Birth Control Implant
 From Mdwiki
From Mdwiki
| Birth control implant | |
|---|---|
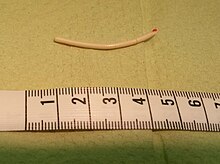
 Delivery device for Nexplanon, an example of an etonogestrel-based birth control implant | |
| Background | |
| Synonyms | Contraceptive implant |
| Trade names | Implanon, Nexplanon, Jadelle, others |
| Type | Long-acting reversible contraception[1] |
| First use | 1983[2] |
| Failure rates (first year) | |
| Perfect use | 0.1%[1] |
| Typical use | 0.1%[1] |
| Usage | |
| Duration effect | 3–5 years[1] |
| Reversibility | Yes[1] |
| User reminders | None[1] |
| Advantages and disadvantages | |
| STI protection | None[1] |
Birth control implant, also known as contraceptive implant, is a medical device placed under the skin for birth control.[3] Effectiveness begins after a week and lasts for at least 3 to 5 years, depending on the type.[1] Though can be removed sooner if pregnancy is desired.[3] About 1 in 1,000 women become pregnant over a year of use.[1] Other uses may include endometriosis and painful periods.[1]
Side effects are few, with no increased risk of blood clots or alteration in body weight.[1] Bruises or pain at the site may occur in a few percent.[1] Irregular vaginal bleeding may occur and periods may stop after a year in up to 20%.[1] They provide no protection again sexually transmitted infections.[1] Use is not recommended in those who have had breast cancer within the last 5 years.[1] It works by releasing hormones which prevent ovulation and blocks the opening of the cervix.[1]
Many types of healthcare providers are able to place the device.[1] Neither a pelvic exam nor pregnancy testing is required before use.[1] Placement is recommended in the subcutaneous tissue over the triceps muscle.[1] It may also be used in women of all weights (BMIs) and placed immediately following childbirth.[1] A number of medications may decrease effectiveness including carbamazapine and St. John's Wort.[1]
The birth control implant was first approved for medical use in Finland in 1983 and the United States in 1990.[2] They are used by about 25 million women globally as of 2020 and are the most common method of birth control at nearly 50% in Guinea-Bissau and Rwanda.[4] In the United States it may cost up to 1,300 USD as of 2024.[3] They differs from intrauterine devices (IUDs), which are placed in the uterus.[5]
Medical use[edit | edit source]
The contraceptive implant is hormone-based and highly effective, approved in more than 60 countries and used by millions of women around the world. The typical implant is a small flexible tube measuring about 40 mm (1.6 in) in length. It is most commonly inserted subdermally in the inner portion of the upper, non-dominant arm by a trained and certified health care provider.[6] After insertion, it prevents pregnancy by releasing progestin which inhibits ovulation.[6][7] The two most common versions are the single-rod etonogestrel implant and the two-rod levonorgestrel implant.[8]
-
Insertion of a contraceptive implant into a woman's arm
-
Removal of a contraceptive implant from a woman's arm
Benefits[edit | edit source]
Some brands of the contraceptive implant, including Nexplanon, are over 99% effective.[9] Benefits of the implant for some include fewer, lighter periods, improved symptoms of premenstrual syndrome, long-lasting up to three to five years, smoker- and breastfeeding-safe, and the convenience of not needing to remember to use it every day. The implant is also useful for women who cannot use contraception that contains oestrogen. The implant can also be removed at any time and natural fertility will return very quickly.[10]
Side effects[edit | edit source]
When the implant is first inserted, it is common to have some bruising, tenderness or swelling around the implant.[10] In some cases, adverse effects do occur, the most common being irregular bleeding or amenorrhea.[6] Although irregularity in bleeding can be troublesome for some women, this also allows for use in treatment of dysmenorrhea, menorrhagia, and endometriosis.[6] Less common symptoms include change in appetite, depression, moodiness, hormonal imbalance, sore breasts, weight gain, dizziness, pregnancy symptoms, and lethargy.[11][12] Although rare, there is also a risk of complications occurring during insertion or removal of the implant.[6] In rare cases, the area of skin where the implant has been inserted can become infected, which can require antibiotics.[10]
Most commonly reported from the levonorgestrel-releasing intrauterine system LNG-IUG contraceptive; breast tenderness, headaches, swelling, and skin irritation.[13] contraceptive also corresponds with earlier waking, frequent mood swings, impaired concentration, and strain.[13] Irregular vaginal uterus lining shedding is a common pattern with Norplant users; if this occurs it will be seen during the first 60 days of use but it can subside or disappear over time.[14] The Implanon also has these negative side effects causing a considerable amount of vaginal bleeding irregularities, and amenorrhea in about 30–40% of its users during the following 90 days of starting use.[14]
