from heat alone, is impossible without difference of temperature. In other words the production of motive power from heat is not merely a question of the consumption of heat, but always requires transference of heat from hot to cold. What then are the conditions which enable the difference of temperature to be most advantageously employed in the production of motive power, and how much motive power can be obtained with a given difference of temperature from a given quantity of heat?
Carnot’s Rule for Maximum Effect.—In order to realize the maximum effect, it is necessary that, in the process employed, there should not be any direct interchange of heat between bodies at different temperatures. Direct transference of heat by conduction or radiation between bodies at different temperatures is equivalent to wasting a difference of temperature which might have been utilized to produce motive power. The working substance must throughout every stage of the process be in equilibrium with itself (i.e. at uniform temperature and pressure) and also with external bodies, such as the boiler and condenser, at such times as it is put in communication with them. In the actual engine there is always some interchange of heat between the steam and the cylinder, and some loss of heat to external bodies. There may also be some difference of temperature between the boiler steam and the cylinder on admission, or between the waste steam and the condenser at release. These differences represent losses of efficiency which may be reduced indefinitely, at least in imagination, by suitable means, and designers had even at that date been very successful in reducing them. All such losses are supposed to be absent in deducing the ideal limit of efficiency, beyond which it would be impossible to go.
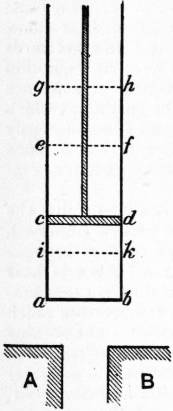
14. Carnot’s Description of his Ideal Cycle.—Carnot first gives a rough illustration of an incomplete cycle, using steam much in the same way as it is employed in an ordinary steam-engine. After expansion down to condenser pressure the steam is completely condensed to water, and is then returned as cold water to the hot boiler. He points out that the last step does not conform exactly to the condition he laid down, because although the water is restored to its initial state, there is direct passage of heat from a hot body to a cold body in the last process. He points out that this difficulty might be overcome by supposing the difference of temperature small, and by employing a series of engines, each working through a small range, to cover a finite interval of temperature. Having established the general notions of a perfect cycle, he proceeds to give a more exact illustration, employing a gas as the working substance. He takes as the basis of his demonstration the well-established experimental fact that a gas is heated by rapid compression and cooled by rapid expansion, and that if compressed or expanded slowly in contact with conducting bodies, the gas will give out heat in compression or absorb heat in expansion while its temperature remains constant. He then goes on to say:—
 |
| Fig 4. |
| Carnot’s Cylinder. |
“This preliminary notion being settled, let us imagine an elastic fluid, atmospheric air for example, enclosed in a cylinder abcd, fig. 4, fitted with a movable diaphragm or piston cd. Let there also be two bodies A, B, each maintained at a constant temperature, that of A being more elevated than that of B. Let us now suppose the following series of operations to be performed:
“1. Contact of the body A with the air contained in the space abcd, or with the bottom of the cylinder, which we will suppose to transmit heat easily. The air is now at the temperature of the body A, and cd is the actual position of the piston.
“2. The piston is gradually raised, and takes the position ef. The air remains in contact with the body A, and is thereby maintained at a constant temperature during the expansion. The body A furnishes the heat necessary to maintain the constancy of temperature.
“3. The body A is removed, and the air no longer being in contact with any body capable of giving it heat, the piston continues nevertheless to rise, and passes from the position ef to gh. The air expands without receiving heat and its temperature falls. Let us imagine that it falls until it is just equal to that of the body B. At this moment the piston is stopped and occupies the position gh.
“4. The air is placed in contact with the body B; it is compressed by the return of the piston, which is brought from the position gh to the position cd. The air remains meanwhile at a constant temperature, because of its contact with the body B to which it gives up its heat.
“5. The body B is removed, and the compression of the air is continued. The air being now isolated, rises in temperature. The compression is continued until the air has acquired the temperature of the body A. The piston passes meanwhile from the position cd to the position ik.
“6. The air is replaced in contact with the body A, and the piston returns from the position ik to the position ef, the temperature remaining invariable.
“7. The period described under (3) is repeated, then successively the periods (4), (5), (6); (3), (4), (5), (6); (3), (4), (5), (6); and so on.
“During these operations the air enclosed in the cylinder exerts an effort more or less great on the piston. The pressure of the air varies both on account of changes of volume and on account of changes of temperature; but it should be observed that for equal volumes, that is to say, for like positions of the piston, the temperature is higher during the dilatation than during the compression. Since the pressure is greater during the expansion, the quantity of motive power produced by the dilatation is greater than that consumed by the compression. We shall thus obtain a balance of motive power, which may be employed for any purpose. The air has served as working substance in a heat-engine; it has also been employed in the most advantageous manner possible, since no useless re-establishment of the equilibrium of heat has been allowed to occur.
“All the operations above described may be executed in the reverse order and direction. Let us imagine that after the sixth period, that is to say, when the piston has reached the position ef, we make it return to the position ik, and that at the same time we keep the air in contact with the hot body A; the heat furnished by this body during the sixth period will return to its source, that is, to the body A, and everything will be as it was at the end of the fifth period. If now we remove the body A, and if we make the piston move from ik to cd, the temperature of the air will decrease by just as many degrees as it increased during the fifth period, and will become that of the body B. We can evidently continue in this way a series of operations the exact reverse of those which were previously described; it suffices to place oneself in the same circumstances and to execute for each period a movement of expansion in place of a movement of compression, and vice versa.
“The result of the first series of operations was the production of a certain quantity of motive power, and the transport of heat from the body A to the body B; the result of the reverse operations is the consumption of the motive power produced in the first case, and the return of heat from the body B to the body A, in such sort that these two series of operations annul and neutralize each other.
“The impossibility of producing by the agency of heat alone a quantity of motive power greater than that which we have obtained in our first series of operations is now easy to prove. It is demonstrated by reasoning exactly similar to that which we have already given. The reasoning will have in this case a greater degree of exactitude; the air of which we made use to develop the motive power is brought back at the end of each cycle of operations precisely to its initial state, whereas this was not quite exactly the case for the vapour of water, as we have already remarked.”
15. Proof of Carnot’s Principle.—Carnot considered the proof too obvious to be worth repeating, but, unfortunately, his previous demonstration, referring to an incomplete cycle, is not so exactly worded that exception cannot be taken to it. We will therefore repeat his proof in a slightly more definite and exact form. Suppose that a reversible engine R, working in the cycle above described, takes a quantity of heat H from the source in each cycle, and performs a quantity of useful work Wr. If it were possible for any other engine S, working with the same two bodies A and B as source and refrigerator, to perform a greater amount of useful work Ws per cycle for the same quantity of heat H taken from the source, it would suffice to take a portion Wr of this motive power (since Ws is by hypothesis greater than Wr) to drive the engine R backwards, and return a quantity of heat H to the source in each cycle. The process might be repeated indefinitely, and we should obtain at each repetition a balance of useful work Ws − Wr, without taking any heat from the source, which is contrary to experience. Whether the quantity of heat taken from the condenser by R is equal to that given to the condenser by S is immaterial. The hot body A might be a comparatively small boiler, since no heat is taken from it. The cold body B might be the ocean, or the whole earth. We might thus obtain without any consumption of fuel a practically unlimited supply of motive power. Which is absurd.
Carnot’s Statement of his Principle.5—If the above reasoning be admitted, we must conclude with Carnot that the motive power obtainable from heat is independent of the agents employed to realize it. The efficiency is fixed solely by the temperatures of the bodies between which, in the last resort, the transfer of heat is effected. “We must understand here that each of the methods of developing motive power attains the perfection of which it is susceptible. This condition is fulfilled if, according to our rule, there is produced in the body no change of temperature that is not due to change of volume, or in other words, if there is no direct interchange of heat between bodies of sensibly different temperatures.”
It is characteristic of a state of frictionless mechanical equilibrium that an indefinitely small difference of pressure suffices to upset the equilibrium and reverse the motion. Similarly in thermal equilibrium between bodies at the same temperature, an indefinitely small difference of temperature suffices to reverse the transfer of heat. Carnot’s rule is therefore the criterion of the reversibility of a cycle of operations as regards transfer of heat. It is assumed that the ideal engine is mechanically reversible, that there is not, for instance, any communication between reservoirs of gas or vapour at sensibly different pressures, and that there is no waste of power in friction. If there is equilibrium both mechanical and thermal at every stage of the cycle, the ideal engine will be perfectly reversible. That is to say, all its operations will be exactly reversed as regards transfer of heat and work, when the operations are performed in the reverse order and direction. On this understanding Carnot’s principle may be put in a different way, which is often adopted, but is really only the same thing put in different words: The efficiency of a perfectly reversible engine is the maximum possible, and is a function solely of the limits of temperature between which it works. This result depends essentially on the existence of a state of thermal equilibrium defined by equality of temperature, and independent, in the majority of cases, of the state of a body in other respects. In order to apply the principle to the calculation and prediction of results, it is sufficient to determine the manner in which the efficiency depends on the temperature for one particular case, since the efficiency must be the same for all reversible engines.
16. Experimental Verification of Carnot’s Principle.—Carnot endeavoured to test his result by the following simple calculations. Suppose that we have a cylinder fitted with a frictionless piston, containing 1 gram of water at 100° C., and that the pressure of the steam, namely 760 mm., is in equilibrium with the external pressure on the piston at this temperature. Place the cylinder in connexion with a boiler or hot body at 101° C. The water will then acquire the temperature of 101° C., and will absorb 1 gram-calorie of heat. Some waste of motive power occurs here because heat is allowed to pass from one body to another at a different temperature, but the waste in this case is so small as to be immaterial. Keep the cylinder in contact with the hot body at 101° C. and allow the piston to rise. It may be made to perform useful work as the pressure is now 27.7 mm. (or 37.7 grams per sq. cm.) in excess of the external pressure. Continue the process till all the water is converted into steam. The heat absorbed from the hot body will be nearly 540 gram-calories, the latent heat of steam at this temperature. The increase of volume will be approximately 1620 c.c., the volume of 1 gram of steam at this pressure and temperature. The work done by the excess pressure will be 37.7 × 1620 = 61,000 gram-centimetres or 0.61 of a kilogrammetre. Remove the hot body, and allow the steam to expand further till its pressure is 760 mm. and its temperature has fallen to 100° C. The work which might be done in this expansion is less than 1⁄1000th part of a kilogrammetre, and may be neglected for the present purpose. Place the cylinder in contact with the cold body at 100° C., and allow the steam to condense at this temperature. No work is done on the piston, because there is equilibrium of pressure, but a quantity of heat equal to the latent heat of steam at 100° C. is given to the cold body. The water is now in its initial condition, and the result of the process has been to gain 0.61 of a kilogrammetre of work by allowing 540 gram-calories of heat to pass from a body at 101° C. to a body at 100° C. by means of an ideally simple steam-engine. The work obtainable in this way from 1000 gram-calories of heat, or 1 kilo-calorie, would evidently be 1.13 kilogrammetre (= 0.61 × 1000⁄540).
 |
| Fig. 5.—Elementary Carnot Cycle for Gas. |
Taking the same range of temperature, namely 101° to 100° C., we may perform a similar series of operations with air in the cylinder, instead of water and steam. Suppose the cylinder to contain 1 gramme of air at 100° C. and 760 mm. pressure instead of water. Compress it without loss of heat (adiabatically), so as to raise its temperature to 101° C. Place it in contact with the hot body at 101° C., and allow it to expand at this temperature, absorbing heat from the hot body, until its volume is increased by 1⁄374th part (the expansion per degree at constant pressure). The quantity of heat absorbed in this expansion, as explained in § 14, will be the difference of the specific heats or the latent heat of expansion R′ = .069 calorie. Remove the hot body, and allow the gas to expand further without gain of heat till its temperature falls to 100° C. Compress it at 100° C. to its original volume, abstracting the heat of compression by contact with the cold body at 100° C. The air is now in its original state, and the process has been carried out in strict accordance with Carnot’s rule. The quantity of external work done in the cycle is easily obtained by the aid of the indicator diagram ABCD (fig. 5), which is approximately a parallelogram in this instance. The area of the diagram is equal to that of the rectangle BEHG, being the product of the vertical height BE, namely, the increase of pressure per 1° at constant volume, by the increase of volume BG, which is 1⁄273rd of the volume at 0° C. and 760 mm., or 2.83 c.c. The increase of pressure BE is 760⁄373, or 2.03 mm., which is equivalent to 2.76 gm. per sq. cm. The work done in the cycle is 2.76 × 2.83 = 7.82 gm. cm., or .0782 gram-metre. The heat absorbed at 101° C. was .069 gram-calorie, so that the work obtained is .0782/.069 or 1.13 gram-metre per gram-calorie, or 1.13 kilogrammetre per kilogram-calorie. This result is precisely the same as that obtained by using steam with the same range of temperature, but a very different kind of cycle. Carnot in making the same calculation did not obtain quite so good an agreement, because the experimental data at that time available were not so accurate. He used the value 1⁄267 for the coefficient of expansion, and .267 for the specific heat of air. Moreover, he did not feel justified in assuming, as above, that the difference of the specific heats was the same at 100° C. as at the ordinary temperature of 15° to 20° C., at which it had been experimentally determined. He made similar calculations for the vapour of alcohol, which differed slightly from the vapour of water. But the agreement he found was close enough to satisfy him that his theoretical deductions were correct, and that the resulting ratio of work to heat should be the same for all substances at the same temperature.
17. Carnot’s Function. Variation of Efficiency with Temperature.—By means of calculations, similar to those given above, Carnot endeavoured to find the amount of motive power obtainable from one unit of heat per degree fall at various temperatures with various substances. The value found above, namely 1.13 kilogrammetre per kilo-calorie per 1° fall, is the value of the efficiency per 1° fall at 100° C. He was able to show that the efficiency per degree fall probably diminished with rise of temperature, but the experimental data at that time were too inconsistent to suggest the true relation. He took as the analytical expression of his principle that the efficiency W/H of a perfect engine taking in heat H at a temperature t° C., and rejecting heat at the temperature 0° C., must be some function Ft of the temperature t, which would be the same for all substances. The efficiency per degree fall at a temperature t he represented by F′t, the derived function of Ft. The function F′t would be the same for all substances at the same temperature, but would have different values at different temperatures. In terms of this function, which is generally known as Carnot’s function, the results obtained in the previous section might be expressed as follows:—
“The increase of volume of a mixture of liquid and vapour per unit-mass vaporized at any temperature, multiplied by the increase of vapour-pressure per degree, is equal to the product of the function F′t by the latent heat of vaporization.
