Fungi
 From Britannica 11th Edition (1911)
From Britannica 11th Edition (1911) Fungi (pl. of Lat. fungus, a mushroom), the botanical name covering in the broad sense all the lower cellular Cryptogams devoid of chlorophyll, which arise from spores, and the thallus of which is either unicellular or composed of branched or unbranched tubes or cell-filaments (hyphae) with apical growth, or of more or less complex wefted sheets or tissue-like masses of such (mycelium). The latter may in certain cases attain large dimensions, and even undergo cell-divisions in their interior, resulting in the development of true tissues. The spores, which may be uni- or multicellular, are either abstricted free from the ends of hyphae (acrogenous), or formed from segments in their course (chlamydospores) or from protoplasm in their interior (endogenous). The want of chlorophyll restricts their mode of life—which is rarely aquatic—since they are therefore unable to decompose the carbon dioxide of the atmosphere, and renders them dependent on other plants or (rarely) animals for their carbonaceous food-materials. These they obtain usually in the form of carbohydrates from the dead remains of other organisms, or in this or other forms from the living cells of their hosts; in the former case they are termed saprophytes, in the latter parasites. While some moulds (Penicillium, Aspergillus) can utilize almost any organic food-materials, other fungi are more restricted in their choice—e.g. insect-parasites, horn- and feather-destroying fungi and parasites generally. It was formerly the custom to include with the Fungi the Schizomycetes or Bacteria, and the Myxomycetes or Mycetozoa; but the peculiar mode of growth and division, the cilia, spores and other peculiarities of the former, and the emission of naked amoeboid masses of protoplasm, which creep and fuse to streaming plasmodia, with special modes of nutrition and spore-formation of the latter, have led to their separation as groups of organisms independent of the true Fungi. On the other hand, lichens, previously regarded as autonomous plants, are now known to be dual organisms—fungi symbiotic with algae.
The number of species in 1889 was estimated by Saccardo at about 32,000, but of these 8500 were so-called Fungi imperfecti—i.e. forms of which we only know certain stages, such as conidia, pycnidia, &c., and which there are reasons for regarding as merely the corresponding stages of higher forms. Saccardo also included about 400 species of Myxomycetes and 650 of Schizomycetes. Allowing for these and for the cases, undoubtedly not few, where one and the same fungus has been described under different names, we obtain Schroeter’s estimate (in 1892) of 20,000 species. In illustration of the very different estimates that have been made, however, may be mentioned that of De Bary in 1872 of 150,000 species, and that of Cooke in 1895 of 40,000, and Massee in 1899 of over 50,000 species, the fact being that no sufficient data are as yet to hand for any accurate census. As regards their geographical distribution, fungi, like flowering plants, have no doubt their centres of origin and of dispersal; but we must not forget that every exchange of wood, wheat, fruits, plants, animals, or other commodities involves transmission of fungi from one country to another; while the migrations of birds and other animals, currents of air and water, and so forth, are particularly efficacious in transmitting these minute organisms. Against this, of course, it may be argued that parasitic forms can only go where their hosts grow, as is proved to be the case by records concerning the introduction of Puccinia malvacearum, Peronospora viticola, Hemileia vastatrix, &c. Some fungi—e.g. moulds and yeasts—appear to be distributed all over the earth. That the north temperate regions appear richest in fungi may be due only to the fact that North America and Europe have been much more thoroughly investigated than other countries; it is certain that the tropics are the home of very numerous species. Again, the accuracy of the statement that the fleshy Agaricini, Polyporei, Pezizae, &c., are relatively rarer in the tropics may depend on the fact that they are more difficult to collect and remit for identification than the abundantly recorded woody and coriaceous forms of these regions. When we remember that many parts of the world are practically unexplored as regards fungi, and that new species are constantly being discovered in the United States, Australia and northern Europe—the best explored of all—it is clear that no very accurate census of fungi can as yet be made, and no generalizations of value as to their geographical distribution are possible.
The existence of fossil fungi is undoubted, though very few of the identifications can be relied on as regards species or genera. They extend back beyond the Carboniferous, where they occur as hyphae, &c., preserved in the fossil woods, but the best specimens are probably those in amber and in siliceous petrifactions of more recent origin.
 |
| Fig. 1.—1, Peronospora parasitica (De Bary). Mycelium with haustoria (h); 2, Erysiphe; A and B, mycelium (m), with haustoria (h). (After De Bary.) |
Organs.—Individual hyphae or their branches often exhibit specializations of form. In many Basidiomycetes minute branches arise below the septa; their tips curve over the outside of the latter, and fuse with the cell above just beyond it, forming a clamp-connexion. Many parasitic hyphae put out minute lateral branches, which pierce the cell-wall of the host and form a peg-like (Trichosphaeria), sessile (Cystopus), or stalked (Hemileia), knot-like, or a more or less branched (Peronospora) or coiled (Protomyces) haustorium. In Rhizopus certain hyphae creep horizontally on the surface of the substratum, and then anchor their tips to it by means of a tuft of short branches (appressorium), the walls of which soften and gum themselves to it, then another branch shoots out from the tuft and repeats the process, like a strawberry-runner. Appressoria are also formed by some parasitic fungi, as a minute flattening of the tip of a very short branch (Erysiphe), or the swollen end of any hypha which comes in contact with the surface of the host (Piptocephalis, Syncephalis), haustoria piercing in each case the cell-wall below. In Botrytis the appressoria assume the form of dense tassels of short branches. In Arthrobotrys side-branches of the mycelium sling themselves around the host (Tylenchus) much as tendrils round a support.
Many fungi (Phallus, Agaricus, Fumago, &c.) when strongly growing put out ribbon-like or cylindrical cords, or sheet-like mycelial plates of numerous parallel hyphae, all growing together equally, and fusing by anastomoses, and in this way extend long distances in the soil, or over the surfaces of leaves, branches, &c. These mycelial strands may be white and tender, or the outer hyphae may be hard and black, and very often the resemblance of the subterranean forms to a root is so marked that they are termed rhizomorphs. The outermost hyphae may even put forth thinner hyphae, radiating into the soil like root-hairs, and the convergent tips may be closely appressed and so divided by septa as to resemble the root-apex of a higher plant (Armillaria mellea).
Sclerotia.—Fungi, like other plants, are often found to store up large quantities of reserve materials (oil, glycogen, carbohydrates, &c.) in special parts of their vegetative tissues, where they lie accumulated between a period of active assimilation and one of renewed activity, forming reserves to be consumed particularly during the formation of large fructifications. These reserve stores may be packed away in single hyphae or in swollen cells, but the hyphae containing them are often gathered into thick cords or mycelial strands (Phallus, mushroom, &c.), or flattened and anastomosing ribbons and plates, often containing several kinds of hyphae (Merulius lacrymans). In other cases the strands undergo differentiation into an outer layer with blackened, hardened cell-walls and a core of ordinary hyphae, and are then termed rhizomorphs (Armillaria mellea), capable not only of extending the fungus in the soil, like roots, but also of lying dormant, protected by the outer casing. Such aggregations of hyphae frequently become knotted up into dense masses of interwoven and closely packed hyphae, varying in size from that of a pin’s head or a pea (Peziza, Coprinus) to that of a man’s fist or head, and weighing 10 to 25 ℔ or more (Polyporus Mylittae, P. tumulosus, Lentinus Woermanni, P. Sapurema, &c.). The interwoven hyphae fuse and branch copiously, filling up all interstices. They also undergo cutting up by numerous septa into short cells, and these often divide again in all planes, so that a pseudoparenchyma results, the walls of which may be thickened and swollen internally, or hardened and black on the exterior. In many cases the swollen cell-walls serve as reserves, and sometimes the substance is so thickly deposited in strata as to obliterate the lumen, and the hyphae become nodular (Polyporus sacer, P. rhinoceros, Lentinus Woermanni). The various sclerotia, if kept moist, give rise to the fructifications of the fungi concerned, much as a potato tuber does to a potato plant, and in the same way the reserve materials are consumed. They are principally Polyporei, Agaricini, Pezizae; none are known among the Phycomycetes, Uredineae or Ustilagineae. The functions of mycelial strands, rhizomorphs and sclerotia are not only to collect and store materials, but also to extend the fungus, and in many cases similar strands act as organs of attack. The same functions of storage in advance of fructification are also exercised by the stromata so common in Ascomycetes.