After delivery[edit | edit source]
With regard to helping women space their pregnancies appropriately, there is some debate about the most effective time to insert contraceptive implants after pregnancy. However, there may be little or no difference between immediate and delayed insertion in terms of continued use of implants at six months or in terms of women's satisfaction.[15] Progestin containing implants (specifically etonogestrel) are safe for immediate insertion in both postpartum individuals and those post-abortion.[6]
Society and culture[edit | edit source]
Brands include: Norplant, Jadelle (Norplant II), Implanon, Nexplanon, Sino-implant (II), Zarin, Femplant and Trust.
Research[edit | edit source]
Several barriers exist to expanding research into implantable and other contraceptive methods for men, including vague regulatory guidelines, long device development timelines, men's attitudes towards convenience, and a lack of funding.[16][17][18] Several implantable devices have been attempted, both hormonal and non-hormonal.
In 2001, Dutch pharmaceutical company Organon announced clinical trials of its implantable etonogestrel-based male contraceptive would begin in Europe and the U.S., anticipating a marketable product as early as 2005.[19][20] Despite promising results, research development stopped, with outside speculation that lack of marketability was a factor. Organon representative Monique Mols stated in 2007 that "[d]espite 20 years of research, the development of a [hormonal] method acceptable to a wide population of men is unlikely".[21] Schering/Bayer had been working on a similar annual implant with quarterly injections but cancelled the research in 2006/2007,[21] declaring that men would most likely view it as "not as convenient as a woman taking a pill once a day."[18]
In 2005, a collaboratory project led by the Population Council, the University of California, Los Angeles, and the Medical Research Council began researching a matchstick-sized implant that contains MENT (7α-methyl-19-nortestosterone or trestolone), a "synthetic steroid that resembles testosterone."[22] Clinical trials were set to begin in 2011 or 2012,[16] and the project was ongoing as of 2016, with hopes of gaining approval as the first reversible male contraceptive.[22]
In 2006, Shepherd Medical Company received FDA approval for a clinical trial of its non-hormonal implant called an intra vas device (IVD), which consists of two plugs that block sperm flow in the vas deferens. Working on the success of its pilot study and solid results from its clinical trials, the company announced it would expand its trials to three U.S. cities later that year. Questions remained about how reversible the procedure would be in the long-term; however, it was expected to be more reversible than a vasectomy. In 2008, the company disbanded due to the economic crisis but has stated it would restart its research with proper funding.[23][24][25]
In January 2016, news broke of a non-hormonal, implantable valve—the Bimek SLV. It included a switch that attaches to the vas deferens, allowing the owner to stop and resume the flow of sperm on demand. A clinical trial of 25 participants was announced to further test the efficacy of the device.[26][27]
Other animals[edit | edit source]
Implantable contraception is also an option for animals, particularly for animal managers at zoos and other captive animal facilities who require reversible contraception methods for managing population growth in limited captive habitat.[28] The Association of Zoos and Aquariums' (AZA) Reproductive Management Center (formerly known as the AZA Wildlife Contraception Center) at the Saint Louis Zoo in St. Louis, Missouri, has played a major role in researching and disseminating contraception information, via its Contraception Database. It houses over 30,000 records for hundreds of species.[28][29] One of the most popular contraceptive methods used by zoos (as well as in domestic animals) is the melengestrol acetate (MGA) implant, a progestin-based hormonal contraceptive developed in the mid-1970s. Other progestin-based implants that have been placed in animals include Norplant, Jadelle, and Implanon. Androgen-based implants that use agonist (stimulating) gonadotropin-releasing hormone (GnRH) and, to a lesser degree, IUDs have also seen use in several domestic and exotic species. Whatever the implant, some care must be taken to minimize the risk of implant migration or loss.[28][30][31]
References[edit | edit source]
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 Cason, Patty; Cwiak, Carrie; Kowal, Deborah; Edelman, Alison (26 September 2023). Contraceptive Technology (22 ed.). Jones & Bartlett Learning. p. 265. ISBN 978-1-284-25503-4.