“The difference of the specific heats, or the latent heat of expansion for any substance multiplied by the function F′t, is equal to the product of the expansion per degree at constant pressure by the increase of pressure per degree at constant volume.”
Since the last two coefficients are the same for all gases if equal volumes are taken, Carnot concluded that: “The difference of the specific heats at constant pressure and volume is the same for equal volumes of all gases at the same temperature and pressure.”
Taking the expression W = RT log er for the whole work done by a gas obeying the gaseous laws pv = RT in expanding at a temperature T from a volume 1 (unity) to a volume r, or for a ratio of expansion r, and putting W′ = R log er for the work done in a cycle of range 1°, Carnot obtained the expression for the heat absorbed by a gas in isothermal expansion
H = R log er/F′t.
He gives several important deductions which follow from this formula, which is the analytical expression of the experimental result already quoted as having been discovered subsequently by Dulong. Employing the above expression for the latent heat of expansion, Carnot deduced a general expression for the specific heat of a gas at constant volume on the basis of the caloric theory. He showed that if the specific heat was independent of the temperature (the hypothesis already adopted by Laplace and Poisson) the function F′t must be of the form
F′t = R/C (t + t0)
where C and t0 are unknown constants. A similar result follows from his expression for the difference of the specific heats. If this is assumed to be constant and equal to C, the expression for F′t becomes R/CT, which is the same as the above if t0 = 273. Assuming the specific heat to be also independent of the volume, he shows that the function F′t should be constant. But this assumption is inconsistent with the caloric theory of latent heat of expansion, which requires the specific heat to be a function of the volume. It appears in fact impossible to reconcile Carnot’s principle with the caloric theory on any simple assumptions. As Carnot remarks: “The main principles on which the theory of heat rests require most careful examination. Many experimental facts appear almost inexplicable in the present state of this theory.”
Carnot’s work was subsequently put in a more complete analytical form by B. P. E. Clapeyron (Journ. de l’Éc. polytechn., Paris, 1832, 14, p. 153), who also made use of Watt’s indicator diagram for the first time in discussing physical problems. Clapeyron gave the general expressions for the latent heat of a vapour, and for the latent heat of isothermal expansion of any substance, in terms of Carnot’s function, employing the notation of the calculus. The expressions he gave are the same in form as those in use at the present day. He also gave the general expression for Carnot’s function, and endeavoured to find its variation with temperature; but having no better data, he succeeded no better than Carnot. Unfortunately, in describing Carnot’s cycle, he assumed the caloric theory of heat, and made some unnecessary mistakes, which Carnot (who, we now know, was a believer in the mechanical theory) had been very careful to avoid. Clapeyron directs one to compress the gas at the lower temperature in contact with the body B until the heat disengaged is equal to that which has been absorbed at the higher temperature.6 He assumes that the gas at this point contains the same quantity of heat as it contained in its original state at the higher temperature, and that, when the body B is removed, the gas will be restored to its original temperature, when compressed to its initial volume. This mistake is still attributed to Carnot, and regarded as a fatal objection to his reasoning by nearly all writers at the present day.
18. Mechanical Theory of Heat.—According to the caloric theory, the heat absorbed in the expansion of a gas became latent, like the latent heat of vaporization of a liquid, but remained in the gas and was again evolved on compressing the gas. This theory gave no explanation of the source of the motive power produced by expansion. The mechanical theory had explained the production of heat by friction as being due to transformation of visible motion into a brisk agitation of the ultimate molecules, but it had not so far given any definite explanation of the converse production of motive power at the expense of heat. The theory could not be regarded as complete until it had been shown that in the production of work from heat, a certain quantity of heat disappeared, and ceased to exist as heat; and that this quantity was the same as that which could be generated by the expenditure of the work produced. The earliest complete statement of the mechanical theory from this point of view is contained in some notes written by Carnot, about 1830, but published by his brother (Life of Sadi Carnot, Paris, 1878). Taking the difference of the specific heats to be .078, he estimated the mechanical equivalent at 370 kilogrammetres. But he fully recognized that there were no experimental data at that time available for a quantitative test of the theory, although it appeared to afford a good qualitative explanation of the phenomena. He therefore planned a number of crucial experiments such as the “porous plug” experiment, to test the equivalence of heat and motive power. His early death in 1836 put a stop to these experiments, but many of them have since been independently carried out by other observers.
The most obvious case of the production of work from heat is in the expansion of a gas or vapour, which served in the first instance as a means of calculating the ratio of equivalence, on the assumption that all the heat which disappeared had been transformed into work and had not merely become latent. Marc Séguin, in his De l’influence des chemins de fer (Paris, 1839), made a rough estimate in this manner of the mechanical equivalent of heat, assuming that the loss of heat represented by the fall of temperature of steam on expanding was equivalent to the mechanical effect produced by the expansion. He also remarks (loc. cit. p. 382) that it was absurd to suppose that “a finite quantity of heat could produce an indefinite quantity of mechanical action, and that it was more natural to assume that a certain quantity of heat disappeared in the very act of producing motive power.” J. R. Mayer (Liebig’s Annalen, 1842, 42, p. 233) stated the equivalence of heat and work more definitely, deducing it from the old principle, causa aequat effectum. Assuming that the sinking of a mercury column by which a gas was compressed was equivalent to the heat set free by the compression, he deduced that the warming of a kilogramme of water 1° C. would correspond to the fall of a weight of one kilogramme from a height of about 365 metres. But Mayer did not adduce any fresh experimental evidence, and made no attempt to apply his theory to the fundamental equations of thermodynamics. It has since been urged that the experiment of Gay-Lussac (1807), on the expansion of gas from one globe to another (see above, § 11), was sufficient justification for the assumption tacitly involved in Mayer’s calculation. But Joule was the first to supply the correct interpretation of this experiment, and to repeat it on an adequate scale with suitable precautions. Joule was also the first to measure directly the amount of heat liberated by the compression of a gas, and to prove that heat was not merely rendered latent, but disappeared altogether as heat, when a gas did work in expansion.
19. Joule’s Determinations of the Mechanical Equivalent.—The honour of placing the mechanical theory of heat on a sound experimental basis belongs almost exclusively to J. P. Joule, who showed by direct experiment that in all the most important cases in which heat was generated by the expenditure of mechanical work, or mechanical work was produced at the expense of heat, there was a constant ratio of equivalence between the heat generated and the work expended and vice versa. His first experiments were on the relation of the chemical and electric energy expended to the heat produced in metallic conductors and voltaic and electrolytic cells; these experiments were described in a series of papers published in the Phil. Mag., 1840-1843. He first proved the relation, known as Joule’s law, that the heat produced in a conductor of resistance R by a current C is proportional to C²R per second. He went on to show that the total heat produced in any voltaic circuit was proportional to the electromotive force E of the battery and to the number of equivalents electrolysed in it. Faraday had shown that electromotive force depends on chemical affinity. Joule measured the corresponding heats of combustion, and showed that the electromotive force corresponding to a chemical reaction is proportional to the heat of combustion of the electrochemical equivalent. He also measured the E.M.F. required to decompose water, and showed that when part of the electric energy EC is thus expended in a voltameter, the heat generated is less than the heat of combustion corresponding to EC by a quantity representing the heat of combustion of the decomposed gases. His papers so far had been concerned with the relations between electrical energy, chemical energy and heat which he showed to be mutually equivalent. The first paper in which he discussed the relation of heat to mechanical power was entitled “On the Calorific Effects of Magneto-Electricity, and on the Mechanical Value of Heat” (Brit. Assoc., 1843; Phil. Mag., 23, p. 263). In this paper he showed that the heat produced by currents generated by magneto-electric induction followed the same law as voltaic currents. By a simple and ingenious arrangement he succeeded in measuring the mechanical power expended in producing the currents, and deduced the mechanical equivalent of heat and of electrical energy. The amount of mechanical work required to raise 1 ℔ of water 1° F. (1 B.Th.U.), as found by this method, was 838 foot-pounds. In a note added to the paper he states that he found the value 770 foot-pounds by the more direct method of forcing water through fine tubes. In a paper “On the Changes of Temperature produced by the Rarefaction and Condensation of Air” (Phil. Mag., May 1845), he made the first direct measurements of the quantity of heat disengaged by compressing air, and also of the heat absorbed when the air was allowed to expand against atmospheric pressure; as the result he deduced the value 798 foot-pounds for the mechanical equivalent of 1 B.Th.U. He also showed that there was no appreciable absorption of heat when air was allowed to expand in such a manner as not to develop mechanical power, and he pointed out that the mechanical equivalent of heat could not be satisfactorily deduced from the relations of the specific heats, because the knowledge of the specific heats of gases at that time was of so uncertain a character. He attributed most weight to his later determinations of the mechanical equivalent made by the direct method of friction of liquids. He showed that the results obtained with different liquids, water, mercury and sperm oil, were the same, namely, 782 foot-pounds; and finally repeating the method with water, using all the precautions and improvements which his experience had suggested, he obtained the value 772 foot-pounds, which was accepted universally for many years, and has only recently required alteration on account of the more exact definition of the heat unit, and the standard scale of temperature (see Calorimetry). The great value of Joule’s work for the general establishment of the principle of the conservation of energy lay in the variety and completeness of the experimental evidence he adduced. It was not sufficient to find the relation between heat and mechanical work or other forms of energy in one particular case. It was necessary to show that the same relation held in all cases which could be examined experimentally, and that the ratio of equivalence of the different forms of energy, measured in different ways, was independent of the manner in which the conversion was effected and of the material or working substance employed.
As the result of Joule’s experiments, we are justified in concluding that heat is a form of energy, and that all its transformations are subject to the general principle of the conservation of energy. As applied to heat, the principle is called the first law of thermodynamics, and may be stated as follows: When heat is transformed into any other kind of energy, or vice versa, the total quantity of energy remains invariable; that is to say, the quantity of heat which disappears is equivalent to the quantity of the other kind of energy produced and vice versa.
The number of units of mechanical work equivalent to one unit of heat is generally called the mechanical equivalent of heat, or Joule’s equivalent, and is denoted by the letter J. Its numerical value depends on the units employed for heat and mechanical energy respectively. The values of the equivalent in terms of the units most commonly employed at the present time are as follows:—
| 777 foot-pounds (Lat. 45°) | are equivalent to | 1 B.Th.U. (℔ deg. Fahr.) |
| 1399 foot-pounds ” | ” ” | 1 ℔ deg. C. |
| 426.3 kilogrammetres | ” ” | 1 kilogram-deg. C. or kilo-calorie. |
| 426.3 grammetres | ” ” | 1 gram-deg. C. or calorie. |
| 4.180 joules | ” ” | 1 gram-deg. C. or calorie. |
The water for the heat units is supposed to be taken at 20° C. or 68° F., and the degree of temperature is supposed to be measured by the hydrogen thermometer. The acceleration of gravity in latitude 45° is taken as 980.7 C.G.S. For details of more recent and accurate methods of determination, the reader should refer to the article Calorimetry, where tables of the variation of the specific heat of water with temperature are also given.
The second law of thermodynamics is a title often used to denote Carnot’s principle or some equivalent mathematical expression. In some cases this title is not conferred on Carnot’s principle itself, but on some axiom from which the principle may be indirectly deduced. These axioms, however, cannot as a rule be directly applied, so that it would appear preferable to take Carnot’s principle itself as the second law. It may be observed that, as a matter of history, Carnot’s principle was established and generally admitted before the principle of the conservation of energy as applied to heat, and that from this point of view the titles, first and second laws, are not particularly appropriate.
20. Combination of Carnot’s Principle with the Mechanical Theory.—A very instructive paper, as showing the state of the science of heat about this time, is that of C. H. A. Holtzmann, “On the Heat and Elasticity of Gases and Vapours” (Mannheim, 1845; Taylor’s Scientific Memoirs, iv. 189). He points out that the theory of Laplace and Poisson does not agree with facts when applied to vapours, and that Clapeyron’s formulae, though probably correct, contain an undetermined function (Carnot’s F′t, Clapeyron’s 1/C) of the temperature. He determines the value of this function to be J/T by assuming, with Séguin and Mayer, that the work done in the isothermal expansion of a gas is a measure of the heat absorbed. From the then accepted value .078 of the difference of the specific heats of air, he finds the numerical value of J to be 374 kilogrammetres per kilo-calorie. Assuming the heat equivalent of the work to remain in the gas, he obtains expressions similar to Clapeyron’s for the total heat and the specific heats. In consequence of this assumption, the formulae he obtained for adiabatic expansion were necessarily wrong, but no data existed at that time for testing them. In applying his formulae to vapours, he obtained an expression for the saturation-pressure of steam, which agreed with the empirical formula of Roche, and satisfied other experimental data on the supposition that the coefficient of expansion of steam was .00423, and its specific heat 1.69—values which are now known to be impossible, but which appeared at the time to give a very satisfactory explanation of the phenomena.
The essay of Hermann Helmholtz, On the Conservation of Force (Berlin, 1847), discusses all the known cases of the transformation of energy, and is justly regarded as one of the chief landmarks in the establishment of the energy-principle. Helmholtz gives an admirable statement of the fundamental principle as applied to heat, but makes no attempt to formulate the correct equations of thermodynamics on the mechanical theory. He points out the fallacy of Holtzmann’s (and Mayer’s) calculation of the equivalent, but admits that it is supported by Joule’s experiments, though he does not seem to appreciate the true value of Joule’s work. He considers that Holtzmann’s formulae are well supported by experiment, and are much preferable to Clapeyron’s, because the value of the undetermined function F′t is found. But he fails to notice that Holtzmann’s equations are fundamentally inconsistent with the conservation of energy, because the heat equivalent of the external work done is supposed to remain in the gas.