Tissue Differentiations.—The simpler mycelia consist of hyphae all alike and thin-walled, or merely differing in the diameter of the branches of various orders, or in their relations to the environment, some plunging into the substratum like roots, others remaining on its surface, and others (aerial hyphae) rising into the air. Such hyphae may be multicellular, or they may consist of simple tubes with numerous nuclei and no septa (Phycomycetes), and are then non-cellular. In the more complex tissue-bodies of higher fungi, however, we find considerable differences in the various layers or strands of hyphae.
An epidermis-like or cortical protective outer layer is very common, and is usually characterized by the close septation of the densely interwoven hyphae and the thickening and dark colour of their outer walls (sclerotia, Xylaria, &c.). Fibre-like hyphae with the lumen almost obliterated by the thick walls occur in mycelial cords (Merulius). Latex-tubes abound in the tissues of Lactarius, Stereum, Mycena, Fistulina, filled with white or coloured milky fluids, and Istvanffvi has shown that similar tubes with fluid or oily contents are widely spread in other Hymenomycetes. Sometimes fatty oil or watery sap is found in swollen hyphal ends, or such tubes contain coloured sap. Cystidia and paraphyses may be also classed here. In Merulius lacrymans Hartig has observed thin-walled hyphae with large lumina, the septa of which are perforated like those of sieve-tubes.
As regards its composition, the cell-wall of fungi exhibits variations of the same kind as those met with in higher plants. While the fundamental constituent is a cellulose in many Mucorini and other Phycomycetes, in others bodies like pectose, callose, &c., commonly occur, and Wisselingh’s researches show that chitin, a gluco-proteid common in animals, forms the main constituent in many cases, and is probably deposited directly as such, though, like the other substances, it may be mixed with cellulose. As in other cell-walls, so here the older membranes may be altered by deposits of various substances, such as resin, calcium oxalate, colouring matters; or more profoundly altered throughout, or in definite layers, by lignification, suberization (Trametes, Daedalea), or swelling to a gelatinous mucilage (Tremella, Gymnosporangium), while cutinization of the outer layers is common. One of the most striking alterations of cell-walls is that termed carbonization, in which the substance gradually turns black, hard and brittle, as if charred—e.g. Xylaria, Ustulina, some sclerotia. At the other extreme the cell-walls of many lichen-fungi are soft and colourless, but turn blue in iodine, as does starch. The young cell-wall is always tenuous and flexible, and may remain so throughout, but in many cases thickenings and structural differentiations, as well as the changes referred to above, alter the primary wall considerably. Such thickening may be localized, and pits (e.g. Uredospores, septa of Basidiomycetes), spirals, reticulations, rings, &c. (capillitium fibres of Podaxon, Calostoma, Battarrea), occur as in the vessels of higher plants, while sculptured networks, pittings and so forth are as common on fungus-spores as they are on pollen grains.
Cell-Contents.—The cells of fungi, in addition to protoplasm, nuclei and sap-vacuoles, like other vegetable cells, contain formed and amorphous bodies of various kinds. Among those directly visible to the microscope are oil drops, often coloured (Uredineae) crystals of calcium oxalate (Phallus, Russula), proteid crystals (Mucor, Pilobolus, &c.) and resin (Polyporei). The oidia of Erysipheae contain fibrosin bodies and the hyphae of Saprolegnieae cellulin bodies, but starch apparently never occurs. Invisible to the microscope, but rendered visible by reagents, are glycogen, Mucor, Ascomycetes, yeast, &c. In addition to these cell-contents we have good indirect evidence of the existence of large series of other bodies, such as proteids, carbohydrates, organic acids, alkaloids, enzymes, &c. These must not be confounded with the numerous substances obtained by chemical analysis of masses of the fungus, as there is often no proof of the manner of occurrence of such bodies, though we may conclude with a good show of probability that some of them also exist preformed in the living cell. Such are sugars (glucose, mannite, &c.), acids (acetic, citric and a whole series of lichen-acids), ethereal oils and resinous bodies, often combined with the intense colours of fungi and lichens, and a number of powerful alkaloid poisons, such as muscarin (Amanita), ergotin (Claviceps), &c.
Among the enzymes already extracted from fungi are invertases (yeasts, moulds, &c.), which split cane-sugar and other complex sugars with hydrolysis into simpler sugars such as dextrose and levulose; diastases, which convert starches into sugars (Aspergillus, &c.); cytases, which dissolve cellulose similarly (Botrytis, &c.); peptases, using the term as a general one for all enzymes which convert proteids into peptones and other bodies (Penicillium, &c.); lipases, which break up fatty oils (Empusa, Phycomyces, &c.); oxydases, which bring about the oxidations and changes of colour observed in Boletus, and zymase, extracted by Buchner from yeast, which brings about the conversion of sugar into alcohol and carbon-dioxide. That such enzymes are formed in the protoplasm is evident from the behaviour of hyphae, which have been observed to pierce cell-membranes, the chitinous coats of insects, artificial collodion films and layers of wax, &c. That a fungus can secrete more than one enzyme, according to the materials its hyphae have to attack, has been shown by the extraction of diastase, inulase, trehalase, invertase, maltase, raffinase, malizitase, emulsin, trypsin and lipase from Aspergillus by Bourquelot, and similar events occur in other fungi. The same fact is indicated by the wide range of organic substances which can be utilized by Penicillium and other moulds, and by the behaviour of parasitic fungi which destroy various cell-contents and tissues. Many of the coloured pigments of fungi are fixed in the cell-walls or excreted to the outside (Peziza aeruginosa). Matruchot has used them for staining the living protoplasm of other fungi by growing the two together. Striking instances of coloured mycella are afforded by Corticium sanguineum, blood-red; Elaphomyces Leveillei, yellow-green; Chlorosplenium aeruginosum, verdigris green; and the Dematei, brown or black.
Nuclei.—Although many fungi have been regarded as devoid of nuclei, and all have not as yet been proved to contain them, the numerous investigations of recent years have revealed them in the cells of all forms thoroughly examined, and we are justified in concluding that the nucleus is as essential to the cell of a fungus as to that of other organisms. The hyphae of many contain numerous, even hundreds of nuclei (Phycomycetes); those of others have several (Aspergillus) in each segment, or only two (Exoascus) or one (Erysiphe) in each cell. Even the isolated cells of the yeast plant have each one nucleus. As a rule the nuclei of the mycelium are very minute (1.5-2 μ in Phycomyces), but those of many asci and spores are large and easily rendered visible. As with other plants, so in fungi the essential process of fertilization consists in the fusion of two nuclei, but owing to the absence of well-marked sexual organs from many fungi, a peculiar interest attaches to certain nuclear fusions in the vegetative cells or in young spores of many forms. Thus in Ustilagineae the chlamydospores, and in Uredineae the teleutospores, each contain two nuclei when young, which fuse as the spores mature. In young asci a similar fusion of two nuclei occurs, and also in basidia, in each case the nucleus of the ascus or of the basidium resulting from the fusion subsequently giving rise by division to the nuclei of the ascospores and basidiospores respectively. The significance of these fusions will be discussed under the various groups. Nuclear division is usually accompanied by all the essential features of karyokinesis.