- ↑ 2.0 2.1 Hohmann, H; Creinin, MD (December 2007). "The contraceptive implant". Clinical obstetrics and gynecology. 50 (4): 907–17. doi:10.1097/GRF.0b013e318159c2f6. PMID 17982333.
- ↑ 3.0 3.1 3.2 "Birth Control Implants | Nexplanon Information". www.plannedparenthood.org. Retrieved 9 April 2024.
- ↑ World Family Planning 2022 (PDF). United Nations. 2022. ISBN 9789211483765. Archived (PDF) from the original on 7 December 2023. Retrieved 22 March 2024.
- ↑ "IUD and Implant: Set It and Don't Sweat It Birth Control". www.plannedparenthood.org. Retrieved 9 April 2024.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Graves, Gillian. "Contraception". login.uml.idm.oclc.org. Retrieved 2021-11-21.
- ↑ "Birth Control Pill (for Teens) - Nemours KidsHealth". kidshealth.org. Retrieved 2022-09-21.
- ↑ French, V.A.; Darney, P.D. (2015). "Chapter 9: Implantable Contraception". In Shoupe, D.; Mishell Jr., D.R. (eds.). The Handbook of Contraception: A Guide for Practical Management (2nd ed.). Humana Press. pp. 139–164. ISBN 9783319201856. Retrieved 17 March 2016.
- ↑ "HOW EFFECTIVE IS NEXPLANON?". nexplanon.com. Retrieved 2023-08-24.
- ↑ 10.0 10.1 10.2 "Contraceptive implant". nhs.uk. 21 December 2017. Retrieved 2023-08-24.
 Text was copied from this source, which is available under an Open Government Licence v3.0. © Crown copyright.
Text was copied from this source, which is available under an Open Government Licence v3.0. © Crown copyright.
- ↑ "Birth Control Methods - Implant". Bedsider.org. National Campaign to Prevent Teen and Unplanned Pregnancy. February 2016. Retrieved 17 March 2016.
- ↑ "What is the Effectiveness of the Birth Control Implant?". www.plannedparenthood.org. Retrieved 2021-12-03.
- ↑ 13.0 13.1 Toffol, E.; Heikinheimo, O.; Koponen, P.; Luoto, R.; Partonen, T. (2011-11-01). "Hormonal contraception and mental health: results of a population-based study". Human Reproduction. 26 (11): 3085–3093. doi:10.1093/humrep/der269. ISSN 0268-1161. PMID 21840911.
- ↑ 14.0 14.1 Ramdhan, Rebecca C; Simonds, Emily; Wilson, Charlotte; Loukas, Marios; Oskouian, Rod J; Tubbs, R. Shane (2018-01-31). "Complications of Subcutaneous Contraception: A Review". Cureus. 10 (1): e2132. doi:10.7759/cureus.2132. ISSN 2168-8184. PMC 5878093. PMID 29610715.