That a quantity of heat equivalent to the work performed actually disappears when a gas does work in expansion, was first shown by Joule in the paper on condensation and rarefaction of air (1845) already referred to. At the conclusion of this paper he felt justified by direct experimental evidence in reasserting definitely the hypothesis of Séguin (loc. cit. p. 383) that “the steam while expanding in the cylinder loses heat in quantity exactly proportional to the mechanical force developed, and that on the condensation of the steam the heat thus converted into power is not given back.” He did not see his way to reconcile this conclusion with Clapeyron’s description of Carnot’s cycle. At a later date, in a letter to Professor W. Thomson (Lord Kelvin) (1848), he pointed out that, since, according to his own experiments, the work done in the expansion of a gas at constant temperature is equivalent to the heat absorbed, by equating Carnot’s expressions (given in § 17) for the work done and the heat absorbed, the value of Carnot’s function F′t must be equal to J/T, in order to reconcile his principle with the mechanical theory.
Professor W. Thomson gave an account of Carnot’s theory (Trans. Roy. Soc. Edin., Jan. 1849), in which he recognized the discrepancy between Clapeyron’s statement and Joule’s experiments, but did not see his way out of the difficulty. He therefore adopted Carnot’s principle provisionally, and proceeded to calculate a table of values of Carnot’s function F′t, from the values of the total-heat and vapour-pressure of steam-then recently determined by Regnault (Mémoires de l’Institut de Paris, 1847). In making the calculation, he assumed that the specific volume v of saturated steam at any temperature T and pressure p is that given by the gaseous laws, pv = RT. The results are otherwise correct so far as Regnault’s data are accurate, because the values of the efficiency per degree F′t are not affected by any assumption with regard to the nature of heat. He obtained the values of the efficiency F′t over a finite range from t to 0° C., by adding up the values of F′t for the separate degrees. This latter proceeding is inconsistent with the mechanical theory, but is the correct method on the assumption that the heat given up to the condenser is equal to that taken from the source. The values he obtained for F′t agreed very well with those previously given by Carnot and Clapeyron, and showed that this function diminishes with rise of temperature roughly in the inverse ratio of T, as suggested by Joule.
R. J. E. Clausius (Pogg. Ann., 1850, 79, p. 369) and W. J. M. Rankine (Trans. Roy. Soc. Edin., 1850) were the first to develop the correct equations of thermodynamics on the mechanical theory. When heat was supplied to a body to change its temperature or state, part remained in the body as intrinsic heat energy E, but part was converted into external work of expansion W and ceased to exist as heat. The part remaining in the body was always the same for the same change of state, however performed, as required by Carnot’s fundamental axiom, but the part corresponding to the external work was necessarily different for different values of the work done. Thus in any cycle in which the body was exactly restored to its initial state, the heat remaining in the body would always be the same, or as Carnot puts it, the quantities of heat absorbed and given out in its diverse transformations are exactly “compensated,” so far as the body is concerned. But the quantities of heat absorbed and given out are not necessarily equal. On the contrary, they differ by the equivalent of the external work done in the cycle. Applying this principle to the case of steam, Clausius deduced a fact previously unknown, that the specific heat of steam maintained in a state of saturation is negative, which was also deduced by Rankine (loc. cit.) about the same time. In applying the principle to gases Clausius assumes (with Mayer and Holtzmann) that the heat absorbed by a gas in isothermal expansion is equivalent to the work done, but he does not appear to be acquainted with Joule’s experiment, and the reasons he adduces in support of this assumption are not conclusive. This being admitted, he deduces from the energy principle alone the propositions already given by Carnot with reference to gases, and shows in addition that the specific heat of a perfect gas must be independent of the density. In the second part of his paper he introduces Carnot’s principle, which he quotes as follows: “The performance of work is equivalent to a transference of heat from a hot to a cold body without the quantity of heat being thereby diminished.” This is not Carnot’s way of stating his principle (see § 15), but has the effect of exaggerating the importance of Clapeyron’s unnecessary assumption. By equating the expressions given by Carnot for the work done and the heat absorbed in the expansion of a gas, he deduces (following Holtzmann) the value J/T for Carnot’s function F′t (which Clapeyron denotes by 1/C). He shows that this assumption gives values of Carnot’s function which agree fairly well with those calculated by Clapeyron and Thomson, and that it leads to values of the mechanical equivalent not differing greatly from those of Joule. Substituting the value J/T for C in the analytical expressions given by Clapeyron for the latent heat of expansion and vaporization, these relations are immediately reduced to their modern form (see Thermodynamics, § 4). Being unacquainted with Carnot’s original work, but recognizing the invalidity of Clapeyron’s description of Carnot’s cycle, Clausius substituted a proof consistent with the mechanical theory, which he based on the axiom that “heat cannot of itself pass from cold to hot.” The proof on this basis involves the application of the energy principle, which does not appear to be necessary, and the axiom to which final appeal is made does not appear more convincing than Carnot’s. Strange to say, Clausius did not in this paper give the expression for the efficiency in a Carnot cycle of finite range (Carnot’s Ft) which follows immediately from the value J/T assumed for the efficiency F′t of a cycle of infinitesimal range at the temperature t C or T Abs.
Rankine did not make the same assumption as Clausius explicitly, but applied the mechanical theory of heat to the development of his hypothesis of molecular vortices, and deduced from it a number of results similar to those obtained by Clausius. Unfortunately the paper (loc. cit.) was not published till some time later, but in a summary given in the Phil. Mag. (July 1851) the principal results were detailed. Assuming the value of Joule’s equivalent, Rankine deduced the value 0.2404 for the specific heat of air at constant pressure, in place of 0.267 as found by Delaroche and Bérard. The subsequent verification of this value by Regnault (Comptes rendus, 1853) afforded strong confirmation of the accuracy of Joule’s work. In a note appended to the abstract in the Phil. Mag. Rankine states that he has succeeded in proving that the maximum efficiency of an engine working in a Carnot cycle of finite range t1 to t0 is of the form (t1 − t0) / (t1 − k), where k is a constant, the same for all substances. This is correct if t represents temperature Centigrade, and k = −273.
Professor W. Thomson (Lord Kelvin) in a paper “On the Dynamical Theory of Heat” (Trans. Roy. Soc. Edin., 1851, first published in the Phil. Mag., 1852) gave a very clear statement of the position of the theory at that time. He showed that the value F′t = J/T, assumed for Carnot’s function by Clausius without any experimental justification, rested solely on the evidence of Joule’s experiment, and might possibly not be true at all temperatures. Assuming the value J/T with this reservation, he gave as the expression for the efficiency over a finite range t1 to t0 C., or T1 to T0 Abs., the result,
W/H = (t1 − t0) / (t1 + 273) = (T1 − T0) / T1
which, he observed, agrees in form with that found by Rankine.
21. The Absolute Scale of Temperature.—Since Carnot’s function is the same for all substances at the same temperature, and is a function of the temperature only, it supplies a means of measuring temperature independently of the properties of any particular substance. This proposal was first made by Lord Kelvin (Phil. Mag., 1848), who suggested that the degree of temperature should be chosen so that the efficiency of a perfect engine at any point of the scale should be the same, or that Carnot’s function F′t should be constant. This would give the simplest expression for the efficiency on the caloric theory, but the scale so obtained, when the values of Carnot’s function were calculated from Regnault’s observations on steam, was found to differ considerably from the scale of the mercury or air-thermometer. At a later date, when it became clear that the value of Carnot’s function was very nearly proportional to the reciprocal of the temperature T measured from the absolute zero of the gas thermometer, he proposed a simpler method (Phil. Trans., 1854), namely, to define absolute temperature θ as proportional to the reciprocal of Carnot’s function. On this definition of absolute temperature, the expression (θ1 − θ0) / θ1 for the efficiency of a Carnot cycle with limits θ1 and θ0 would be exact, and it became a most important problem to determine how far the temperature T by gas thermometer differed from the absolute temperature θ. With this object he devised a very delicate method, known as the “porous plug experiment” (see Thermodynamics) of testing the deviation of the gas thermometer from the absolute scale. The experiments were carried out in conjunction with Joule, and finally resulted in showing (Phil. Trans., 1862, “On the Thermal Effects of Fluids in Motion”) that the deviations of the air thermometer from the absolute scale as above defined are almost negligible, and that in the case of the gas hydrogen the deviations are so small that a thermometer containing this gas may be taken for all practical purposes as agreeing exactly with the absolute scale at all ordinary temperatures. For this reason the hydrogen thermometer has since been generally adopted as the standard.
22. Availability of Heat of Combustion.—Taking the value 1.13 kilogrammetres per kilo-calorie for 1° C. fall of temperature at 100° C., Carnot attempted to estimate the possible performance of a steam-engine receiving heat at 160° C. and rejecting it at 40° C. Assuming the performance to be simply proportional to the temperature fall, the work done for 120° fall would be 134 kilogrammetres per kilo-calorie. To make an accurate calculation required a knowledge of the variation of the function F′t with temperature. Taking the accurate formula of § 20, the work obtainable is 118 kilogrammetres per kilo-calorie, which is 28% of 426, the mechanical equivalent of the kilo-calorie in kilogrammetres. Carnot pointed out that the fall of 120° C. utilized in the steam-engine was only a small fraction of the whole temperature fall obtainable by combustion, and made an estimate of the total power available if the whole fall could be utilized, allowing for the probable diminution of the function F′t with rise of temperature. His estimate was 3.9 million kilogrammetres per kilogramme of coal. This was certainly an over-estimate, but was surprisingly close, considering the scanty data at his disposal.
In reality the fraction of the heat of combustion available, even in an ideal engine and apart from practical limitations, is much less than might be inferred from the efficiency formula of the Carnot cycle. In applying this formula to estimate the availability of the heat it is usual to take the temperature obtainable by the combustion of the fuel as the upper limit of temperature in the formula. For carbon burnt in air at constant pressure without any loss of heat, the products of combustion might be raised 2300° C. in temperature, assuming that the specific heats of the products were constant and that there was no dissociation. If all the heat could be supplied to the working fluid at this temperature, that of the condenser being 40° C., the possible efficiency by the formula of § 20 would be 89%. But the combustion obviously cannot maintain so high a temperature if heat is being continuously abstracted by a boiler. Suppose that θ′ is the maximum temperature of combustion as above estimated, θ” the temperature of the boiler, and θ0 that of the condenser. Of the whole heat supplied by combustion represented by the rise of temperature θ′ − θ0, the fraction (θ′ − θ″) / (θ′ − θ0) is the maximum that could be supplied to the boiler, the fraction (θ″ − θ0) / (θ′ − θ0) being carried away with the waste gases. Of the heat supplied to the boiler, the fraction (θ′ − θ0) / θ″ might theoretically be converted into work. The problem in the case of an engine using a separate working fluid, like a steam-engine, is to find what must be the temperature θ″ of the boiler in order to obtain the largest possible fraction of the heat of combustion in the form of work. It is easy to show that θ” must be the geometric mean of θ′ and θ0, or θ″ = √θ′θ0. Taking θ′ − θ0 = 2300° C., and θ0 = 313° Abs. as before, we find θ″ = 903° Abs. or 630° C. The heat supplied to the boiler is then 74.4% of the heat of combustion, and of this 65.3% is converted into work, giving a maximum possible efficiency of 49% in place of 89%. With the boiler at 160° C., the possible efficiency, calculated in a similar manner, would be 26.3%, which shows that the possible increase of efficiency by increasing the temperature range is not so great as is usually supposed. If the temperature of the boiler were raised to 300° C., corresponding to a pressure of 1260 ℔ per sq. in., which is occasionally surpassed in modern flash-boilers, the possible efficiency would be 40%. The waste heat from the boiler, supposed perfectly efficient, would be in this case 11%, of which less than a quarter could be utilized in the form of work. Carnot foresaw that in order to utilize a larger percentage of the heat of combustion it would be necessary to employ a series of working fluids, the waste heat from one boiler and condenser serving to supply the next in the series. This has actually been effected in a few cases, e.g. steam and SO2, when special circumstances exist to compensate for the extra complication. Improvements in the steam-engine since Carnot’s time have been mainly in the direction of reducing waste due to condensation and leakage by multiple expansion, superheating, &c. The gain by increased temperature range has been comparatively small owing to limitations of pressure, and the best modern steam-engines do not utilize more than 20% of the heat of combustion. This is in reality a very respectable fraction of the ideal limit of 40% above calculated on the assumption of 1260 ℔ initial pressure, with a perfectly efficient boiler and complete expansion, and with an ideal engine which does not waste available motive power by complete condensation of the steam before it is returned to the boiler.