Spores.—No agreement has ever been arrived at regarding the consistent use of the term spore. This is apparently owing to the facts that too much has been attempted in the definition, and that differences arise according as we aim at a morphological or a physiological definition. Physiologically, any cell or group of cells separated off from a hypha or unicellular fungus, and capable of itself growing out—germinating—to reproduce the fungus, is a spore; but it is evident that so wide a definition does not exclude the ordinary vegetative cells of sprouting fungi, such as yeasts, or small sclerotium like cell-aggregates of forms like Coniothecium. Morphologically considered, spores are marked by peculiarities of form, size, colour, place of origin, definiteness in number, mode of preparation, and so forth, such that they can be distinguished more or less sharply from the hyphae which produce them. The only physiological peculiarity exhibited in common by all spores is that they germinate and initiate the production of a new fungus-plant. Whether a spore results from the sexual union of two similar gametes (zygospore) or from the fertilization of an egg-cell by the protoplasm of a male organ (oospore); or is developed asexually as a motile (zoospore) or a quiescent body cut off from a hypha (conidium) or developed along its course (oidium or chlamydospore), or in its protoplasm (endospore), are matters of importance which have their uses in the classification and terminology of spores, though in many respects they are largely of academic interest.
 |
| Fig. 2.—Peronospora parasitica (De Bary). Conidiophore with conidia. |
Klebs has attempted to divide spores into three categories as follows: (1) kinospores, arising by relatively simple cell-divisions and subserving rapid dissemination and propagation, e.g. zoospores, conidia, endogonidia, stylospores, &c.; (2) paulospores, due to simple rearrangement of cell-contents, and subserving the persistence of the fungus through periods of exigency, e.g. gemmae, chlamydospores, resting-cells, cysts, &c.; (3) carpospores, produced by a more or less complex formative process, often in special fructifications, and subserving either or both multiplication and persistence, e.g. zygospores, oospores, brand-spores, aecidiospores, ascospores, basidiospores, &c. Little or nothing is gained by these definitions, however, which are especially physiological. In practice these various kinds of spores of fungi receive further special names in the separate groups, and names, moreover, which will appear, to those unacquainted with the history, to have been given without any consistency or regard to general principles; nevertheless, for ordinary purposes these names are far more useful in most cases, owing to their descriptive character, than the proposed new names, which have been only partially accepted.
Sporophores.—In some of the simpler fungi the spores are not borne on or in hyphae which can be distinguished from the vegetative parts or mycelium, but in the vast majority of cases the sporogenous hyphae either ascend free into the air or radiate into the surrounding water as distinct branches, or are grouped into special columns, cushions, layers or complex masses obviously different in colour, consistency, shape and other characters from the parts which gather up and assimilate the food-materials. The term “receptacle” sometimes applied to these spore-bearing hyphae is better replaced by sporophore. The sporophore is obsolete when the spore-bearing hyphae are not sharply distinct from the mycelium, simple when the constituent hyphae are isolated, and compound when the latter are conjoined. The chief distinctive characters of the sporogenous hyphae are their orientation, usually vertical; their limited apical growth; their peculiar branching, form, colour, contents, consistency; and their spore-production. According to the characters of the last, we might theoretically divide them into conidiophores, sporangiophores, gametophores, oidiophores, &c.; but since the two latter rarely occur, and more than one kind of spore or spore-case may occur on a sporophore, it is impossible to carry such a scheme fully into practice.
A simple sporophore may be merely a single short hypha, the end of which stops growing and becomes cut off as a conidium by the formation of a septum, which then splits and allows the conidium to fall. More generally the hypha below the septum grows forwards again, and repeats this process several times before the terminal conidium falls, and so a chain of conidia results, the oldest of which terminates the series (Erysiphe); when the primary branch has thus formed a basipetal series, branches may arise from below and again repeat this process, thus forming a tuft (Penicillium). Or the primary hypha may first swell at its apex, and put forth a series of short peg-like branches (sterigmata) from the increased surface thus provided, each of which develops a similar basipetal chain of conidia (Aspergillus), and various combinations of these processes result in the development of numerous varieties of exquisitely branched sporophores of this type (Botrytis, Botryosporium, Verticillium, &c.).
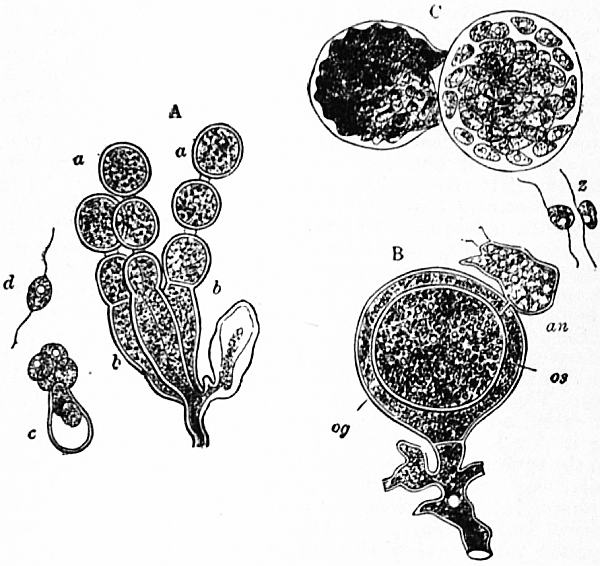 | |
| Fig. 3.—Cystopus candidus. | |
|
A. a, Conidia. b, Conidiophores. c, Conidium emitting zoospores. d, Free zoospore. B.og, Oogonium. |
os, Oosphere. an, Antheridium. C. Formation of zoospores by oospores. z, Free zoospores. (After De Bary.) |
A second type is developed as follows: the primary hypha forms a septum below its apex as before, and the terminal conidium, thus abstricted, puts out a branch at its apex, which starts as a mere point and rapidly swells to a second conidium; this repeats the process, and so on, so that we now have a chain of conidia developed in acropetal succession, the oldest being below, and, as in Penicillium, &c., branches put forth lower down may repeat the process (Hormodendron). In all these cases we may speak of simple conidiophores. The simple sporophore does not necessarily terminate in conidia, however. In Mucor, for example, the end of the primary hypha swells into a spheroidal head (sporangium), the protoplasm of which undergoes segmentation into more or less numerous globular masses, each of which secretes an enveloping cell-wall and becomes a spore (endospore), and branched systems of sporangia may arise as before (Thamnidium). Such may be termed sporangiophores. In Sporodinia the branches give rise also to short branches, which meet and fuse their contents to form zygospores. In Peronospora, Saprolegnia, &c., the ends of the branches swell up into sporangia, which develop zoospores in their interior (zoosporangia), or their contents become oospheres, which may be fertilized by the contents of other branches (antheridia) and so form egg-cases (oogonia). Since in such cases the sporophore bears sexual cells, they may be conveniently termed gametophores.
Compound sporophores arise when any of the branched or unbranched types of spore-bearing hyphae described above ascend into the air in consort, and are more or less crowded into definite layers, cushions, columns or other complex masses. The same laws apply to the individual hyphae and their branches as to simple sporophores, and as long as the conidia, sporangia, gametes, &c., are borne on their external surfaces, it is quite consistent to speak of these as compound sporophores, &c., in the sense described, however complex they may become. Among the simplest cases are the sheet-like aggregates of sporogenous hyphae in Puccinia, Uromyces, &c., or of basidia in Exobasidium, Corticium, &c., or of asci in Exoascus, Ascocorticium, &c. In the former, where the layer is small, it is often termed a sorus, but where, as in the latter, the sporogenous layer is extensive, and spread out more or less sheet-like on the supporting tissues, it is more frequently termed a hymenium. Another simple case is that of the columnar aggregates of sporogenous hyphae in forms like Stilbum, Coremium, &c. These lead us to cases where the main mass of the sporophore forms a supporting tissue of closely crowded or interwoven hyphae, the sporogenous terminal parts of the hyphae being found at the periphery or apical regions only. Here we have the cushion-like type (stroma) of Nectria and many Pyrenomycetes, the clavate “receptacle” of Clavaria, &c., passing into the complex forms met with in Sparassis, Xylaria, Polyporei, and Agaricini, &c. In these cases the compound sporophore is often termed the hymenophore, and its various parts demand special names (pileus, stipes, gills, pores, &c.) to denote peculiarities of distribution of the hymenium over the surface.
Other series of modifications arise in which the tissues corresponding to the stroma invest the sporogenous hyphal ends, and thus enclose the spores, asci, basidia, &c., in a cavity. In the simplest case the stroma, after bearing its crop of conidia or oidia, develops ascogenous branches in the loosened meshes of its interior (e.g. Onygena). Another simple case is where the plane or slightly convex surface of the stroma rises at its margins and overgrows the sporogenous hyphal ends, so that the spores, asci, &c., come to lie in the depression of a cavity—e.g. Solenia, Cyphella—and even simpler cases are met with in Mortierella, where the zygospore is invested by the overgrowth of a dense mat of closely branching hyphae, and in Gymnoascus, where a loose mat of similarly barren hyphae covers in the tufts of asci as they develop.
