Oxygen Difluoride
 From Handwiki
From Handwiki 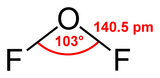
| |
| |
| Names | |
|---|---|
| IUPAC name
Oxygen difluoride
| |
Other names
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
OF 2 |
| Molar mass | 53.9962 g/mol |
| Appearance | colorless gas, pale yellow liquid when condensed |
| Odor | peculiar, foul |
| Density |
|
| Melting point | −223.8 °C (−370.8 °F; 49.3 K) |
| Boiling point | −144.75 °C (−228.55 °F; 128.40 K) |
Solubility in water
|
hydrolyzes[1] slowly |
| Vapor pressure | 48.9 atm (at −58.0 °C or −72.4 °F or 215.2 K[lower-alpha 1]) |
| Thermochemistry | |
Heat capacity (C)
|
43.3 J/mol K |
Std molar
entropy (S |
246.98 J/mol K |
Std enthalpy of
formation (ΔfH⦵298) |
−24.5 kJ mol−1 |
Gibbs free energy (ΔfG˚)
|
42.5 kJ/mol |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | danger |
GHS precautionary statements
|
Template:PPhrases |
| NFPA 704 (fire diamond) | 
0
4
3 OX |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
|
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.05 ppm (0.1 mg/m3)[2] |
REL (Recommended)
|
C 0.05 ppm (0.1 mg/m3)[2] |
IDLH (Immediate danger)
|
0.5 ppm[2] |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
- SizeSet
Oxygen difluoride is a chemical compound with the formula OF
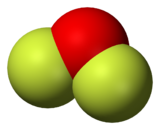
2. As predicted by VSEPR theory, the molecule adopts a bent molecular geometry. It is a strong oxidizer and has attracted attention in rocketry for this reason.[5] With a boiling point of −144.75 °C, OF2 is the most volatile (isolable) triatomic compound.[6] The compound is one of many known oxygen fluorides.
Preparation
Oxygen difluoride was first reported in 1929; it was obtained by the electrolysis of molten potassium fluoride and hydrofluoric acid containing small quantities of water.[7][8] The modern preparation entails the reaction of fluorine with a dilute aqueous solution of sodium hydroxide, with sodium fluoride as a side-product:
- 2 F
2 + 2 NaOH → OF
2 + 2 NaF + H
2O
Structure and bonding
It is a covalently bonded molecule with a bent molecular geometry and a F-O-F bond angle of 103 degrees. Its powerful oxidizing properties are suggested by the oxidation number of +2 for the oxygen atom instead of its normal −2.
Reactions
Above 200 °C, OF
2 decomposes to oxygen and fluorine by a radical mechanism.
- 2 OF
2 → O
2 + 2 F
2
OF
2 reacts with many metals to yield oxides and fluorides. Nonmetals also react: phosphorus reacts with OF
2 to form PF
5 and POF
3; sulfur gives SO
2 and SF
4; and unusually for a noble gas, xenon reacts (at elevated temperatures) yielding XeF
4 and xenon oxyfluorides.
Oxygen difluoride reacts very slowly with water to form hydrofluoric acid:
- OF
2 + H
2O → 2 HF + O
2
It can oxidize sulphur dioxide to sulfur trioxide and elemental fluorine:
- OF
2 + SO
2 → SO
3 + F
2
However, in the presence of UV radiation, the products are sulfuryl fluoride (SO
2F
2) and pyrosulfuryl fluoride (S
2O
5F
2):
- OF
2 + 2 SO
2 → S
2O
5F
2
Safety
Oxygen difluoride is considered an unsafe gas due to its oxidizing properties. Hydrofluoric acid produced by the hydrolysis of OF
2 with water is highly corrosive and toxic, capable of causing necrosis, leaching calcium from the bones and causing cardiovascular damage, among a host of other highly toxic effects.
Popular culture
In Robert L. Forward's science fiction novel Camelot 30K, oxygen difluoride was used as a biochemical solvent by fictional life forms living in the solar system's Kuiper belt. While OF
2 would be a solid at 30 K, the fictional alien lifeforms were described as endothermic, maintaining elevated body temperatures and liquid OF
2 blood by radiothermal heating.
Notes
- ↑ This is its critical temperature, which is below ordinary room temperature.
References
- ↑ "difluorine monoxide; oxygen difluoride, physical properties, suppliers, CAS, MSDS, structure, Molecular Formula, Molecular Weight, Solubility, boiling point, melting point". http://www.chemyq.com/En/xz/xz1/2818mqnrv.htm.
- ↑ 2.0 2.1 2.2 NIOSH Pocket Guide to Chemical Hazards. "#0475". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0475.html.
- ↑ "Oxygen difluoride". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/7783417.html.
- ↑ GHS: GESTIS 570242
- ↑ "Oxygen Difluoride - an overview | ScienceDirect Topics". https://www.sciencedirect.com/topics/chemistry/oxygen-difluoride.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 819. ISBN 978-0-08-037941-8.
- ↑ Lebeau, P.; Damiens, A. (1929). "Sur un nouveau mode de préparation du fluorure d'oxygène" (in fr). Comptes rendus hebdomadaires des séances de l'Académie des Sciences 188: 1253–1255. http://visualiseur.bnf.fr/CadresFenetre?O=NUMM-3141&I=1253&M=chemindefer. Retrieved February 21, 2013.
- ↑ Lebeau, P.; Damiens, A. (1927). "Sur l'existence d'un composé oxygéné du fluor" (in fr). Comptes rendus hebdomadaires des séances de l'Académie des Sciences 185: 652–654. http://visualiseur.bnf.fr/CadresFenetre?O=NUMM-3138&I=652&M=tdm. Retrieved February 21, 2013.
External links
- National Pollutant Inventory - Fluoride and compounds fact sheet
- WebBook page for OF
2 - CDC - NIOSH Pocket Guide to Chemical Hazards
 |
Categories: [Rocket oxidizers]
↧ Download as ZWI file | Last modified: 11/07/2025 15:48:24 | 11 views
☰ Source: https://handwiki.org/wiki/Chemistry:Oxygen_difluoride | License: CC BY-SA 3.0

 KSF
KSF