23. Advantages of Internal Combustion.—As Carnot pointed out, the chief advantage of using atmospheric air as a working fluid in a heat-engine lies in the possibility of imparting heat to it directly by internal combustion. This avoids the limitation imposed by the use of a separate boiler, which as we have seen reduces the possible efficiency at least 50%. Even with internal combustion, however, the full range of temperature is not available, because the heat cannot conveniently in practice be communicated to the working fluid at constant temperature, owing to the large range of expansion at constant temperature required for the absorption of a sufficient quantity of heat. Air-engines of this type, such as Stirling’s or Ericsson’s, taking in heat at constant temperature, though theoretically the most perfect, are bulky and mechanically inefficient. In practical engines the heat is generated by the combustion of an explosive mixture at constant volume or at constant pressure. The heat is not all communicated at the highest temperature, but over a range of temperature from that of the mixture at the beginning of combustion to the maximum temperature. The earliest instance of this type of engine is the lycopodium engine of M. M. Niepce, discussed by Carnot, in which a combustible mixture of air and lycopodium powder at atmospheric pressure was ignited in a cylinder, and did work on a piston. The early gas-engines of E. Lenoir (1860) and N. Otto and E. Langen (1866), operated in a similar manner with illuminating gas in place of lycopodium. Combustion in this case is effected practically at constant volume, and the maximum efficiency theoretically obtainable is 1 − loger / (r − 1), where r is the ratio of the maximum temperature θ′ to the initial temperature θ0. In order to obtain this efficiency it would be necessary to follow Carnot’s rule, and expand the gas after ignition without loss or gain of heat from θ′ down to θ0, and then to compress it at θ0 to its initial volume. If the rise of temperature in combustion were 2300° C., and the initial temperature were 0° C. or 273° Abs., the theoretical efficiency would be 73.3%, which is much greater than that obtainable with a boiler. But in order to reach this value, it would be necessary to expand the mixture to about 270 times its initial volume, which is obviously impracticable. Owing to incomplete expansion and rapid cooling of the heated gases by the large surface exposed, the actual efficiency of the Lenoir engine was less than 5%, and of the Otto and Langen, with more rapid expansion, about 10%. Carnot foresaw that in order to render an engine of this type practically efficient, it would be necessary to compress the mixture before ignition. Compression is beneficial in three ways: (1) it permits a greater range of expansion after ignition; (2) it raises the mean effective pressure, and thus improves the mechanical efficiency and the power in proportion to size and weight; (3) it reduces the loss of heat during ignition by reducing the surface exposed to the hot gases. In the modern gas or petrol motor, compression is employed as in Carnot’s cycle, but the efficiency attainable is limited not so much by considerations of temperature as by limitations of volume. It is impracticable before combustion at constant volume to compress a rich mixture to much less than 1⁄5th of its initial volume, and, for mechanical simplicity, the range of expansion is made equal to that of compression. The cycle employed was patented in 1862 by Beau de Rochas (d. 1892), but was first successfully carried out by Otto (1876). It differs from the Carnot cycle in employing reception and rejection of heat at constant volume instead of at constant temperature. This cycle is not so efficient as the Carnot cycle for given limits of temperature, but, for the given limits of volume imposed, it gives a much higher efficiency than the Carnot cycle. The efficiency depends only on the range of temperature in expansion and compression, and is given by the formula (θ′ − θ″) / θ′, where θ′ is the maximum temperature, and θ″ the temperature at the end of expansion. The formula is the same as that for the Carnot cycle with the same range of temperature in expansion. The ratio θ′ / θ″ is rγ−1, where r is the given ratio of expansion or compression, and γ is the ratio of the specific heats of the working fluid. Assuming the working fluid to be a perfect gas with the same properties as air, we should have γ = 1.41. Taking r = 5, the formula gives 48% for the maximum possible efficiency. The actual products of combustion vary with the nature of the fuel employed, and have different properties from air, but the efficiency is found to vary with compression in the same manner as for air. For this reason a committee of the Institution of Civil Engineers in 1905 recommended the adoption of the air-standard for estimating the effects of varying the compression ratio, and defined the relative efficiency of an internal combustion engine as the ratio of its observed efficiency to that of a perfect air-engine with the same compression.
24. Effect of Dissociation, and Increase of Specific Heat.—One of the most important effects of heat is the decomposition or dissociation of compound molecules. Just as the molecules of a vapour combine with evolution of heat to form the more complicated molecules of the liquid, and as the liquid molecules require the addition of heat to effect their separation into molecules of vapour; so in the case of molecules of different kinds which combine with evolution of heat, the reversal of the process can be effected either by the agency of heat, or indirectly by supplying the requisite amount of energy by electrical or other methods. Just as the latent heat of vaporization diminishes with rise of temperature, and the pressure of the dissociated vapour molecules increases, so in the case of compound molecules in general the heat of combination diminishes with rise of temperature, and the pressure of the products of dissociation increases. There is evidence that the compound carbon dioxide, CO2, is partly dissociated into carbon monoxide and oxygen at high temperatures, and that the proportion dissociated increases with rise of temperature. There is a very close analogy between these phenomena and the vaporization of a liquid. The laws which govern dissociation are the same fundamental laws of thermodynamics, but the relations involved are necessarily more complex on account of the presence of different kinds of molecules, and present special difficulties for accurate investigation in the case where dissociation does not begin to be appreciable until a high temperature is reached. It is easy, however, to see that the general effect of dissociation must be to diminish the available temperature of combustion, and all experiments go to show that in ordinary combustible mixtures the rise of temperature actually attained is much less than that calculated as in § 22, on the assumption that the whole heat of combustion is developed and communicated to products of constant specific heat. The defect of temperature observed can be represented by supposing that the specific heat of the products of combustion increases with rise of temperature. This is the case for CO2 even at ordinary temperatures, according to Regnault, and probably also for air and steam at higher temperatures. Increase of specific heat is a necessary accompaniment of dissociation, and from some points of view may be regarded as merely another way of stating the facts. It is the most convenient method to adopt in the case of products of combustion consisting of a mixture of CO2 and steam with a large excess of inert gases, because the relations of equilibrium of dissociated molecules of so many different kinds would be too complex to permit of any other method of expression. It appears from the researches of Dugald Clerk, H. le Chatelier and others that the apparent specific heat of the products of combustion in a gas-engine may be taken as approximately .34 to .33 in place of .24 at working temperatures between 1000° C. and 1700° C., and that the ratio of the specific heats is about 1.29 in place of 1.41. This limits the availability of the heat of combustion by reducing the rise of temperature actually obtainable in combustion at constant volume by 30 or 40%, and also by reducing the range of temperature θ′ / θ″ for a given ratio of expansions r from r.41 to r.29. The formula given in § 21 is no longer quite exact, because the ratio of the specific heats of the mixture during compression is not the same as that of the products of combustion during expansion. But since the work done depends principally on the expansion curve, the ratio of the range of temperature in expansion (θ′ − θ″) to the maximum temperature θ′ will still give a very good approximation to the possible efficiency. Taking r = 5, as before, for the compression ratio, the possible efficiency is reduced from 48% to 38%, if γ = 1.29 instead of 1.41. A large gas-engine of the present day with r = 5 may actually realize as much as 34% indicated efficiency, which is 90% of the maximum possible, showing how perfectly all avoidable heat losses have been minimized.
It is often urged that the gas-engine is relatively less efficient than the steam-engine, because, although it has a much higher absolute efficiency, it does not utilize so large a fraction of its temperature range, reckoning that of the steam-engine from the temperature of the boiler to that of the condenser, and that of the gas-engine from the maximum temperature of combustion to that of the air. This is not quite fair, and has given rise to the mistaken notion that “there is an immense margin for improvement in the gas-engine,” which is not the case if the practical limitations of volume are rightly considered. If expansion could be carried out in accordance with Carnot’s principle of maximum efficiency, down to the lower limit of temperature θ0, with rejection of heat at θ0 during compression to the original volume V0, it would no doubt be possible to obtain an ideal efficiency of nearly 80%. But this would be quite impracticable, as it would require expansion to about 100 times v0, or 500 times the compression volume. Some advantage no doubt might be obtained by carrying the expansion beyond the original volume. This has been done, but is not found to be worth the extra complication. A more practical method, which has been applied by Diesel for liquid fuel, is to introduce the fuel at the end of compression, and adjust the supply in such a manner as to give combustion at nearly constant pressure. This makes it possible to employ higher compression, with a corresponding increase in the ratio of expansion and the theoretical efficiency. With a compression ratio of 14, an indicated efficiency of 40% has been obtained In this way, but owing to additional complications the brake efficiency was only 31%, which is hardly any improvement on the brake efficiency of 30% obtained with the ordinary type of gas-engine. Although Carnot’s principle makes it possible to calculate in every case what the limiting possible efficiency would be for any kind of cycle if all heat losses were abolished, it is very necessary, in applying the principle to practical cases, to take account of the possibility of avoiding the heat losses which are supposed to be absent, and of other practical limitations in the working of the actual engine. An immense amount of time and ingenuity has been wasted in striving to realize impossible margins of ideal efficiency, which a close study of the practical conditions would have shown to be illusory. As Carnot remarks at the conclusion of his essay: “Economy of fuel is only one of the conditions a heat-engine must satisfy; in many cases it is only secondary, and must often give way to considerations of safety, strength and wearing qualities of the machine, of smallness of space occupied, or of expense in erecting. To know how to appreciate justly in each case the considerations of convenience and economy, to be able to distinguish the essential from the accessory, to balance all fairly, and finally to arrive at the best result by the simplest means, such must be the principal talent of the man called on to direct and co-ordinate the work of his fellows for the attainment of a useful object of any kind.”
Transference of Heat
25. Modes of Transference.—There are three principal modes of transference of heat, namely (1) convection, (2) conduction, and (3) radiation.
(1) In convection, heat is carried or conveyed by the motion of heated masses of matter. The most familiar illustrations of this method of transference are the heating of buildings by the circulation of steam or hot water, or the equalization of temperature of a mass of unequally heated liquid or gas by convection currents, produced by natural changes of density or by artificial stirring. (2) In conduction, heat is transferred by contact between contiguous particles of matter and is passed on from one particle to the next without visible relative motion of the parts of the body. A familiar illustration of conduction is the passage of heat through the metal plates of a boiler from the fire to the water inside, or the transference of heat from a soldering bolt to the solder and the metal with which it is placed in contact. (3) In radiation, the heated body gives rise to a motion of vibration in the aether, which is propagated equally in all directions, and is reconverted into heat when it encounters any obstacle capable of absorbing it. Thus radiation differs from conduction and convection in taking place most perfectly in the absence of matter, whereas conduction and convection require material communication between the bodies concerned.
In the majority of cases of transference of heat all three modes of transference are simultaneously operative in a greater or less degree, and the combined effect is generally of great complexity. The different modes of transference are subject to widely different laws, and the difficulty of disentangling their effects and subjecting them to calculation is often one of the most serious obstacles in the experimental investigation of heat. In space void of matter, we should have pure radiation, but it is difficult to obtain so perfect a vacuum that the effects of the residual gas in transferring heat by conduction or convection are inappreciable. In the interior of an opaque solid we should have pure conduction, but if the solid is sensibly transparent in thin layers there must also be an internal radiation, while in a liquid or a gas it is very difficult to eliminate the effects of convection. These difficulties are well illustrated in the historical development of the subject by the experimental investigations which have been made to determine the laws of heat-transference, such as the laws of cooling, of radiation and of conduction.
26. Newton’s Law of Cooling.—There is one essential condition common to all three modes of heat-transference, namely, that they depend on difference of temperature, that the direction of the transfer of heat is always from hot to cold, and that the rate of transference is, for small differences, directly proportional to the difference of temperature. Without difference of temperature there is no transfer of heat. When two bodies have been brought to the same temperature by conduction, they are also in equilibrium as regards radiation, and vice versa. If this were not the case, there could be no equilibrium of heat defined by equality of temperature. A hot body placed in an enclosure of lower temperature, e.g. a calorimeter in its containing vessel, generally loses heat by all three modes simultaneously in different degrees. The loss by each mode will depend in different ways on the form, extent and nature of its surface and on that of the enclosure, on the manner in which it is supported, on its relative position and distance from the enclosure, and on the nature of the intervening medium. But provided that the difference of temperature is small, the rate of loss of heat by all modes will be approximately proportional to the difference of temperature, the other conditions remaining constant. The rate of cooling or the rate of fall of temperature will also be nearly proportional to the rate of loss of heat, if the specific heat of the cooling body is constant, or the rate of cooling at any moment will be proportional to the difference of temperature. This simple relation is commonly known as Newton’s law of cooling, but is limited in its application to comparatively simple cases such as the foregoing. Newton himself applied it to estimate the temperature of a red-hot iron ball, by observing the time which it took to cool from a red heat to a known temperature, and comparing this with the time taken to cool through a known range at ordinary temperatures. According to this law if the excess of temperature of the body above its surroundings is observed at equal intervals of time, the observed values will form a geometrical progression with a common ratio. Supposing, for instance, that the surrounding temperature were 0° C., that the red-hot ball took 25 minutes to cool from its original temperature to 20° C., and 5 minutes to cool from 20° C. to 10° C., the original temperature is easily calculated on the assumption that the excess of temperature above 0° C. falls to half its value in each interval of 5 minutes. Doubling the value 20° at 25 minutes five times, we arrive at 640° C. as the original temperature. No other method of estimation of such temperatures was available in the time of Newton, but, as we now know, the simple law of proportionality to the temperature difference is inapplicable over such large ranges of temperature. The rate of loss of heat by radiation, and also by convection and conduction to the surrounding air, increases much more rapidly than in simple proportion to the temperature difference, and the rate of increase of each follows a different law. At a later date Sir John Herschel measured the intensity of the solar radiation at the surface of the earth, and endeavoured to form an estimate of the temperature of the sun by comparison with terrestrial sources on the assumption that the intensity of radiation was simply proportional to the temperature difference. He thus arrived at an estimate of several million degrees, which we now know would be about a thousand times too great. The application of Newton’s law necessarily leads to absurd results when the difference of temperature is very large, but the error will not in general exceed 2 to 3% if the temperature difference does not exceed 10° C., and the percentage error is proportionately much smaller for smaller differences.
27. Dulong and Petit’s Empirical Laws of Cooling.—One of the most elaborate experimental investigations of the law of cooling was that of Dulong and Petit (Ann. Chim. Phys., 1817, 7, pp. 225 and 337), who observed the rate of cooling of a mercury thermometer from 300° C. in a water-jacketed enclosure at various temperatures from 0° C. to 80° C. In order to obtain the rate of cooling by radiation alone, they exhausted the enclosure as perfectly as possible after the introduction of the thermometer, but with the imperfect appliances available at that time they were not able to obtain a vacuum better than about 3 or 4 mm. of mercury. They found that the velocity of cooling V in a vacuum could be represented by a formula of the type
V = A (at − at0)
in which t is the temperature of the thermometer, and t0 that of the enclosure, a is a constant having the value 1.0075, and the coefficient A depends on the form of the bulb and the nature of its surface. For the ranges of temperature they employed, this formula gives much better results than Newton’s, but it must be remembered that the temperatures were expressed on the arbitrary scale of the mercury thermometer, and were not corrected for the large and uncertain errors of stem-exposure (see Thermometry). Moreover, although the effects of cooling by convection currents are practically eliminated by exhausting to 3 or 4 mm. (since the density of the gas is reduced to 1⁄200th while its viscosity is not appreciably affected), the rate of cooling by conduction is not materially diminished, since the conductivity, like the viscosity, is nearly independent of pressure. It has since been shown by Sir William Crookes (Proc. Roy. Soc., 1881, 21, p. 239) that the rate of cooling of a mercury thermometer in a vacuum suffers a very great diminution when the pressure is reduced from 1 mm. to .001 mm., at which pressure the effect of conduction by the residual gas has practically disappeared.
Dulong and Petit also observed the rate of cooling under the same conditions with the enclosure filled with various gases. They found that the cooling effect of the gas could be represented by adding to the term already given as representing radiation, an expression of the form
V′ = Bpc (t − t0)1.233.