In such examples as the above we may regard the hymenium (Solenia, Cyphella), zygospores, or asci as truly invested by later growth, but in the vast majority of cases the processes which result in the enclosure of the spores, asci, &c., in a “fructification” are much more involved, inasmuch as the latter is developed in the interior of hyphal tissues, which are by no means obviously homologous with a stroma. Thus in Penicillium, Eurotium, Erysiphe, &c., hyphal ends which are the initials of ascogenous branches, are invested by closely packed branches at an early stage of development, and the asci develop inside what has by that time become a complete investment. Whether a true sexual process precedes these processes or not does not affect the present question, the point being that the resulting spheroidal “fructification” (cleistocarp, perithecium) has a definite wall of its own not directly comparable with a stroma. In other cases (Hypomyces, Nectria) the perithecia arise on an already mature stroma, while yet more numerous examples can be given (Poronia, Hypoxylon, Claviceps, &c.) where the perithecia originate below the surface of a stroma formed long before. Similarly with the various types of conidial or oidial “fructifications,” termed pycnidia, spermogonia, aecidia, &c. In the simplest of these cases—e.g. Fumago—a single mycelial cell divides by septa in all three planes until a more or less solid clump results. Then a hollow appears in the centre owing to the more rapid extension of the outer parts, and into this hollow the cells lining it put forth short sporogenous branches, from the tips of which the spores (stylospores, conidia, spermatia) are abstricted. In a similar way are developed the pycnidia of Cicinnobolus, Pleospora, Cucurbitaria, Leptosphaeria and others. In other cases (Diplodia, Aecidium, &c.) conidial or oidial “fructifications” arise by a number of hyphae interweaving themselves into a knot, as if they were forming a Sclerotium. The outer parts of the mass then differentiate as a wall or investment, and the interior becomes a hollow, into which hyphal ends grow and abstrict the spores. Much more complicated are the processes in a large series of “fructifications,” where the mycelium first develops a densely packed mass of hyphae, all alike, in which labyrinths of cavities subsequently form by separation of hyphae in the previously homogeneous mass, and the hymenium covers the walls of these cavities and passages as with a lining layer. Meanwhile differences in consistency appear in various strata, and a dense outer protective layer (peridium), soft gelatinous layers, and so on are formed, the whole eventually attaining great complexity—e.g. puff-balls, earth-stars and various Phalloideae.
Spore-Distribution.—Ordinary conidia and similarly abstricted dry spores are so minute, light and numerous that their dispersal is ensured by any current of air or water, and we also know that rats and other burrowing animals often carry them on their fur; similarly with birds, insects, slugs, worms, &c., on claws, feathers, proboscides, &c., or merely adherent to the slimy body. In addition to these accidental modes of dispersal, however, there is a series of interesting adaptations on the part of the fungus itself. Passing over the locomotor activity of zoospores (Pythium, Peronospora, Saprolegnia) we often find spores held under tension in sporangia (Pilobolus) or in asci (Peziza) until ripe, and then forcibly shot out by the sudden rupture of the sporangial wall under the pressure of liquid behind—mechanism comparable to that of a pop-gun, if we suppose air replaced by watery sap. Even a single conidium, held tense to the last moment by the elastic cell-wall, may be thus shot forward by a spurt of liquid under pressure in the hypha abstricting it (e.g. Empusa), and similarly with basidiospores (Coprinus, Agaricus, &c.). A more complicated case is illustrated by Sphaerobolus, where the entire mass of spores, enclosed in its own peridium, is suddenly shot up into the air like a bomb from a mortar by the elastic retroversion of a peculiar layer which, up to the last moment, surrounded the bomb, and then suddenly splits above, turns inside out, and drives the former as a projectile from a gun. Gelatinous or mucilaginous degenerations of cell-walls are frequently employed in the interests of spore dispersal. The mucilage surrounding endospores of Mucor, conidia of Empusa, &c., serves to gum the spore to animals. Such gums are formed abundantly in pycnidia, and, absorbing water, swell and carry out the spores in long tendrils, which emerge for days and dry as they reach the air, the glued spores gradually being set free by rain, wind, &c. In oidial chains (Sclerotinia) a minute double wedge of wall-substance arises in the middle lamella between each pair of contiguous oidia, and by its enlargement splits the separating lamella. These disjunctors serve as points of application for the elastic push of the swelling spore-ends, and as the connecting outer lamella of cell-wall suddenly gives way, the spores are jerked asunder. In many cases the slimy masses of spermatia (Uredineae), conidia (Claviceps), basidiospores (Phallus, Coprinus), &c., emit more or less powerful odours, which attract flies or other insects, and it has been shown that bees carry the fragrant oidia of Sclerotinia to the stigma of Vaccinium and infect it, and that flies carry away the foetid spores of Phallus, just as pollen is dispersed by such insects. Whether the strong odour of trimethylamine evolved by the spores of Tilletia attracts insects is not known.
The recent observations and exceedingly ingenious experiments of Falck have shown that the sporophores of the Basidiomycetes—especially the large sporophores of such forms as Boletus, Polyporus—contain quantities of reserve combustible material which are burnt up by the active metabolism occurring when the fruit-body is ripe. By this means the temperature of the sporophore is raised and the difference between it and the surrounding air may be one of several degrees. As a result convection currents are produced in the air which are sufficient to catch the basidiospores in their fall and carry them, away from the regions of comparative atmospheric stillness near the ground, to the upper air where more powerful air-currents can bring about their wide distribution.
Classification.—It has been accepted for some time now that the majority of the fungi proper fall into three main groups, the Phycomycetes, Ascomycetes and Basidiomycetes, the Schizomycetes and Myxomycetes (Mycetozoa) being considered as independent groups not coming under the true fungi.
The chief schemes of classification put forward in detail have been those of P.A. Saccardo (1882-1892), of Oskar Brefeld and Von Tavel (1892), of P.E.L. Van Tieghem (1893) and of J. Schroeter (1892). The scheme of Brefeld, which was based on the view that the Ascomycetes and Basidiomycetes were completely asexual and that these two groups had been derived from one division (Zygomycetes) of the Phycomycetes, has been very widely accepted. The recent work of the last twelve years has shown, however, that the two higher groups of fungi exhibit distinct sexuality, of either a normal or reduced type, and has also rendered very doubtful the view of the origin of these two groups from the Phycomycetes. The real difficulty of classification of the fungi lies in the polyphyletic nature of the group. There is very little doubt that the primitive fungi have been derived by degradation from the lower algae. It appears, however, that such a degradation has occurred not only once in evolution but on several occasions, so that we have in the Phycomycetes not a series of naturally related forms, but groups which have arisen perfectly independently of one another from various groups of the algae. It is also possible in the absence of satisfactory intermediate forms that the Ascomycetes and Basidiomycetes have also been derived from the algae independently of the Phycomycetes, and perhaps of one another.
A natural classification on these lines would obviously be very complicated, so that in the present state of our knowledge it will be best to retain the three main groups mentioned above, bearing in mind that the Phycomycetes especially are far from being a natural group. The following gives a tabular survey of the scheme adopted in the present article:
A. Phycomycetes. Alga-like fungi with unicellular thallus and well-marked sexual organs.
Class I.—Oomycetes. Mycelium usually well developed, but sometimes poor or absent. Sexual reproduction by oogonia and antheridia; asexual reproduction by zoospores or conidia.
1. Monoblepharidineae. Mycelium present, antheridia with antherozoids, oogonium with single oosphere: Monoblepharidaceae.
2. Peronosporineae. Mycelium present; antheridia but no antherozoids; oogonia with one or more oospheres: Peronosporaceae, Saprolegniaceae.
3. Chytridineae. Mycelium poorly developed or absent; oogonia and antheridia (without antherozoids) known in some cases; zoospores common: Chytridiaceae. Ancylistaceae.
Class II.—Zygomycetes. Mycelium well developed; sexual reproduction by zygospores; asexual reproduction by sporangia and conidia.
1. Mucorineae. Sexual reproduction as above, asexual by sporangia or conidia or both: Mucoraceae. Mortierellaceae, Chaetocladiaceae, Piptocephalidaceae.
2. Entomophthorineae. Sexual reproduction typical but with sometimes inequality of the fusing gametes (gametangia ?): Entomophthoraceae.
B. Higher Fungi. Fungi with segmental thallus; sexual reproduction sometimes with typical antheridia and oogonia (ascogonia) but usually much reduced.
Class I.—Ustilaginales. Forms with septate thallus, and reproduction by chlamydospores which on germination produce sporidia; sexuality doubtful.