- ↑ Sothornwit, Jen; Kaewrudee, Srinaree; Lumbiganon, Pisake; Pattanittum, Porjai; Averbach, Sarah H. (2022-10-27). "Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception". The Cochrane Database of Systematic Reviews. 2022 (10): CD011913. doi:10.1002/14651858.CD011913.pub3. ISSN 1469-493X. PMC 9612833. PMID 36302159.
{{cite journal}}: Check|pmc=value (help) - ↑ 16.0 16.1 Bai, N. (14 June 2011). "Beyond Condoms: The Long Quest for a Better Male Contraceptive". Scientific American. Nature America, Inc. Retrieved 17 March 2016.
- ↑ Fawcett, K. (26 February 2015). "The Future of Male Birth Control". U.S. News & World Report. U.S. News & World Report LP. Retrieved 17 March 2016.
- ↑ 18.0 18.1 Khazan, O. (March 2015). "Block That Sperm!". The Atlantic. The Atlantic Monthly Group. Retrieved 17 March 2016.
- ↑ "Male Contraceptive Implant Gets Trial Run". abcnews.go.com. ABC News Internet Ventures. 11 July 2001. Retrieved 17 March 2016.
- ↑ Jones, N. (11 July 2001). "Contraceptive implant for men in trials". New Scientist. Reed Business Information Ltd. Retrieved 17 March 2016.
- ↑ 21.0 21.1 Goodman, A. (3 August 2008). "The Long Wait for Male Birth Control". Time. Time, Inc. Retrieved 17 March 2016.
- ↑ 22.0 22.1 "MENT: Subdermal Implants for Men". PopCouncil.org. The Population Council, Inc. Archived from the original on 30 December 2017. Retrieved 17 March 2016.
- ↑ "New male contraceptive targets sperm, not hormones". EurekAlert!. American Association for the Advancement of Science. 5 May 2006. Retrieved 17 March 2016.
- ↑ "Male contraceptive study expands to 4 US cities". Psych Central. October 2006. Retrieved 17 March 2016.
- ↑ "Shug Product Report" (PDF). Calliope, The Contraceptive Pipeline Database. Contraceptive Technology Innovation Exchange. 21 May 2015. Retrieved 17 March 2016.
- ↑ Atkin, C. (8 January 2016). "The male contraception that makes you infertile by flicking a switch". The Independent. Independent Digital News and Media Limited. Retrieved 17 March 2016.
- ↑ "FAQ - Science and Research". PES Innovation AG. Retrieved 17 March 2016.
- ↑ 28.0 28.1 28.2 Asa, C.S.; Porton, I.J. (2010). "Chapter 34: Contraception as a Management Tool for Controlling Surplus Animals". In Kleiman, D.G.; Thompson, K.V.; Baer, C.K. (eds.). Wild Mammals in Captivity: Principles and Techniques for Zoo Management (2nd ed.). Chicago, IL: University of Chicago Press. pp. 469–482. ISBN 9780226440118. Retrieved 17 March 2016.
- ↑ "AZA Reproductive Management Center". STLZoo.org. Saint Louis Zoo. Retrieved 17 March 2016.
- ↑ "AZA Reproductive Management Center - Contraception Methods". STLZoo.org. Saint Louis Zoo. Retrieved 17 March 2016.
- ↑ Concannon, P.W. (2013). "Chapter 215: Estrus Suppression in the Bitch". In Bonagura, J.D.; Twedt, D.C. (eds.). Kirk's Current Veterinary Therapy XV. Elsevier Health Sciences. pp. 984–989. ISBN 9780323227629. Retrieved 17 March 2016.
External links[edit | edit source]
Categories: [Articles containing video clips] [Birth control] [Implants (medicine)] [Methods of birth control] [RTT] [WHRTT]
↧ Download as ZWI file | Last modified: 04/10/2024 15:32:02 | 3 views
☰ Source: https://mdwiki.org/wiki/Birth_control_implant | License: CC BY-SA 3.0
 KSF
KSF