They found that the cooling effect of convection, unlike that of radiation, was independent of the nature of the surface of the thermometer, whether silvered or blackened, that it varied as some power c of the pressure p, and that it was independent of the absolute temperature of the enclosure, but varied as the excess temperature (t − t0) raised to the power 1.233. This highly artificial result undoubtedly contains some elements of truth, but could only be applied to experiments similar to those from which it was derived. F. Hervé de la Provostaye and P. Q. Desains (Ann. Chim. Phys., 1846, 16, p. 337), in repeating these experiments under various conditions, found that the coefficients A and B were to some extent dependent on the temperature, and that the manner in which the cooling effect varied with the pressure depended on the form and size of the enclosure. It is evident that this should be the case, since the cooling effect of the gas depends partly on convective currents. which are necessarily greatly modified by the form of the enclosure in a manner which it would appear hopeless to attempt to represent by any general formula.
28. Surface Emissivity.—The same remark applies to many attempts which have since been made to determine the general value of the constant termed by Fourier and early writers the “exterior conductibility,” but now called the surface emissivity. This coefficient represents the rate of loss of heat from a body per unit area of surface per degree excess of temperature, and includes the effects of radiation, convection and conduction. As already pointed out, the combined effect will be nearly proportional to the excess of temperature in any given case provided that the excess is small, but it is not necessarily proportional to the extent of surface exposed except in the case of pure radiation. The rate of loss by convection and conduction varies greatly with the form of the surface, and, unless the enclosure is very large compared with the cooling body, the effect depends also on the size and form of the enclosure. Heat is necessarily communicated from the cooling body to the layer of gas in contact with it by conduction. If the linear dimensions of the body are small, as in the case of a fine wire, or if it is separated from the enclosure by a thin layer of gas, the rate of loss depends chiefly on conduction. For very fine metallic wires heated by an electric current, W. E. Ayrton and H. Kilgour (Phil. Trans., 1892) showed that the rate of loss is nearly independent of the surface, instead of being directly proportional to it. This should be the case, as Porter has shown (Phil. Mag., March 1895), since the effect depends mainly on conduction. The effects of conduction and radiation may be approximately estimated if the conductivity of the gas and the nature and forms of the surfaces of the body and enclosure are known, but the effect of convection in any case can be determined only by experiment. It has been found that the rate of cooling by a current of air is approximately proportional to the velocity of the current, other things being equal. It is obvious that this should be the case, but the result cannot generally be applied to convection currents. Values which are commonly given for the surface emissivity must therefore be accepted with great reserve. They can be regarded only as approximate, and as applicable only to cases precisely similar to those for which they were experimentally obtained. There cannot be said to be any general law of convection. The loss of heat is not necessarily proportional to the area of the surface, and no general value of the coefficient can be given to suit all cases. The laws of conduction and radiation admit of being more precisely formulated, and their effects predicted, except in so far as they are complicated by convection.
29. Conduction of Heat.—The laws of transference of heat in the interior of a solid body formed one of the earliest subjects of mathematical and experimental treatment in the theory of heat. The law assumed by Fourier was of the simplest possible type, but the mathematical application, except in the simplest cases, was so difficult as to require the development of a new mathematical method. Fourier succeeded in showing how, by his method of analysis, the solution of any given problem with regard to the flow of heat by conduction in any material could be obtained in terms of a physical constant, the thermal conductivity of the material, and that the results obtained by experiment agreed in a qualitative manner with those predicted by his theory. But the experimental determination of the actual values of these constants presented formidable difficulties which were not surmounted till a later date. The experimental methods and difficulties are discussed in a special article on Conduction of Heat. It will suffice here to give a brief historical sketch, including a few of the more important results by way of illustration.
30. Comparison of Conducting Powers.—That the power of transmitting heat by conduction varied widely in different materials was probably known in a general way from prehistoric times. Empirical knowledge of this kind is shown in the construction of many articles for heating, cooking, &c., such as the copper soldering bolt, or the Norwegian cooking-stove. One of the earliest experiments for making an actual comparison of conducting powers was that suggested by Franklin, but carried out by Jan Ingenhousz (Journ. de phys., 1789, 34, pp. 68 and 380). Exactly similar bars of different materials, glass, wood, metal, &c., thinly coated with wax, were fixed in the side of a trough of boiling water so as to project for equal distances through the side of the trough into the external air. The wax coating was observed to melt as the heat travelled along the bars, the distance from the trough to which the wax was melted along each affording an approximate indication of the distribution of temperature. When the temperature of each bar had become stationary the heat which it gained by conduction from the trough must be equal to the heat lost to the surrounding air, and must therefore be approximately proportional to the distance to which the wax had melted along the bar. But the temperature fall per unit length, or the temperature-gradient, in each bar at the point where it emerged from the trough would be inversely proportional to the same distance. For equal temperature-gradients the quantities of heat conducted (or the relative conducting powers of the bars) would therefore be proportional to the squares of the distances to which the wax finally melted on each bar. This was shown by Fourier and Despretz (Ann. chim. phys., 1822, 19, p. 97).
31. Diffusion of Temperature.—It was shown in connexion with this experiment by Sir H. Davy, and the experiment was later popularized by John Tyndall, that the rate at which wax melted along the bar, or the rate of propagation of a given temperature, during the first moments of heating, as distinguished from the melting-distance finally attained, depended on the specific heat as well as the conductivity. Short prisms of iron and bismuth coated with wax were placed on a hot metal plate. The wax was observed to melt first on the bismuth, although its conductivity is less than that of iron. The reason is that its specific heat is less than that of iron in the proportion of 3 to 11. The densities of iron and bismuth being 7.8 and 9.8, the thermal capacities of equal prisms will be in the ratio .86 for iron to .29 for bismuth. If the prisms receive heat at equal rates, the bismuth will reach the temperature of melting wax nearly three times as quickly as the iron. It is often stated on the strength of this experiment that the rate of propagation of a temperature wave, which depends on the ratio of the conductivity to the specific heat per unit volume, is greater in bismuth than in iron (e.g. Preston, Heat, p. 628). This is quite incorrect, because the conductivity of iron is about six times that of bismuth, and the rate of propagation of a temperature wave is therefore twice as great in iron as in bismuth. The experiment in reality is misleading because the rates of reception of heat by the prisms are limited by the very imperfect contact with the hot metal plate, and are not proportional to the respective conductivities. If the iron and bismuth bars are properly faced and soldered to the top of a copper box (in order to ensure good metallic contact, and exclude a non-conducting film of air), and the box is then heated by steam, the rates of reception of heat will be nearly proportional to the conductivities, and the wax will melt nearly twice as fast along the iron as along the bismuth. A bar of lead similarly treated will show a faster rate of propagation than iron, because, although its conductivity is only half that of iron, its specific heat per unit volume is 2.5 times smaller.
32. Bad Conductors. Liquids and Gases.—Count Rumford (1792) compared the conducting powers of substances used in clothing, such as wool and cotton, fur and down, by observing the time which a thermometer took to cool when embedded in a globe filled successively with the different materials. The times of cooling observed for a given range varied from 1300 to 900 seconds for different materials. The low conducting power of such materials is principally due to the presence of air in the interstices, which is prevented from forming convection currents by the presence of the fibrous material. Finely powdered silica is a very bad conductor, but in the compact form of rock crystal it is as good a conductor as some of the metals. According to the kinetic theory of gases, the conductivity of a gas depends on molecular diffusion. Maxwell estimated the conductivity of air at ordinary temperatures at about 20,000 times less than that of copper. This has been verified experimentally by Kundt and Warburg, Stefan and Winkelmann, by taking special precautions to eliminate the effects of convection currents and radiation. It was for some time doubted whether a gas possessed any true conductivity for heat. The experiment of T. Andrews, repeated by Grove, and Magnus, showing that a wire heated by an electric current was raised to a higher temperature in air than in hydrogen, was explained by Tyndall as being due to the greater mobility of hydrogen which gave rise to stronger convection currents. In reality the effect is due chiefly to the greater velocity of motion of the ultimate molecules of hydrogen, and is most marked if molar (as opposed to molecular) convection is eliminated. Molecular convection or diffusion, which cannot be distinguished experimentally from conduction, as it follows the same law, is also the main cause of conduction of heat in liquids. Both in liquids and gases the effects of convection currents are so much greater than those of diffusion or conduction that the latter are very difficult to measure, and, except in special cases, comparatively unimportant as affecting the transference of heat. Owing to the difficulty of eliminating the effects of radiation and convection, the results obtained for the conductivities of liquids are somewhat discordant, and there is in most cases great uncertainty whether the conductivity increases or diminishes with rise of temperature. It would appear, however, that liquids, such as water and glycerin, differ remarkably little in conductivity in spite of enormous differences of viscosity. The viscosity of a liquid diminishes very rapidly with rise of temperature, without any marked change in the conductivity, whereas the viscosity of a gas increases with rise of temperature, and is always nearly proportional to the conductivity.
33. Difficulty of Quantitative Estimation of Heat Transmitted.—The conducting powers of different metals were compared by C. M. Despretz, and later by G. H. Wiedemann and R. Franz, employing an extension of the method of Jan Ingenhousz, in which the temperatures at different points along a bar heated at one end were measured by thermometers or thermocouples let into small holes in the bars, instead of being measured at one point only by means of melting wax. These experiments undoubtedly gave fairly accurate relative values, but did not permit the calculation of the absolute amounts of heat transmitted. This was first obtained by J. D. Forbes (Brit. Assoc. Rep., 1852; Trans. Roy. Soc. Ed., 1862, 23, p. 133) by deducing the amount of heat lost to the surrounding air from a separate experiment in which the rate of cooling of the bar was observed (see Conduction of Heat). Clément (Ann. chim. phys., 1841) had previously attempted to determine the conductivities of metals by observing the amount of heat transmitted by a plate with one side exposed to steam at 100° C., and the other side cooled by water at 28° C. Employing a copper plate 3 mm. thick, and assuming that the two surfaces of the plate were at the same temperatures as the water and the steam to which they were exposed, or that the temperature-gradient in the metal was 72° in 3 mm., he had thus obtained a value which we now know to be nearly 200 times too small. The actual temperature difference in the metal itself was really about 0.36° C. The remainder of the 72° drop was in the badly conducting films of water and steam close to the metal surface. Similarly in a boiler plate in contact with flame at 1500° C. on one side and water at, say, 150° C. on the other, the actual difference of temperature in the metal, even if it is an inch thick, is only a few degrees. The metal, unless badly furred with incrustation, is but little hotter than the water. It is immaterial so far as the transmission of heat is concerned, whether the plates are iron or copper. The greater part of the resistance to the passage of heat resides in a comparatively quiescent film of gas close to the surface, through which film the heat has to pass mainly by conduction. If a Bunsen flame, preferably coloured with sodium, is observed impinging on a cold metal plate, it will be seen to be separated from the plate by a dark space of a millimetre or less, throughout which the temperature of the gas is lowered by its own conductivity below the temperature of incandescence. There is no abrupt change of temperature in passing from the gas to the metal, but a continuous temperature-gradient from the temperature of the metal to that of the flame. It is true that this gradient may be upwards of 1000° C. per mm., but there is no discontinuity.
34. Resistance of a Gas Film to the Passage of Heat.—It is possible to make a rough estimate of the resistance of such a film to the passage of heat through it. Taking the average conductivity of the gas in the film as 10,000 times less than that of copper (about double the conductivity of air at ordinary temperatures) a millimetre film would be equivalent to a thickness of 10 metres of copper, or about 1.2 metres of iron. Taking the temperature-gradient as 1000° C. per mm. such a film would transmit 1 gramme-calorie per sq. cm. per sec., or 36,000 kilo-calories per sq. metre per hour. With an area of 100 sq. cms. the heat transmitted at this rate would raise a litre of water from 20° C. to 100° C. in 800 secs. By experiment with a strong Bunsen flame it takes from 8 to 10 minutes to do this, which would indicate that on the above assumptions the equivalent thickness of quiescent film should be rather less than 1 mm. in this case. The thickness of the film diminishes with the velocity of the burning gases impinging on the surface. This accounts for the rapidity of heating by a blowpipe flame, which is not due to any great increase in temperature of the flame as compared with a Bunsen. Similarly the efficiency of a boiler is but slightly reduced if half the tubes are stopped up, because the increase of draught through the remainder compensates partly for the diminished heating surface. Some resistance to the passage of heat into a boiler is also due to the water film on the inside. But this is of less account, because the conductivity of water is much greater than that of air, and because the film is continually broken up by the formation of steam, which abstracts heat very rapidly.
35. Heating by Condensation of Steam.—It is often stated that the rate at which steam will condense on a metal surface at a temperature below that corresponding to the saturation pressure of the steam is practically infinite (e.g. Osborne Reynolds, Proc. Roy. Soc. Ed., 1873, p. 275), and conversely that the rate at which water will abstract heat from a metal surface by the formation of steam (if the metal is above the temperature of saturation of the steam) is limited only by the rate at which the metal can supply heat by conduction to its surface layer. The rate at which heat can be supplied by condensation of steam appears to be much greater than that at which heat can be supplied by a flame under ordinary conditions, but there is no reason to suppose that it is infinite, or that any discontinuity exists. Experiments by H. L. Callendar and J. T. Nicolson by three independent methods (Proc. Inst. Civ. Eng., 1898, 131, p. 147; Brit. Assoc. Rep. p. 418) appear to show that the rate of abstraction of heat by evaporation, or that of communication of heat by condensation, depends chiefly on the difference of temperature between the metal surface and the saturated steam, and is nearly proportional to the temperature difference (not to the pressure difference, as suggested by Reynolds) for such ranges of pressure as are common in practice. The rate of heat transmission they observed was equivalent to about 8 calories per sq. cm. per sec., for a difference of 20° C. between the temperature of the metal surface and the saturation temperature of the steam. This would correspond to a condensation of 530 kilogrammes of steam at 100° C. per sq. metre per hour, or 109 ℔ per sq. ft. per hour for the same difference of temperature, values which are many times greater than those actually obtained in ordinary surface condensers. The reason for this is that there is generally some air mixed with the steam in a surface condenser, which greatly retards the condensation. It is also difficult to keep the temperature of the metal as much as 20° C. below the temperature of the steam unless a very free and copious circulation of cold water is available. For the same difference of temperature, steam can supply heat by condensation about a thousand times faster than hot air. This rate is not often approached in practice, but the facility of generation and transmission of steam, combined with its high latent heat and the accuracy of control and regulation of temperature afforded, render it one of the most convenient agents for the distribution of large quantities of heat in all kinds of manufacturing processes.