Class II.—Ascomycetes. Thallus septate; spores developed in special type of sporangium, the ascus, the number of spores being usually eight. Sexual reproduction sometimes typical, usually reduced.
Exoascineae, Saccharomycetineae, Perisporinea, Discomycetes, Pyrenomycetes, Tuberineae, Laboulbeniineae.
Class III.—Basidiales. Thallus septate. Conidia (basidiospores) borne in fours on a special conidiophore, the basidium. Sexual reproduction always much reduced.
1. Uredineae. Life-history in some cases very complex and with well-marked sexual process and alternation of generations, in others much reduced; basidium (promycelium) derived usually from a thick-walled spore (teleutospore).
2. Basidiomycetes. Life-history always very simple, no well-marked alternation of generations; basidium borne directly on the mycelium.
(A) Protobasidiomycetes. Basidia septate. Auriculariaceae, Pilacreaceae, Tremellinaceae.
(B) Autobasidiomycetes. Basidia non-septate. Hymenomycetes, Gasteromycetes.
A. Phycomycetes.—Most of the recent work of importance in this group deals with the cytology of sexual reproduction and of spore-formation, and the effect of external conditions on the production of reproductive organs.
Monoblepharidaceae consists of a very small group of aquatic forms living on fallen twigs in ponds and ditches. Only one genus, Monoblepharis, can certainly be placed here, though a somewhat similar genus, Myrioblepharis, with a peculiar multiciliate zoospore like that of Vaucheria, is provisionally placed in the same group. Monoblepharis was first described by Cornu in 1871, but from that time until 1895 when Roland Thaxter described several species from America the genus was completely lost sight of. Monoblepharis has oogonia with single oospheres and antheridia developing a few amoeboid uniciliate antherozoids; these creep to the opening of the oogonium and then swim in. The resemblance between this genus and Oedogonium among the algae is very striking, as is also that of Myrioblepharis and Vaucheria.
Peronosporaceae are a group of endophytic parasites—about 100 species—of great importance as comprising the agents of “damping off” disease (Pythium), vine-mildew (Plasmopara), potato disease (Phytophthora), onion-mildew (Peronospora). Pythium is a semi-aquatic form attacking seedlings which are too plentifully supplied with water; its hyphae penetrate the cell-walls and rapidly destroy the watery tissues of the living plant; then the fungus lives in the dead remains. When the free ends of the hyphae emerge again into the air they swell up into spherical bodies which may either fall off and behave as conidia, each putting out a germ-tube and infecting the host; or the germ-tube itself swells up into a zoosporangium which develops a number of zoospores. In the rotting tissues branches of the older mycelium similarly swell up and form antheridia and oogonia (fig. 4). The contents of the antheridium are not set free, but that organ penetrates the oogonium by means of a narrow outgrowth, the fertilizing tube, and a male nucleus then passes over into the single oosphere, which at first multinucleate becomes uninucleate before fertilization. Pythium is of interest as illustrating the dependence of zoospore-formation on conditions and the indeterminate nature of conidia. The other genera are more purely parasitic; the mycelium usually sends haustoria into the cells of the host and puts out branched, aerial conidiophores through the stomata, the branches of which abstrict numerous “conidia”; these either germinate directly or their contents break up into zoospores (fig. 5). The development of the “conidia” as true conidial spores or as zoosporangia may occur in one and the same species (Cystopus candidus, Phytophthora infestans) as in Pythium described above; in other cases the direct conidial germination is characteristic of genera—e.g. Peronospora; while others emit zoospores—e.g. Plasmopara, &c. In Cystopus (Albugo) the “conidia” are abstricted in basipetal chain-like series from the ends of hyphae which come to the surface in tufts and break through the epidermis as white pustules. Each “conidium” contains numerous nuclei and is really a zoosporangium, as after dispersal it breaks up into a number of zoospores. The Peronosporaceae reproduce themselves sexually by means of antheridia and oogonia as described in Pythium. In Cystopus Bliti the oosphere contains numerous nuclei, and all the male nuclei from the antheridium pass into it, the male and female nuclei then fusing in pairs. We thus have a process of “multiple fertilization”; the oosphere really represents a large number of undifferentiated gametes and has been termed a coenogamete. Between Cystopus Bliti on the one hand and Pythium de Baryanum on the other a number of cytologically intermediate forms are known. The oospore on germination usually gives origin to a zoosporangium, but may form directly a germ tube which infects the host.
 | |
| From Strasburger’s Lehrbuch der Botanik, by permission of Gustav Fischer. | |
| Fig. 4.—Fertilization of the Peronosporeae. After Wager. | |
|
1, Peronospora parasitica. Young multinucleate oogonium (og) and antheridium (an). 2, Albugo candida. Oogonium with the central uninucleate oosphere and the fertilizing tube (a) of the antheridium which introduces the male nucleus. |
3, The same. Fertilized egg-cell (o) surrounded by the periplasm (p). |
 | |
| Fig. 5.—Phytophthora infestans. Fungus of Potato Disease. | |
|
A, B, Section of Leaf of Potato with sporangiophores of Phytophthora infestans passing through the stomata D, on the under surface of the leaf. E, Sporangia. F, G, H, J, Further development of the sporangia. |
K, Germination of the zoospores formed in the sporangia. L, M, N, Fertilization of the oogonium and development of the oospore in Peronospora. |
Saprolegniaceae are aquatic forms found growing usually on dead insects lying in water but occasionally on living fish (e.g. the salmon disease associated with Saprolegnia ferax). The chief genera are Saprolegnia, Achlya, Pythiopsis, Dictyuchus, Aplanes. Motile zoospores which escape from the zoosporangium are present except in Aplanes. The sexual reproduction shows all transitions between forms which are normally sexual, like the Peronosporaceae, to forms in which no antheridium is developed and the oospheres develop parthenogenetically. The oogonia, unlike the Peronosporaceae, contain more than one oosphere. Klebs has shown that the development of zoosporangia or of oogonia and pollinodia respectively in Saprolegnia is dependent on the external conditions; so long as a continued stream of suitable food-material is ensured the mycelium grows on without forming reproductive organs, but directly the supplies of nitrogenous and carbonaceous food fall below a certain degree of concentration sporangia are developed. Further reduction of the supplies of food effects the formation of oogonia. This explains the sequence of events in the case of a Saprolegnia-mycelium radiating from a dead fly in water. Those parts nearest the fly and best supplied develop barren hyphae only; in a zone at the periphery, where the products of putrefaction dissolved in the water form a dilute but easily accessible supply, the zoosporangia are developed in abundance; oogonia, however, are only formed in the depths of this radiating mycelium, where the supplies of available food materials are least abundant.
Chytridineae.—These parasitic and minute, chiefly aquatic, forms may be looked upon as degenerate Oomycetes, since a sexual process and feeble unicellular mycelium occur in some; or they may be regarded as series of primitive forms leading up to higher members. There is no means of deciding the question. They are usually included in Oomycetes, but their simple structure, minute size, usually uniciliate zoospores, and their negative characters would justify their retention as a separate group. It contains less than 200 species, chiefly parasitic on or in algae and other water-plants or animals, of various kinds, or in other fungi, seedlings, pollen and higher plants. They are often devoid of hyphae, or put forth fine protoplasmic filaments into the cells of their hosts. After absorbing the cell-contents of the latter, which it does in a few hours or days, the fungus puts out a sporangium, the contents of which break up into numerous minute swarm-spores, usually one-ciliate, rarely two-ciliate. Any one of these soon comes to rest on a host-cell, and either pierces it and empties its contents into its cavity, where the further development occurs (Olpidium), or merely sends in delicate protoplasmic filaments (Rhizophydium) or a short hyphal tube of, at most, two or three cells, which acts as a haustorium, the further development taking place outside the cell-wall of the host (Chytridium). In some cases resting spores are formed inside the host (Chytridium), and give rise to zoosporangia on germination. In a few species a sexual process is described, consisting in the conjugation of similar cells (Zygochytrium) or the union of two dissimilar ones (Polyphagus). In the development of distinct antheridial and oogonial cells the allied Ancylistineae show close alliances to Pythium and the Oomycetes. On the other hand, the uniciliate zoospores of Polyphagus have slightly amoeboid movements, and in this and the pseudopodium-like nature of the protoplasmic processes, such forms suggest resemblances to the Myxomycetes. Opinions differ as to whether the Chytridineae are degraded or primitive forms, and the group still needs critical revision. Many new forms will doubtless be discovered, as they are rarely collected on account of their minuteness. Some forms cause damping off of seedlings—e.g. Olpidium Brassicae; others discoloured spots and even tumour-like swellings—e.g. Synchytium Scabiosae, S. Succisae, Urophlyctis, &c., on higher plants. Analogies have been pointed out between Chytridiaceae and unicellular algae, such as Chlorosphaeraceae, Protococcaceae, “Palmellaceae,” &c., some of which are parasitic, and suggestions may be entertained as to possible origin from such algae.
