Chromium(Iii) Sulfide
 From Handwiki
From Handwiki 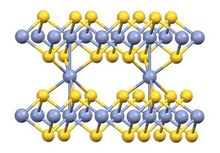
| |
| Identifiers | |
|---|---|
| EC Number |
|
PubChem CID
|
|
| Properties | |
| Cr2S3 | |
| Molar mass | 200.19 g/mol |
| Appearance | Brown to black powder |
| Odor | odorless |
| Density | 3.77 g/cm3 |
| Melting point | 1350 °C |
| insoluble | |
| +2390.0·10−6 cm3/mol | |
| Hazards | |
| Safety data sheet | [1] |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3[1] |
REL (Recommended)
|
TWA 0.5 mg/m3[1] |
IDLH (Immediate danger)
|
250 mg/m3[1] |
| Related compounds | |
Other anions
|
Chromium(III) oxide Chromium(III) telluride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chromium(III) sulfide is the inorganic compound with the formula Cr2S3. It is a brown-black solid. Chromium sulfides are usually nonstoichiometric compounds, with formulas ranging from CrS to Cr0.67S (corresponding to Cr2S3).
Preparation
Chromium(III) sulfide can be prepared through the reaction of a stoichiometric mixture of the elements at 1000 °C[2]
- [math]\displaystyle{ \mathrm{2 \ Cr + 3 \ S \longrightarrow Cr_2S_3} }[/math]
It is a solid that is insoluble in water. According to X-ray crystallography, its structure is a combination of that of nickel arsenide (1:1 stoichiometry) and Cd(OH)2 (1:2 stoichiometry). Some metal-metal bonding is indicated by the short Cr-Cr distance of 2.78 Å.[3]
See also
- Brezinaite, a mineral with the formula Cr3S4
References
- ↑ 1.0 1.1 1.2 NIOSH Pocket Guide to Chemical Hazards. "#0141". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0141.html.
- ↑ Georg Brauer: Handbuch der präparativen anorganischen Chemie. 3., umgearb. Auflage. Band III. Enke, Stuttgart 1981, ISBN:3-432-87823-0, S. 1493
- ↑ Jellinek, F. "The structures of the Chromium Sulphides" Acta Crystallographica 1957, volume 10, p620-p628 doi:10.1107/S0365110X57002200
 |
Categories: [Chromium(III) compounds] [Sesquisulfides]
↧ Download as ZWI file | Last modified: 03/05/2025 16:33:25 | 14 views
☰ Source: https://handwiki.org/wiki/Chemistry:Chromium(III)_sulfide | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]


 KSF
KSF