36. Spheroidal State.—An interesting contrast to the extreme rapidity with which heat is abstracted by the evaporation of a liquid in contact with a metal plate, is the so-called spheroidal state. A small drop of liquid thrown on a red-hot metal plate assumes a spheroidal form, and continues swimming about for some time, while it slowly evaporates at a temperature somewhat below its boiling-point. The explanation is simply that the liquid itself cannot come in actual contact with the metal plate (especially if the latter is above the critical temperature), but is separated from it by a badly conducting film of vapour, through which, as we have seen, the heat is comparatively slowly transmitted even if the difference of temperature is several hundred degrees. If the metal plate is allowed to cool gradually, the drop remains suspended on its cushion of vapour, until, in the case of water, a temperature of about 200° C. is reached, at which the liquid comes in contact with the plate and boils explosively, reducing the temperature of the plate, if thin, almost instantaneously to 100° C. The temperature of the metal is readily observed by a thermo-electric method, employing a platinum dish with a platinum-rhodium wire soldered with gold to its under side. The absence of contact between the liquid and the dish in the spheroidal state may also be shown by connecting one terminal of a galvanometer to the drop and the other through a battery to the dish, and observing that no current passes until the drop boils.
37. Early Theories of Radiation.—It was at one time supposed that there were three distinct kinds of radiation—thermal, luminous and actinic, combined in the radiation from a luminous source such as the sun or a flame. The first gave rise to heat, the second to light and the third to chemical action. The three kinds were partially separated by a prism, the actinic rays being generally more refracted, and the thermal rays less refracted than the luminous. This conception arose very naturally from the observation that the feebly luminous blue and violet rays produced the greatest photographic effects, which also showed the existence of dark rays beyond the violet, whereas the brilliant yellow and red were practically without action on the photographic plate. A thermometer placed in the blue or violet showed no appreciable rise of temperature, and even in the yellow the effect was hardly discernible. The effect increased rapidly as the light faded towards the extreme red, and reached a maximum beyond the extreme limits of the spectrum (Herschel), showing that the greater part of the thermal radiation was altogether non-luminous. It is now a commonplace that chemical action, colour sensation and heat are merely different effects of one and the same kind of radiation, the particular effect produced in each case depending on the frequency and intensity of the vibration, and on the nature of the substance on which it falls. When radiation is completely absorbed by a black substance, it is converted into heat, the quantity of heat produced being equivalent to the total energy of the radiation absorbed, irrespective of the colour or frequency of the different rays. The actinic or chemical effects, on the other hand, depend essentially on some relation between the period of the vibration and the properties of the substance acted on. The rays producing such effects are generally those which are most strongly absorbed. The spectrum of chlorophyll, the green colouring matter of plants, shows two very strong absorption bands in the red. The red rays of corresponding period are found to be the most active in promoting the growth of the plant. The chemically active rays are not necessarily the shortest. Even photographic plates may be made to respond to the red rays by staining them with pinachrome or some other suitable dye.
The action of light rays on the retina is closely analogous to the action on a photographic plate. The retina, like the plate, is sensitive only to rays within certain restricted limits of frequency. The limits of sensitiveness of each colour sensation are not exactly defined, but vary slightly from one individual to another, especially in cases of partial colour-blindness, and are modified by conditions of fatigue. We are not here concerned with these important physiological and chemical effects of radiation, but rather with the question of the conversion of energy of radiation into heat, and with the laws of emission and absorption of radiation in relation to temperature. We may here also assume the identity of visible and invisible radiations from a heated body in all their physical properties. It has been abundantly proved that the invisible rays, like the visible, (1) are propagated in straight lines in homogeneous media; (2) are reflected and diffused from the surface of bodies according to the same law; (3) travel with the same velocity in free space, but with slightly different velocities in denser media, being subject to the same law of refraction; (4) exhibit all the phenomena of diffraction and interference which are characteristic of wave-motion in general; (5) are capable of polarization and double refraction; (6) exhibit similar effects of selective absorption. These properties are more easily demonstrated in the case of visible rays on account of the great sensitiveness of the eye. But with the aid of the thermopile or other sensitive radiometer, they may be shown to belong equally to all the radiations from a heated body, even such as are thirty to fifty times slower in frequency than the longest visible rays. The same physical properties have also been shown to belong to electromagnetic waves excited by an electric discharge, whatever the frequency, thus including all kinds of aetherial radiation in the same category as light.
38. Theory of Exchanges.—The apparent concentration of cold by a concave mirror, observed by G. B. Porta and rediscovered by M. A. Pictet, led to the enunciation of the theory of exchanges by Pierre Prevost in 1791. Prevost’s leading idea was that all bodies, whether cold or hot, are constantly radiating heat. Heat equilibrium, he says, consists in an equality of exchange. When equilibrium is interfered with, it is re-established by inequalities of exchange. If into a locality at uniform temperature a refracting or reflecting body is introduced, it has no effect in the way of changing the temperature at any point of that locality. A reflecting body, heated or cooled in the interior of such an enclosure, will acquire the surrounding temperature more slowly than would a non-reflector, and will less affect another body placed at a little distance, but will not affect the final equality of temperature. Apparent radiation of cold, as from a block of ice to a thermometer placed near it, is due to the fact that the thermometer being at a higher temperature sends more heat to the ice than it received back from it. Although Prevost does not make the statement in so many words, it is clear that he regards the radiation from a body as depending only on its own nature and temperature, and as independent of the nature and presence of any adjacent body. Heat equilibrium in an enclosure of constant temperature such as is here postulated by Prevost, has often been regarded as a consequence of Carnot’s principle. Since difference of temperature is required for transforming heat into work, no work could be obtained from heat in such a system, and no spontaneous changes of temperature can take place, as any such changes might be utilized for the production of work. This line of reasoning does not appear quite satisfactory, because it is tacitly assumed, in the reasoning by which Carnot’s principle was established, as a result of universal experience, that a number of bodies within the same impervious enclosure, which contains no source of heat, will ultimately acquire the same temperature, and that difference of temperature is required to produce flow of heat. Thus although we may regard the equilibrium in such an enclosure as being due to equal exchanges of heat in all directions, the equal and opposite streams of radiation annul and neutralize each other in such a way that no actual transfer of energy in any direction takes place. The state of the medium is everywhere the same in such an enclosure, but its energy of agitation per unit volume is a function of the temperature, and is such that it would not be in equilibrium with any body at a different temperature.
39. ”Full” and Selective Radiation. Correspondence of Emission and Absorption.—The most obvious difficulties in the way of this theory arise from the fact that nearly all radiation is more or less selective in character, as regards the quality and frequency of the rays emitted and absorbed. It was shown by J. Leslie, M. Melloni and other experimentalists that many substances such as glass and water, which are very transparent to visible rays, are extremely opaque to much of the invisible radiation of lower frequency; and that polished metals, which are perfect reflectors, are very feeble radiators as compared with dull or black bodies at the same temperature. If two bodies emit rays of different periods in different proportions, it is not at first sight easy to see how their radiations can balance each other at the same temperature. The key to all such difficulties lies in the fundamental conception, so strongly insisted on by Balfour Stewart, of the absolute uniformity (qualitative as well as quantitative) of the full or complete radiation stream inside an impervious enclosure of uniform temperature. It follows from this conception that the proportion of the full radiation stream absorbed by any body in such an enclosure must be exactly compensated in quality as well as quantity by the proportion emitted, or that the emissive and absorptive powers of any body at a given temperature must be precisely equal. A good reflector, like a polished metal, must also be a feeble radiator and absorber. Of the incident radiation it absorbs a small fraction and reflects the remainder, which together with the radiation emitted (being precisely equal to that absorbed) makes up the full radiation stream. A partly transparent material, like glass, absorbs part of the full radiation and transmits part. But it emits rays precisely equal in quality and intensity to those which it absorbs, which together with the transmitted portion make up the full stream. The ideal black body or perfect radiator is a body which absorbs all the radiation incident on it. The rays emitted from such a body at any temperature must be equal to the full radiation stream in an isothermal enclosure at the same temperature. Lampblack, which may absorb between 98 to 99% of the incident radiation, is generally taken as the type of a black body. But a closer approximation to full radiation may be obtained by employing a hollow vessel the internal walls of which are blackened and maintained at a uniform temperature by a steam jacket or other suitable means. If a relatively small hole is made in the side of such a vessel, the radiation proceeding through the aperture will be the full radiation corresponding to the temperature. Such a vessel is also a perfect absorber. Of radiation entering through the aperture an infinitesimal fraction only could possibly emerge by successive reflection even if the sides were of polished metal internally. A thin platinum tube heated by an electric current appears feebly luminous as compared with a blackened tube at the same temperature. But if a small hole is made in the side of the polished tube, the light proceeding through the hole appears brighter than the blackened tube, as though the inside of the tube were much hotter than the outside, which is not the case to any appreciable extent if the tube is thin. The radiation proceeding through the hole is nearly that of a perfectly black body if the hole is small. If there were no hole the internal stream of radiation would be exactly that of a black body at the same temperature however perfect the reflecting power, or however low the emissive power of the walls, because the defect in emissive power would be exactly compensated by the internal reflection.
Balfour Stewart gave a number of striking illustrations of the qualitative identity of emission and absorption of a substance. Pieces of coloured glass placed in a fire appear to lose their colour when at the same temperature as the coals behind them, because they compensate exactly for their selective absorption by radiating chiefly those colours which they absorb. Rocksalt is remarkably transparent to thermal radiation of nearly all kinds, but it is extremely opaque to radiation from a heated plate of rocksalt, because it emits when heated precisely those rays which it absorbs. A plate of tourmaline cut parallel to the axis absorbs almost completely light polarized in a plane parallel to the axis, but transmits freely light polarized in a perpendicular plane. When heated its radiation is polarized in the same plane as the radiation which it absorbs. In the case of incandescent vapours, the exact correspondence of emission and absorption as regards wave-length of frequency of the light emitted and absorbed forms the foundation of the science of spectrum analysis. Fraunhofer had noticed the coincidence of a pair of bright yellow lines seen in the spectrum of a candle flame with the dark D lines in the solar spectrum, a coincidence which was afterwards more exactly verified by W. A. Miller. Foucault found that the flame of the electric arc showed the same lines bright in its spectrum, and proved that they appeared as dark lines in the otherwise continuous spectrum when the light from the carbon poles was transmitted through the arc. Stokes gave a dynamical explanation of the phenomenon and illustrated it by the analogous case of resonance in sound. Kirchhoff completed the explanation (Phil. Mag., 1860) of the dark lines in the solar spectrum by showing that the reversal of the spectral lines depended on the fact that the body of the sun giving the continuous spectrum was at a higher temperature than the absorbing layer of gases surrounding it. Whatever be the nature of the selective radiation from a body, the radiation of light of any particular wave-length cannot be greater than a certain fraction E of the radiation R of the same wave-length from a black body at the same temperature. The fraction E measures the emissive power of the body for that particular wave-length, and cannot be greater than unity. The same fraction, by the principle of equality of emissive and absorptive powers, will measure the proportion absorbed of incident radiation R′. If the black body emitting the radiation R′ is at the same temperature as the absorbing layer, R = R′, the emission balances the absorption, and the line will appear neither bright nor dark. If the source and the absorbing layer are at different temperatures, the radiation absorbed will be ER′, and that transmitted will be R′ − ER′. To this must be added the radiation emitted by the absorbing layer, namely ER, giving R′ − E(R′ − R). The lines will appear darker than the background R′ if R′ is greater than R, but bright if the reverse is the case. The D lines are dark in the sun because the photosphere is much hotter than the reversing layer. They appear bright in the candle-flame because the outside mantle of the flame, in which the sodium burns and combustion is complete, is hotter than the inner reducing flame containing the incandescent particles of carbon which give rise to the continuous spectrum. This qualitative identity of emission and absorption as regards wave-length can be most exactly and easily verified for luminous rays, and we are justified in assuming that the relation holds with the same exactitude for non-luminous rays, although in many cases the experimental proof is less complete and exact.