The Zygomycetes, of which about 200 species are described, are especially important from a theoretical standpoint, since they furnished the series whence Brefeld derived the vast majority of the fungi. They are characterized especially by the zygospores, but the asexual organs (sporangia) exhibit interesting series of changes, beginning with the typical sporangium of Mucor containing numerous endospores, passing to cases where, as in Thamnidium, these are accompanied with more numerous small sporangia (sporangioles) containing few spores, and thence to Chaetocladium and Piptocephalis, where the sporangioles form but one spore and fall and germinate as a whole; that is to say, the monosporous sporangium has become a conidium, and Brefeld regarded these and similar series of changes as explaining the relation of ascus to conidium in higher fungi. According to his view, the ascus is in effect the sporangium with several spores, the conidium the sporangiole with but one spore, and that not loose but fused with the sporangiole wall. On this basis, with other interesting morphological comparisons, Brefeld erected his hypothesis, now untenable, that the Ascomycetes and Basidiomycetes diverge from the Zygomycetes, the former having particularly specialized the ascus (sporangial) mode of reproduction, the latter having specialized the conidial (indehiscent one-spored sporangiole) mode. In addition to sporangia and the conidial spores referred to, some Mucorini show a peculiar mode of vegetative reproduction by means of gemmae or chlamydospores—i.e. short segments of the hyphae become stored with fatty reserves and act as spores. The gemmae formed on submerged Mucors may bud like a yeast, and even bring about alcoholic fermentation in a saccharine solution.
 |
| From Strasburger’s Lehrbuch der Botanik, by permission of Gustav Fischer. |
| Fig. 6.—Mucor Mucedo. Different stages in the formation and germination of the zygospore. (After Brefeld, 1-4. 5 from v. Tavel, Pilze.) |
|
1, Two conjugating branches in contact. 2, Septation of the conjugating cells (a) from the suspensors (b). 3, More advanced stage, the conjugating cells (a) are still distinct from one another; the warty thickenings of their walls have commenced to form. 4, Ripe zygospore (b) between the suspensors (a). 5, Germinating zygospore with a germ-tube bearing a sporangium. |
The segments of the hyphae in this group usually contain several nuclei. At the time of sporangial formation the protoplasm with numerous nuclei streams into the swollen end of the sporangiophore and there becomes cut off by a cell-wall to form the sporangium. The protoplasm then becomes cut up by a series of clefts into a number of smaller and smaller pieces which are unicellular in Pilobolus, multicellular in Sporodinia. These then become surrounded by a cell-wall and form the spores. This mode of spore-formation is totally different from that in the ascus; hence one of the difficulties of the acceptance of Brefeld’s view of the homology of ascus and sporangium. The cytology of zygospore-formation is not known in detail; the so-called gametes which fuse are multinucleate and are no doubt of the nature of gametangia. The fate of these nuclei is doubtful, probably they fuse in pairs (fig. 6).
Blakeslee has lately made some very important observations of the Zygomycetes. It is well known that while in some forms, e.g. Spordinia, zygospores are easily obtained, in others, e.g. most species of Mucor, they are very erratic in their appearance. This has now been explained by Blakeslee, who finds that the Mucorinae can be divided into two groups, termed homothallic and heterothallic respectively. In the first group zygospores can arise by the union of branches from the same mycelium and so can be produced by the growth from a single spore; this group includes Spordinia grandis, Spinellus fusiger, some species of Mucor, &c. The majority of forms, however, fall into the heterothallic group, in which the association of branches from two mycelia different in nature is necessary for the formation of zygospores. These structures cannot then be produced from the product of a single spore nor even from the thalli derived from any two spores. The two kinds of thalli Blakeslee considers to have a differentiation of the nature of sex and he distinguishes them as (+) and (−) forms; the former being usually distinguished by a somewhat greater luxuriance of growth.
The classification of the Mucorini depends on the prevalence and characters of the conidia, and of the sporangia and zygospores—e.g. the presence or absence of a columella in the former, the formation of an investment round the latter. Most genera are saprophytes, but some—Chaetocladium, Piptocephalis—are parasites on other Mucorini, and one or two are associated casually with the rotting of tomatoes and other fruits, bulbs, &c., the fleshy parts of which are rapidly destroyed if once the hyphae gain entrance. Even more important is the question of mycosis in man and other animals, referred to species of Mucor, and investigated by Lucet and Costantin. Klebs has concluded that transpiration is the important factor in determining the formation of sporangia, while zygote-development depends on totally different conditions; these results have been called in question by Falck.
The Entomophthoraceae contain three genera, Empusa, Entomophthora and Basidiobolus. The two first genera consist of forms which are parasitic on insects. Empusa Muscae causes the well-known epidemic in house-flies during the autumn; the dead, affected flies are often found attached to the window surrounded by a white halo of conidia. B. ranarum is found in the alimentary canal of the frog and growing on its excrement. In these three genera the conidia are cast off with a jerk somewhat in the same way as the sporangium of Pilobolus.
B. Higher Fungi.—Now that Brefeld’s view of the origin of these forms from the Zygomycetes has been overthrown, the relationship of the higher and lower forms of fungi is left in obscurity. The term Eumycetes is sometimes applied to this group to distinguish them from the Phycomycetes, but as the same name is also applied to the fungi as a whole to differentiate them from the Mycetozoa and Bacteria, the term had best be dropped. The Higher Fungi fall into three groups: the Ustilaginales, of doubtful position, and the two very sharply marked groups Basidiales and Ascomycetes.
 |
| From Vine’s Students’ Text Book of Botany, by permission of Swan Sonnenschein & Co. |
| Fig. 7.—Germinating resting-gonidia. A, of Ustilago receptaculorum; B, of Tilletia Caries. |
|
sp, The gonidium. pm, The promycelium. d, The sporidia: in B the sporidia have coalesced in pairs at v. |
I. Ustilaginales.—This includes two families Ustilaginaceae (smuts) and Tilletiaceae (bunts). The bunts and smuts which damage our grain and fodder plants comprise about 400 species of internal parasites, found in all countries on herbaceous plants, and especially on Monocotyledons. They are remarkable for their dark spores developed in gall-like excrescences on the leaves, stems, &c., or in the fruits of the host. The discovery of the yeast-conidia of these fungi, and their thorough investigation by Brefeld, have thrown new lights on the group, as also have the results elucidating the nature of the ordinary dark spores—smuts, bunt, &c.—which by their mode of origin and development are chlamydospores. When the latter germinate a slender “promycelium” is put out; in Ustilago and its allies this is transversely septate, and bears lateral conidia (sporidia); in Tilletia and its allies non-septate, and bears a terminal tuft of conidia (sporidia) (fig. 7). Brefeld regarded the promycelium as a kind of basidium, bearing lateral or terminal conidia (comparable to basidiospores), but since the number of basidiospores is not fixed, and the basidium has not yet assumed very definite morphological characters, Brefeld termed the group Hemibasidii, and regarded them as a half-way stage in the evolution of the true Basidiomycetes from Phycomycetes, the Tilletia type leading to the true basidium (Autobasidium), the Ustilago type to the protobasidium, with lateral spores; but this view is based on very poor evidence, so that it is best to place these forms as a separate group, the Ustilaginales. The yeast-conidia, which bud off from the conidia or their resulting mycelium when sown in nutrient solutions, are developed in successive crops by budding exactly as in the yeast plant, but they cannot ferment sugar solutions. It is the rapid spread of these yeast-conidia in manure and soil waters which makes it so difficult to get rid of smuts, &c., in the fields, and they, like the ordinary conidia, readily infect the seedling wheat, oats, barley or other cereals. Infection in these cases occurs in the seedling at the place where root and shoot meet, and the infecting hypha having entered the plant goes on living in it and growing up with it as if it had no parasitic action at all. When the flowers form, however, the mycelium sends hyphae into the young ovaries and rapidly replaces the stores of sugar and starch, &c., which would have gone to make the grain, by the soot-like mass of spores so well known as smut, &c. These spores adhere to the grain, and unless destroyed, by “steeping” or other treatment, are sown with it, and again produce sporidia and yeast-conidia which infect the seedlings. In other species the infection occurs through the style of the flower, but the fungus after reaching the ovule develops no further during that year but remains dormant in the embryo of the seed. On germination, however, the fungus behaves in the same way as one which has entered in the seedling stage. The cytology of these forms is very little known; Dangeard states that there is a fusion of two nuclei in the chlamydospore, but this requires confirmation. Apart from this observation there is no other trace of sexuality in the group.