40. Diathermancy.—A great array of data with regard to the transmissive power or diathermancy of transparent substances for the heat radiated from various sources at different temperatures were collected by Melloni, Tyndall, Magnus and other experimentalists. The measurements were chiefly of a qualitative character, and were made by interposing between the source and a thermopile a layer or plate of the substance to be examined. This method lacked quantitative precision, but led to a number of striking and interesting results, which are admirably set forth in Tyndall’s Heat. It also gave rise to many curious discrepancies, some of which were recognized as being due to selective absorption, while others are probably to be explained by imperfections in the methods of experiment adopted. The general result of such researches was to show that substances, like water, alum and glass, which are practically opaque to radiation from a source at low temperature, such as a vessel filled with boiling water, transmit an increasing percentage of the radiation when the temperature of the source is increased. This is what would be expected, as these substances are very transparent to visible rays. That the proportion transmitted is not merely a question of the temperature of the source, but also of the quality of the radiation, was shown by a number of experiments. For instance, K. H. Knoblauch (Pogg. Ann., 1847) found that a plate of glass interposed between a spirit lamp and a thermopile intercepts a larger proportion of the radiation from the flame itself than of the radiation from a platinum spiral heated in the flame, although the spiral is undoubtedly at a lower temperature than the flame. The explanation is that the spiral is a fairly good radiator of the visible rays to which the glass is transparent, but a bad radiator of the invisible rays absorbed by the glass which constitute the greater portion of the heat-radiation from the feebly luminous flame.
 |
| Fig. 6.—Tyndall’s Apparatus for observing absorption of heat by gas and vapours. |
Assuming that the radiation from the source under investigation is qualitatively determinate, like that of a black body at a given temperature, the proportion transmitted by plates of various substances may easily be measured and tabulated for given plates and sources. But owing to the highly selective character of the radiation and absorption, it is impossible to give any general relation between the thickness of the absorbing plate or layer and the proportion of the total energy absorbed. For these reasons the relative diathermancies of different materials do not admit of any simple numerical statement as physical constants, though many of the qualitative results obtained are very striking. Among the most interesting experiments were those of Tyndall, on the absorptive powers of gases and vapours, which led to a good deal of controversy at the time, owing to the difficulty of the experiments, and the contradictory results obtained by other observers. The arrangement employed by Tyndall for these measurements is shown in Fig. 6. A brass tube AB, polished inside, and closed with plates of highly diathermanous rocksalt at either end, was fitted with stopcocks C and D for exhausting and admitting air or other gases or vapours. The source of heat S was usually a plate of copper heated by a Bunsen burner, or a Leslie cube containing boiling water as shown at E. To obtain greater sensitiveness for differential measurements, the radiation through the tube AB incident on one face of the pile P was balanced against the radiation from a Leslie cube on the other face of the pile by means of an adjustable screen H. The radiation on the two faces of the pile being thus balanced with the tube exhausted, Tyndall found that the admission of dry air into the tube produced practically no absorption of the radiation, whereas compound gases such as carbonic acid, ethylene or ammonia absorbed 20 to 90%, and a trace of aqueous vapour in the air increased its absorption 50 to 100 times. H. G. Magnus, on the other hand, employing a thermopile and a source of heat, both of which were enclosed in the same exhausted receiver, in order to avoid interposing any rocksalt or other plates between the source and the pile, found an absorption of 11% on admitting dry air, but could not detect any difference whether the air were dry or moist. Tyndall suggested that the apparent absorption observed by Magnus may have been due to the cooling of his radiating surface by convection, which is a very probable source of error in this method of experiment. Magnus considered that the remarkable effect of aqueous vapour observed by Tyndall might have been caused by condensation on the polished internal walls of his experimental tube, or on the rocksalt plates at either end.7 The question of the relative diathermancy of air and aqueous vapour for radiation from the sun to the earth and from the earth into space is one of great interest and importance in meteorology. Assuming with Magnus that at least 10% of the heat from a source at 100° C. is absorbed in passing through a single foot of air, a very moderate thickness of atmosphere should suffice to absorb practically all the heat radiated from the earth into space. This could not be reconciled with well-known facts in regard to terrestrial radiation, and it was generally recognized that the result found by Magnus must be erroneous. Tyndall’s experiment on the great diathermancy of dry air agreed much better with meteorological phenomena, but he appears to have exaggerated the effect of aqueous vapour. He concluded from his experiments that the water vapour present in the air absorbs at least 10% of the heat radiated from the earth within 10 ft. of its surface, and that the absorptive power of the vapour is about 17,000 times that of air at the same pressure. If the absorption of aqueous vapour were really of this order of magnitude, it would exert a far greater effect in modifying climate than is actually observed to be the case. Radiation is observed to take place freely through the atmosphere at times when the proportion of aqueous vapour is such as would practically stop all radiation if Tyndall’s results were correct. The very careful experiments of E. Lecher and J. Pernter (Phil. Mag., Jan. 1881) confirmed Tyndall’s observations on the absorptive powers of gases and vapours satisfactorily in nearly all cases with the single exception of aqueous vapour. They found that there was no appreciable absorption of heat from a source at 100° C. in passing through 1 ft. of air (whether dry or moist), but that CO and CO2 at atmospheric pressure absorbed about 8%, and ethylene (olefiant gas) about 50% in the same distance; the vapours of alcohol and ether showed absorptive powers of the same order as that of ethylene. They confirmed Tyndall’s important result that the absorption does not diminish in proportion to the pressure, being much greater in proportion for smaller pressures in consequence of the selective character of the effect. They also supported his conclusion that absorptive power increases with the complexity of the molecule. But they could not detect any absorption by water vapour at a pressure of 7 mm., though alcohol at the same pressure absorbed 3% and acetic acid 10%. Later researches, especially those of S. P. Langley with the spectro-bolometer on the infra-red spectrum of sunlight, demonstrated the existence of marked absorption bands, some of which are due to water vapour. From the character of these bands and the manner in which they vary with the state of the air and the thickness traversed, it may be inferred that absorption by water vapour plays an important part in meteorology, but that it is too small to be readily detected by laboratory experiments in a 4 ft. tube, without the aid of spectrum analysis.
41. Relation between Radiation and Temperature.—Assuming, in accordance with the reasoning of Balfour Stewart and Kirchhoff, that the radiation stream inside an impervious enclosure at a uniform temperature is independent of the nature of the walls of the enclosure, and is the same for all substances at the same temperature, it follows that the full stream of radiation in such an enclosure, or the radiation emitted by an ideal black body or full radiator, is a function of the temperature only. The form of this function may be determined experimentally by observing the radiation between two black bodies at different temperatures, which will be proportional to the difference of the full radiation streams corresponding to their several temperatures. The law now generally accepted was first proposed by Stefan as an empirical relation. Tyndall had found that the radiation from a white hot platinum wire at 1200° C. was 11.7 times its radiation when dull red at 525° C. Stefan (Wien. Akad. Ber., 1879, 79, p. 421) noticed that the ratio 11.7 is nearly that of the fourth power of the absolute temperatures as estimated by Tyndall. On making the somewhat different assumption that the radiation between two bodies varied as the difference of the fourth powers of their absolute temperatures, he found that it satisfied approximately the experiments of Dulong and Petit and other observers. According to this law the radiation between a black body at a temperature θ and a black enclosure or a black radiometer at a temperature θ0 should be proportional to (θ4 − θ04). The law was very simple and convenient in form, but it rested so far on very insecure foundations. The temperatures given by Tyndall were merely estimated from the colour of the light emitted, and might have been some hundred degrees in error. We now know that the radiation from polished platinum is of a highly selective character, and varies more nearly as the fifth power of the absolute temperature. The agreement of the fourth power law with Tyndall’s experiment appears therefore to be due to a purely accidental error in estimating the temperatures of the wire. Stefan also found a very fair agreement with Draper’s observations of the intensity of radiation from a platinum wire, in which the temperature of the wire was deduced from the expansion. Here again the apparent agreement was largely due to errors in estimating the temperature, arising from the fact that the coefficient of expansion of platinum increases considerably with rise of temperature. So far as the experimental results available at that time were concerned, Stefan’s law could be regarded only as an empirical expression of doubtful significance. But it received a much greater importance from theoretical investigations which were even then in progress. James Clerk Maxwell (Electricity and Magnetism, 1873) had shown that a directed beam of electromagnetic radiation or light incident normally on an absorbing surface should produce a mechanical pressure equal to the energy of the radiation per unit volume. A. G. Bartoli (1875) took up this idea and made it the basis of a thermodynamic treatment of radiation. P. N. Lebedew in 1900, and E. F. Nichols and G. F. Hull in 1901, proved the existence of this pressure by direct experiments. L. Boltzmann (1884) employing radiation as the working substance in a Carnot cycle, showed that the energy of full radiation at any temperature per unit volume should be proportional to the fourth power of the absolute temperature. This law was first verified in a satisfactory manner by Heinrich Schneebeli (Wied. Ann., 1884, 22, p. 30). He observed the radiation from the bulb of an air thermometer heated to known temperatures through a small aperture in the walls of the furnace. With this arrangement the radiation was very nearly that of a black body. Measurements by J. T. Bottomley, August Schleiermacher, L. C. H. F. Paschen and others of the radiation from electrically heated platinum, failed to give concordant results on account of differences in the quality of the radiation, the importance of which was not fully realized at first. Later researches by Paschen with improved methods verified the law, and greatly extended our knowledge of radiation in other directions. One of the most complete series of experiments on the relation between full radiation and temperature is that of O. R. Lummer and Ernst Pringsheim (Ann. Phys., 1897, 63, p. 395). They employed an aperture in the side of an enclosure at uniform temperature as the source of radiation, and compared the intensities at different temperatures by means of a bolometer. The fourth power law was well satisfied throughout the whole range of their experiments from −190° C. to 2300° C. According to this law, the rate of loss of heat by radiation R from a body of emissive power E and surface S at a temperature θ in an enclosure at θ0 is given by the formula
R = σES (θ4 − θ04),
where σ is the radiation constant. The absolute value of σ was determined by F. Kurlbaum using an electric compensation method (Wied. Ann., 1898, 65, p. 746), in which the radiation received by a bolometer from a black body at a known temperature was measured by finding the electric current required to produce the same rise of temperature in the bolometer. K. Ångstrom employed a similar method for solar radiation. Kurlbaum gives the value σ = 5.32 × 10−5 ergs per sq. cm. per sec. C. Christiansen (Wied. Ann., 1883, 19, p. 267) had previously found a value about 5% smaller, by observing the rate of cooling of a copper plate of known thermal capacity, which is probably a less accurate method.
42. Theoretical Proof of the Fourth Power Law.—The proof given by Boltzmann may be somewhat simplified if we observe that full radiation in an enclosure at constant temperature behaves exactly like a saturated vapour, and must therefore obey Carnot’s or Clapeyron’s equation given in section 17. The energy of radiation per unit volume, and the radiation-pressure at any temperature, are functions of the temperature only, like the pressure of a saturated vapour. If the volume of the enclosure is increased by any finite amount, the temperature remaining the same, radiation is given off from the walls so as to fill the space to the same pressure as before. The heat absorbed when the volume is increased corresponds with the latent heat of vaporization. In the case of radiation, as in the case of a vapour, the latent heat consists partly of internal energy of formation and partly of external work of expansion at constant pressure. Since in the case of full or undirected radiation the pressure is one-third of the energy per unit volume, the external work for any expansion is one-third of the internal energy added. The latent heat absorbed is, therefore, four times the external work of expansion. Since the external work is the product of the pressure P and the increase of volume V, the latent heat per unit increase of volume is four times the pressure. But by Carnot’s equation the latent heat of a saturated vapour per unit increase of volume is equal to the rate of increase of saturation-pressure per degree divided by Carnot’s function or multiplied by the absolute temperature. Expressed in symbols we have,
θ (dP/dθ) = L/V = 4P,
where (dP/dθ) represents the rate of increase of pressure. This equation shows that the percentage rate of increase of pressure is four times the percentage rate of increase of temperature, or that if the temperature is increased by 1%, the pressure is increased by 4%. This is equivalent to the statement that the pressure varies as the fourth power of the temperature, a result which is mathematically deduced by integrating the equation.
43. Wien’s Displacement Law.—Assuming that the fourth power law gives the quantity of full radiation at any temperature, it remains to determine how the quality of the radiation varies with the temperature, since as we have seen both quantity and quality are determinate. This question may be regarded as consisting of two parts. (1) How is the wave-length or frequency of any given kind of radiation changed when its temperature is altered? (2) What is the form of the curve expressing the distribution of energy between the various wave-lengths in the spectrum of full radiation, or what is the distribution of heat in the spectrum? The researches of Tyndall, Draper, Langley and other investigators had shown that while the energy of radiation of each frequency increased with rise of temperature, the maximum of intensity was shifted or displaced along the spectrum in the direction of shorter wave-lengths or higher frequencies. W. Wien (Ann. Phys., 1898, 58, p. 662), applying Doppler’s principle to the adiabatic compression of radiation in a perfectly reflecting enclosure, deduced that the wave-length of each constituent of the radiation should be shortened in proportion to the rise of temperature produced by the compression, in such a manner that the product λθ of wave-length and the absolute temperature should remain constant. According to this relation, which is known as Wien’s Displacement Law, the frequency corresponding to the maximum ordinate of the energy curve of the normal spectrum of full radiation should vary directly (or the wave-length inversely) as the absolute temperature, a result previously obtained by H. F. Weber (1888). Paschen, and Lummer and Pringsheim verified this relation by observing with a bolometer the intensity at different points in the spectrum produced by a fluorite prism. The intensities were corrected and reduced to a wave-length scale with the aid of Paschen’s results on the dispersion formula of fluorite (Wied. Ann., 1894, 53, p. 301). The curves in fig. 7 illustrate results obtained by Lummer and Pringsheim (Ber. deut. phys. Ges., 1899, 1, p. 34) at three different temperatures, namely 1377°, 1087° and 836° absolute, plotted on a wave-length base with a scale of microns (μ) or millionths of a metre. The wave-lengths Oa, Ob, Oc, corresponding to the maximum ordinates of each curve, vary inversely as the absolute temperatures given. The constant value of the product λθ at the maximum point is found to be 2920. Thus for a temperature of 1000° Abs. the maximum is at wave-length 2.92 μ; at 2000° the maximum is at 1.46 μ.
44. Form of the Curve representing the Distribution of Energy in the Spectrum.—Assuming Wien’s displacement law, it follows that the form of the curve representing the distribution of energy in the spectrum of full radiation should be the same for different temperatures with the maximum displaced in proportion to the absolute temperature, and with the total area increased in proportion to the fourth power of the absolute temperature. Observations taken with a bolometer along the length of a normal or wave-length spectrum, would give the form of the curve plotted on a wave-length base. The height of the ordinate at each point would represent the energy included between given limits of wave-length, depending on the width of the bolometer strip and the slit. Supposing that the bolometer strip had a width corresponding to .01 μ, and were placed at 1.0 μ in the spectrum of radiation at 2000° Abs., it would receive the energy corresponding to wave-lengths between 1.00 and 1.01 μ. At a temperature of 1000° Abs. the corresponding part of the energy, by Wien’s displacement law, would lie between the limits 2.00 and 2.02 μ, and the total energy between these limits would be 16 times smaller. But the bolometer strip placed at 2.0 μ would now receive only half of the energy, or the energy in a band .01 μ wide, and the deflection would be 32 times less. Corresponding ordinates of the curves at different temperatures will therefore vary as the fifth power of the temperature, when the curves are plotted on a wave-length base. The maximum ordinates in the curves already given are found to vary as the fifth powers of the corresponding temperatures. The equation representing the distribution of energy on a wave-length base must be of the form
E = Cλ−5F (λθ) = Cθ5 (λθ)−5F (λθ)
 |
| Fig. 7.—Distribution of energy in the spectrum of a black body. |
 |
| Fig. 8.—Distribution of energy in the spectrum of full radiation at 2000° Abs. according to formulae of Planck & Wien. |
where F (λθ) represents some function of the product of the wave-length and temperature, which remains constant for corresponding wave-lengths when θ is changed. If the curves were plotted on a frequency base, owing to the change of scale, the maximum ordinates would vary as the cube of the temperature instead of the fifth power, but the form of the function F would remain unaltered. Reasoning on the analogy of the distribution of velocities among the particles of a gas on the kinetic theory, which is a very similar problem, Wien was led to assume that the function F should be of the form e−c/λθ, where e is the base of Napierian logarithms, and c is a constant having the value 14,600 if the wave-length is measured in microns μ. This expression was found by Paschen to give a very good approximation to the form of the curve obtained experimentally for those portions of the visible and infra-red spectrum where observations could be most accurately made. The formula was tested in two ways: (1) by plotting the curves of distribution of energy in the spectrum for constant temperatures as illustrated in fig. 7; (2) by plotting the energy corresponding to a given wave-length as a function of the temperature. Both methods gave very good agreement with Wien’s formula for values of the product λθ not much exceeding 3000. A method of isolating rays of great wave-length by successive reflection was devised by H. Rubens and E. F. Nichols (Wied. Ann., 1897, 60, p. 418). They found that quartz and fluorite possessed the property of selective reflection for rays of wave-length 8.8μ and 24μ to 32μ respectively, so that after four to six reflections these rays could be isolated from a source at any temperature in a state of considerable purity. The residual impurity at any stage could be estimated by interposing a thin plate of quartz or fluorite which completely reflected or absorbed the residual rays, but allowed the impurity to pass. H. Beckmann, under the direction of Rubens, investigated the variation with temperature of the residual rays reflected from fluorite employing sources from −80° to 600° C., and found the results could not be represented by Wien’s formula unless the constant c were taken as 26,000 in place of 14,600. In their first series of observations extending to 6μ O. R. Lummer and E. Pringsheim (Deut. phys. Ges., 1899, 1, p. 34) found systematic deviations indicating an increase in the value of the constant c for long waves and high temperatures. In a theoretical discussion of the subject, Lord Rayleigh (Phil. Mag., 1900, 49, p. 539) pointed out that Wien’s law would lead to a limiting value Cλ−5, of the radiation corresponding to any particular wave-length when the temperature increased to infinity, whereas according to his view the radiation of great wave-length should ultimately increase in direct proportion to the temperature. Lummer and Pringsheim (Deut. phys. Ges., 1900, 2, p. 163) extended the range of their observations to 18 μ by employing a prism of sylvine in place of fluorite. They found deviations from Wien’s formula increasing to nearly 50% at 18μ, where, however, the observations were very difficult on account of the smallness of the energy to be measured. Rubens and F. Kurlbaum (Ann. Phys., 1901, 4, p. 649) extended the residual reflection method to a temperature range from −190° to 1500° C., and employed the rays reflected from quartz 8.8μ, and rocksalt 51μ, in addition to those from fluorite. It appeared from these researches that the rays of great wave-length from a source at a high temperature tended to vary in the limit directly as the absolute temperature of the source, as suggested by Lord Rayleigh, and could not be represented by Wien’s formula with any value of the constant c. The simplest type of formula satisfying the required conditions is that proposed by Max Planck (Ann. Phys., 1901, 4, p. 553) namely,
E = Cλ−5 (ec/λθ − 1)−1,
 |
| Fig. 9.—Variation of energy of radiation corresponding to wave-length 30μ, with temperature of source. |
which agrees with Wien’s formula when θ is small, where Wien’s formula is known to be satisfactory, but approaches the limiting form E = Cλ−4θ/c, when θ is large, thus satisfying the condition proposed by Lord Rayleigh. The theoretical interpretation of this formula remains to some extent a matter of future investigation, but it appears to satisfy experiment within the limits of observational error. In order to compare Planck’s formula graphically with Wien’s, the distribution curves corresponding to both formulae are plotted in fig. 8 for a temperature of 2000° abs., taking the value of the constant c = 14,600 with a scale of wave-length in microns μ. The curves in fig. 9 illustrate the difference between the two formulae for the variation of the intensity of radiation corresponding to a fixed wave-length 30μ. Assuming Wien’s displacement law, the curves may be applied to find the energy for any other wave-length or temperature, by simply altering the wave-length scale in inverse ratio to the temperature, or vice versa. Thus to find the distribution curve for 1000° abs., it is only necessary to multiply all the numbers in the wave-length scale of fig. 8 by 2; or to find the variation curve for wave-length 60μ, the numbers on the temperature scale of fig. 9 should be divided by 2. The ordinate scales must be increased in proportion to the fifth power of the temperature, or inversely as the fifth power of the wave-length respectively in figs. 8 and 9 if comparative results are required for different temperatures or wave-lengths. The results hitherto obtained for cases other than full radiation are not sufficiently simple and definite to admit of profitable discussion in the present article.
Bibliography.—It would not be possible, within the limits of an article like the present, to give tables of the specific thermal properties of different substances so far as they have been ascertained by experiment. To be of any use, such tables require to be extremely detailed, with very full references and explanations with regard to the value of the experimental evidence, and the limits within which the results may be relied on. The quantity of material available is so enormous and its value so varied, that the most elaborate tables still require reference to the original authorities. Much information will be found collected in Landolt and Bornstein’s Physical and Chemical Tables (Berlin, 1905). Shorter tables, such as Everett’s Units and Physical Constants, are useful as illustrations of a system, but are not sufficiently complete for use in scientific investigations. Some of the larger works of reference, such as A. A. Winkelmann’s Handbuch der Physik, contain fairly complete tables of specific properties, but these tables occupy so much space, and are so misleading if incomplete, that they are generally omitted in theoretical textbooks.
Among older textbooks on heat, Tyndall’s Heat may be recommended for its vivid popular interest, and Balfour Stewart’s Heat for early theories of radiation. Maxwell’s Theory of Heat and Tait’s Heat give a broad and philosophical survey of the subject. Among modern textbooks, Preston’s Theory of Heat and Poynting and Thomson’s Heat are the best known, and have been brought well up to date. Sections on heat are included in all the general textbooks of Physics, such as those of Deschanel (translated by Everett), Ganot (translated by Atkinson), Daniell, Watson, &c. Of the original investigations on the subject, the most important have already been cited. Others will be found in the collected papers of Joule, Kelvin and Maxwell. Treatises on special branches of the subject, such as Fourier’s Conduction of Heat, are referred to in the separate articles in this encyclopaedia dealing with recent progress, of which the following is a list: Calorimetry, Condensation of Gases, Conduction of Heat, Diffusion, Energetics, Fusion, Liquid Gases, Radiation, Radiometer, Solution, Thermodynamics, Thermoelectricity, Thermometry, Vaporization. For the practical aspects of heating see Heating.
1 Units of Work, Energy and Power.—In English-speaking countries work is generally measured in foot-pounds. Elsewhere it is generally measured in kilogrammetres, or in terms of the work done in raising 1 kilogramme weight through the height of 1 metre. In the middle of the 19th century the terms “force” and “motive power” were commonly employed in the sense of “power of doing work.” The term “energy” is now employed in this sense. A quantity of energy is measured by the work it is capable of performing. A body may possess energy in virtue of its state (gas or steam under pressure), or in virtue of its position (a raised weight), or in various other ways, when at rest. In these cases it is said to possess potential energy. It may also possess energy in virtue of its motion or rotation (as a fly-wheel or a cannon-ball). In this case it is said to possess kinetic energy, or energy of motion. In many cases the energy (as in the case of a vibrating body, like a pendulum) is partly kinetic and partly potential, and changes continually from one to the other throughout the motion. For instance, the energy of a pendulum is wholly potential when it is momentarily at rest at the top of its swing, but is wholly kinetic when the pendulum is moving with its maximum velocity at the lowest point of its swing. The whole energy at any moment is the sum of the potential and kinetic energy, and this sum remains constant so long as the amplitude of the vibration remains the same. The potential energy of a weight W ℔ raised to a height h ft. above the earth, is Wh foot-pounds. If allowed to fall freely, without doing work, its kinetic energy on reaching the earth would be Wh foot-pounds, and its velocity of motion would be such that if projected upwards with the same velocity it would rise to the height h from which it fell. We have here a simple and familiar case of the conversion of one kind of energy into a different kind. But the two kinds of energy are mechanically equivalent, and they can both be measured in terms of the same units. The units already considered, namely foot-pounds or kilogrammetres, are gravitational units, depending on the force of gravity. This is the most obvious and natural method of measuring the potential energy of a raised weight, but it has the disadvantage of varying with the force of gravity at different places. The natural measure of the kinetic energy of a moving body is the product of its mass by half the square of its velocity, which gives a measure in kinetic or absolute units independent of the force of gravity. Kinetic and gravitational units are merely different ways of measuring the same thing. Just as foot-pounds may be reduced to kilogrammetres by dividing by the number of foot-pounds in one kilogrammetre, so kinetic may be reduced to gravitational units by dividing by the kinetic measure of the intensity of gravity, namely, the work in kinetic units done by the weight of unit mass acting through unit distance. For scientific purposes, it is necessary to take account of the variation of gravity. The scientific unit of energy is called the erg. The erg is the kinetic energy of a mass of 2 gm. moving with a velocity of 1 cm. per sec. The work in ergs done by a force acting through a distance of 1 cm. is the absolute measure of the force. A force equal to the weight of 1 gm. (in England) acting through a distance of 1 cm. does 981 ergs of work. A force equal to the weight of 1000 gm. (1 kilogramme) acting through a distance of 1 metre (100 cm.) does 98.1 million ergs of work. As the erg is a very small unit, for many purposes, a unit equal to 10 million ergs, called a joule, is employed. In England, where the weight of 1 gm. is 981 ergs per cm., a foot-pound is equal to 1.356 joules, and a kilogrammetre is equal to 9.81 joules.
The term power is now generally restricted to mean “rate of working.” Watt estimated that an average horse was capable of raising 550 ℔ 1 ft. in each second, or doing work at the rate of 550 foot-pounds per second, or 33,000 foot-pounds per minute. This conventional horse-power is the unit commonly employed for estimating the power of engines. The horse-power-hour, or the work done by one horse-power in one hour, is nearly 2 million foot-pounds. For electrical and scientific purposes the unit of power employed is called the watt. The watt is the work per second done by an electromotive force of 1 volt in driving a current of 1 ampere, and is equal to 10 million ergs or 1 joule per second. One horse-power is 746 watts or nearly ¾ of a kilowatt. The kilowatt-hour, which is the unit by which electrical energy is sold, is 3.6 million joules or 2.65 million foot-pounds, or 366,000 kilogrammetres, and is capable of raising nearly 19 ℔ of water from the freezing to the boiling point.
2 In an essay on “Heat, Light, and Combinations of Light,” republished in Sir H. Davy’s Collected Works, ii. (London, 1836).
3 For instance a mass of compressed air, if allowed to expand in a cylinder at the ordinary temperature, will do work, and will at the same time absorb a quantity of heat which, as we now know, is the thermal equivalent of the work done. But this work cannot be said to have been produced solely from the heat absorbed in the process, because the air at the end of the process is in a changed condition, and could not be restored to its original state at the same temperature without having work done upon it precisely equal to that obtained by its expansion. The process could not be repeated indefinitely without a continual supply of compressed air. The source of the work in this case is work previously done in compressing the air, and no part of the work is really generated at the expense of heat alone, unless the compression is effected at a lower temperature than the expansion.
4 Clausius (Pogg. Ann. 79, p. 369) and others have misinterpreted this assumption, and have taken it to mean that the quantity of heat required to produce any given change of state is independent of the manner in which the change is effected, which Carnot does not here assume.
5 Carnot’s description of his cycle and statement of his principle have been given as nearly as possible in his own words, because some injustice has been done him by erroneous descriptions and statements.
6 It was for this reason that Professor W. Thomson (Lord Kelvin) stated (Phil. Mag., 1852, 4) that “Carnot’s original demonstration utterly fails,” and that he introduced the “corrections” attributed to James Thomson and Clerk Maxwell respectively. In reality Carnot’s original demonstration requires no correction.
7 In reference to this objection, Tyndall remarks (Phil. Mag., 1862, p. 422; Heat, p. 385); “In the first place the plate of salt nearest the source of heat is never moistened, unless the experiments are of the roughest character. Its proximity to the source enables the heat to chase away every trace of humidity from its surface.” He therefore took precautions to dry only the circumferential portions of the plate nearest the pile, assuming that the flux of heat through the central portions would suffice to keep them dry. This reasoning is not at all satisfactory, because rocksalt is very hygroscopic and becomes wet, even in unsaturated air, if the vapour pressure is greater than that of a saturated solution of salt at the temperature of the plate. Assuming that the vapour pressure of the saturated salt solution is only half that of pure water, it would require an elevation of temperature of 10° C. to dry the rocksalt plates in saturated air at 15° C. It is only fair to say that the laws of the vapour pressures of solutions were unknown in Tyndall’s time, and that it was usual to assume that the plates would not become wetted until the dew-point was reached. The writer has repeated Tyndall’s experiments with a facsimile of one of Tyndall’s tubes in the possession of the Royal College of Science, fitted with plates of rocksalt cut from the same block as Tyndall’s, and therefore of the same hygroscopic quality. Employing a reflecting galvanometer in conjunction with a differential bolometer, which is quicker in its action than Tyndall’s pile, there appears to be hardly any difference between dry and moist air, provided that the latter is not more than half saturated. Using saturated air with a Leslie cube as source of heat, both rocksalt plates invariably become wet in a minute or two and the absorption rises to 10 or 20% according to the thickness of the film of deposited moisture. Employing the open tube method as described by Tyndall, without the rocksalt plates, the absorption is certainly less than 1% in 3 ft. of air saturated at 20° C., unless condensation is induced on the walls of the tube. It is possible that the walls of Tyndall’s tube may have become covered with a very hygroscopic film from the powder of the calcium chloride which he was in the habit of introducing near one end. Such a film would be exceedingly difficult to remove, and would account for the excessive precautions which he found necessary in drying the air in order to obtain the same transmitting power as a vacuum. It is probable that Tyndall’s experiments on aqueous vapour were effected by experimental errors of this character.
↧ Download as ZWI file | Last modified: 11/17/2022 15:23:41 | 53 views
☰ Source: https://oldpedia.org/article/britannica11/Heat | License: Public domain in the USA. Project Gutenberg License
 ZWI signed:
ZWI signed: KSF
KSF