II. Ascomycetes.—This, except in the case of a few of the simpler forms, is a very sharply marked group characterized by a special type of sporangium, the ascus. In the development of the ascus we find two nuclei at the base which fuse together to form the single nucleus of the young ascus. The single nucleus divides by three successive divisions to form eight nuclei lying free in the protoplasm of the ascus. Then by a special method, described first by Harper, a mass of protoplasm is cut out round each nucleus; thus eight uninucleate ascospores are formed by free-cell formation. The protoplasm remaining over is termed epiplasm and often contains glycogen (fig. 8). In some cases nuclear division is carried further before spore-formation occurs, and the number of spores is then 16, 32 and 64, &c.; in a few cases the number of spores is less than eight by abortion of some of the eight nuclei. The ascus is thus one of the most sharply characterized structures among the fungi.
 |
| From Strasburger’s Lehrbuch der Botanik, by permission of Gustav Fischer. |
| Fig. 8.—Development of the Ascus. |
|
A-C, Pyronema confluens. (After Harper.) D, Young ascus of Boudiera with eight spores. (After Claussen.) |
In some forms we find definite male and female sexual organs (Sphaerotheca, Pyronema, &c.), in others the antheridium is abortive or absent, but the ascogonium (oogonium) is still present and the female nuclei fuse in pairs (Lachnea stercorea, Humaria granulata, Ascobolus furfuraceus); while in other forms ascogonium and antheridium are both absent and fusion occurs between vegetative nuclei (Humaria rutilans, and probably the majority of other forms). In other cases the sexual fusion is apparently absent altogether, as in Exoascus. In the first case (fig. 9) we have a true sexual process, while in the second and third cases we have a reduced sexual process in which the fusion of other nuclei has replaced the fusion of the normal male and female nuclei. It is to be noted that all the forms exhibit the fusion of nuclei in the ascus, so that those with the normal or reduced sexual process described above have two nuclear fusions in their life-history. The advantage or significance of the second (ascus) fusion is not clearly understood.
The group of the Hemiasci was founded by Brefeld to include forms which were supposed to be a connecting link between Phycomycetes and Ascomycetes. As mentioned before, the connexion between these two groups is very doubtful, and the derivation of the ascus from an ordinary sporangium of the Zygomycetes cannot be accepted. The majority of the forms which were formerly included in this group have been shown to be either true Phycomycetes (like Ascoidea) or true Ascomycetes (like Thelebolus). Eremascus and Dipodascus, which are often placed among the Hemiasci, possibly do not belong to the Ascomycetes series at all.
 | |
| From Strasburger’s Lehrbuch der Botanik, by permission of Gustav Fischer. | |
| Fig. 9.—Sphaerotheca Castagnei. Fertilization and Development of the Perithecium. (After Harper.) | |
|
1, Oogonium (og) with the antheridial branch (az) applied to its surface 2, Separation of antheridium (an). 3, Passage of the antheridial nucleus towards that of the oogonium. |
4, Union of the nuclei. 5, Fertilized oogonium surrounded by two layers of hyphae derived from the stalk-cell (st). 6, The multicellular ascogonium derived by division from the oogonium; the terminal cell with the two nuclei (as) gives rise to the ascus. |
Exoascaceae are a small group of doubtful extent here used to include Exoascus, Taphrina, Ascorticium and Endomyces. The mycelium is very much reduced in extent. The asci are borne directly on the mycelium and are therefore fully exposed, being devoid from the beginning of any investment. The Taphrineae, which include Exoascus and Taphrina, are important parasites—e.g. pocket-plums and witches’ brooms on birches, &c., are due to their action (fig. 10). Exoascus and Ascorticium present interesting parallels to Exobasidium and Corticium among the Basidiomycetes.
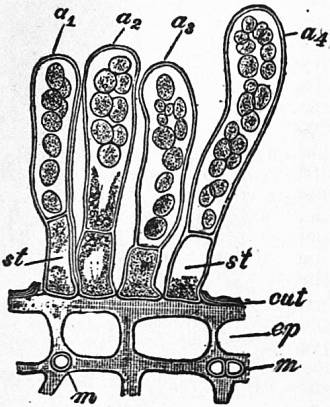 |
| From Strasburger’s Lehrbuch der Botanik, by permission of Gustav Fischer. |
| Fig. 10.—Taphrina Pruni. Transverse section through the epidermis of an infected plum. Four ripe asci, a1, a2, with eight spores, a3, a4, with yeast-like conidia abstricted from the spores. After Sadebeck. |
st, Stalk-cells of the asci. m, Filaments of the mycelium cut transversely. cut, Cuticle. sp, Epidermis. |
Saccharomycetaceae include the well-known yeasts which belong mainly to the genus Saccharomyces. They are characterized by their unicellular nature, their power of rapid budding, their capacity for fermenting various sugars, and their power of forming endogenous spores. The sporangium with its endogenous spores has been compared with an ascus, and on these grounds the group is placed among the Ascomycetes—a very doubtful association. The group has attained an importance of late even beyond that to which it was brought by Pasteur’s researches on alcoholic fermentation, chiefly owing to the exact results of the investigations of Hansen, who first applied the methods of pure cultures to the study of these organisms, and showed that many of the inconsistencies hitherto existing in the literature were due to the coexistence in the cultures of several species or races of yeasts morphologically almost indistinguishable, but physiologically very different. About fifty species of Saccharomyces are described more or less completely, but since many of these cannot be distinguished by the microscope, and some have been found to develop physiological races or varieties under special conditions of growth, the limits are still far too ill-defined for complete botanical treatment of the genus. A typical yeast is able to develop new cells by budding when submerged in a saccharine solution, and to ferment the sugar—i.e. so to break up its molecules that, apart from small quantities used for its own substance, masses of it out of all proportion to the mass of yeast used become resolved into other bodies, such as carbon dioxide and alcohol, the process requiring little or no oxygen. Brefeld regards the budding process as the formation of conidia. Under other conditions, of which the temperature is an important one, the nucleus in the yeast-cell divides, and each daughter-nucleus again, and four spores are formed in the mother cell, a process obviously comparable to the typical development of ascospores in an ascus. Under yet other conditions the quiescent yeast-cells floating on the surface of the fermented liquor grow out into elongated sausage-shaped or cylindrical cells and branching cell-series, which mat together into mycelium-like veils. At the bottom of the fermented liquor the cells often obtain fatty contents and thick walls, and behave as resting cells (chlamydospores). The characters employed by experts for determining a species of yeast are the sum of its peculiarities as regards form and size: the shapes, colours, consistency, &c., of the colonies grown on certain definite media; the optimum temperature for spore-formation, and for the development of the “veils”; and the behaviour as regards the various sugars.
The following summary of some of the principal characteristics of half-a-dozen species will serve to show how such peculiarities can be utilized for systematic purposes:
| Species. | Optimum Temperature for | Characters of | Sugars Fermented and Products, &c. | |||
| Spores. | Veils. | Fermentation. | Cells. | Spores. | ||
| S. cereviseae I. | 30° | 20°-28° | High | Rounded | Globoid | Inverts maltose and saccharose and form alcohol 4-6 vol. %. |
| S. Pastorianus I | 27°-5° | 26°-28° | Low | Rounded | Globoid | |
| S. ellipsoideus | 25° | 33°-34° | Low | Rounded | Globoid | |
| S. anomalus | 28°-31° | ? | High | Elliptical | Hat-shaped | Ditto, and evolves a fragrant ether. |
| S. Ludwigii | 30°-31° | ? | ? | Elongated | Globoid | Will not invert maltose. |
| S. membranaefaciens | 30° | ? | High | Elongated | Globoid | Inverts neither maltose nor saccharose. |
Two questions of great theoretical importance have been raised over and over again in connexion with yeasts, namely, (1) the morphological one as to whether yeasts are merely degraded forms of higher fungi, as would seem implied by their tendency to form elongated, hypha-like cells in the veils, and their development of “ascospores” as well as by the wide occurrence of yeast-like “sprouting forms” in other fungi (e.g. Mucor, Exoasci, Ustilagineae, higher Ascomycetes and Basidiomycetes); and (2) the question as to the physiological nature and meaning of fermentation. With regard to the first question no satisfactory proof has as yet been given that Saccharomycetes are derivable by culture from any higher form, the recent statements to that effect not having been confirmed. At the same time there are strong grounds for insisting on the resemblances between Endomyces, a hyphal fungus bearing yeast-like asci, and such a form as Saccharomyces anomalus. Concerning the second question, the recent investigations of Buchner and others have shown that a ferment (zymase) can be extracted from yeast-cells which causes sugar to break up into carbon dioxide and alcohol. It has since been shown by Buchner and Albert that yeast-cells which have been killed by alcohol and ether, or with acetone, still retain the enzyme. Such material is far more active than the zymase obtained originally by Buchner from the expressed juice of yeast-cells. Thus alcoholic fermentation is brought into line with the other fermentations.
Schizosaccharomyces includes a few species in which the cells do not “bud” but become elongated and then divide transversely. In the formation of sporangia two cells fuse together by means of outgrowths, in a manner very similar to that of Spirogyra; sometimes, however, the wall between two cells merely breaks down. The fused cell becomes a sporangium, and in it eight spores are developed. In certain cases single cells develop parthenogenetically, without fusion, each cell producing, however, only four spores. In Zygosaccharomyces described by Barker (1901) we have a form of the usual sprouting type, but here again there is a fusion of two cells to form a sporangium.
Cytology.—The study of the nucleus of yeast-cells is rendered difficult by the presence of other deeply staining granules termed by Guillermond metachromatic granules. These have often been mistaken for nuclei and have to be carefully distinguished by differential stains. In the process of budding the nucleus divides apparently by a process of direct division. In the formation of spores the nucleus of the cell divides, the protoplasm collects round the nuclei to form the spores by free-cell formation; the protoplasm (epiplasm) not used in this process becomes disorganized. A fusion of nuclei was originally described by Jansens and Leblanc, but it was observed neither by Wager nor Guillermond and is probably absent. In Schizosaccharomyces and Zygosaccharomyces, however, we have a fusion of nuclei in connexion with the conjugation of cells which precedes sporangium-formation. The theory may be put forward that the ordinary forms have been derived from sexual forms like Schizosaccharomyces and Zygosaccharomyces by a loss of sexuality, the sporangium being formed parthenogenetically without any nuclear fusion. This suggests a possible relationship to Eremascus, which can only doubtfully be placed in the Ascomycetes (vide supra).
Carpoascomycetes.—The other divisions of the Ascomycetes may be distinguished as Carpoascomycetes because they do not bear the asci free on the mycelium but enclosed in definite fruit bodies or ascocarps. The ascocarps can be distinguished into two portions, a mass of sterile or vegetative hyphae forming the main mass of the fruit body, and surrounding the fertile ascogenous hyphae which bear at their ends the asci. When the ascogonium (female organ) is present the ascogenous hyphae arise from it, with or without its previous fusion with an antheridium. In other cases the ascogenous hyphae arise directly from the vegetative hyphae. In connexion with this condition of reduction a fusion of nuclei has been observed in Humaria rutilans and is probably of frequent occurrence. The asci may be derived from the terminal cell of the branches of the ascogenous hyphae, but usually they are derived from the penultimate cell, the tip curving over to form the so-called crozier. By this means the ascus cell is brought uppermost, and after the fusion of the two nuclei it develops enormously and produces the ascospores. The ascospores escape from the asci in various ways, sometimes by a special ejaculation-mechanism. The Ascomycetes, at least the Carpoascomycetes, exhibit a well-marked alternation of sexual and asexual generations. The ordinary mycelium is the gametophyte since it bears the ascogonia and antheridia when present; the ascogenous hyphae with their asci represent the sporophyte since they are derived from the fertilized ascogonium. The matter is complicated by the apogamous transition from gametophyte to sporophyte in the absence of the ascogonium; also by the fact that there are normally two fusions in the life-history as mentioned earlier. If there are two fusions one would expect two reductions, and Harper has suggested that the division of the nuclei into eight in the ascus, instead of into four spores as in most reduction processes, is associated with a double reduction process in the ascus. Miss Fraser in Humaria rutilans finds two reductions: a normal synaptic reduction in the first nuclear division of the ascus, and a peculiar reduction division termed brachymeiosis in the third ascus division.
Various types of ascocarp are characteristic of the different divisions of the Carpoascomycetes: the cleistothecium, apothecium and perithecium.
Perisporineae.—This includes two chief families, Erysiphaceae and Perisporiaceae. They are characterized by an ascocarp without any opening to the exterior, the ascospores being set free by the decay or rupture of the ascocarp wall; such a fruit-body is termed a cleistothecium (cleistocarp). The Erysiphaceae are a sharply marked group of forms which live as parasites. They form a superficial mycelium on the surface of the plant, the hyphae not usually penetrating the tissues but merely sending haustoria into the epidermal cells. Only in rare cases is the mycelium intercellular. Owing to their appearance they go by the popular name of mildews. Sphaerotheca Humuli is the well known hop-mildew, Sphaerotheca Mors-Uvae is the gooseberry mildew, the recent advent of which has led to special legislation in Great Britain to prevent its spreading, as when rampant it makes the culture of gooseberries impossible. Erysiphe, Uncinula and Phyllactinia are other well-known genera. The form of the fruit body, the difference and the nature of special outgrowths upon it—the appendages—are characteristic of the various genera. Besides peritheca the members of the Erysiphaceae possess conidia borne in simple chains. De Bary brought forward very strong evidence for the origin of the ascocarp in Sphaerotheca and Erysiphe by a sexual process, but Harper in 1895 was the first to prove conclusively, by the observation of the nuclear fusion, that there was a definite fertilization in Sphaerotheca Humuli by the fusion of a male (antheridial) nucleus with a female, ascogonial (oogonial) nucleus. Since then Harper has shown that the same process occurs in Erysiphe and Phyllactinia.
 | |
| Fig. 11.—Development of Eurotium repens. (After De Bary.) | |
|
A, Small portion of mycelium with conidiophore (c), and archicarp (as). B, The spiral archicarp (as), with the antheridium (p). D, The same, beginning to be surrounded by the hyphae forming the perithecium wall. D, The perithecium. |
E, F, Sections of young perithecia. w, Parietal cells. f, Pseudo-parenchyma. as, Ascogonium. G, An ascus. H, An ascospore. |
The Perisporiaceae are saprophytic forms, the two chief genera being Aspergillus and Penicillium. The blue-green mould P. crustaceum and the green mould A. herbariorium (= Eurotium herbariorum) are extraordinarily widely distributed, moulds being found on almost any food-material which is exposed to the air. They have characteristic conidiophores bearing numerous conidia, and also cleistothecia which are spherical in form and yellowish in colour. The latter arise from the crown of a spirally coiled archicarp (bearing an ascogonium at its end) and a straight antheridium. Vegetative hyphae then grow up and surround these and enclose them in a continuous sheath of plectenchyma (fig. 11). It has lately been shown by Fraser and Chambers that in Eurotium both ascogonium and antheridium contain a number of nuclei (i.e. are coenogametes), but that the antheridium disorganizes without passing its contents into the ascogonium. There is apparently a reduced sexual process by the fusion of the ascogonial (female) nuclei in pairs. Aspergillus Oryzae plays an important part in saccharifying the starch of rice, maize, &c., by means of the abundant diastase it secretes, and, in symbiosis with a yeast which ferments the sugar formed, has long been used by the Japanese for the preparation of the alcoholic liquor saké. The process has now been successfully introduced into European commerce.
 |
 |
| From Strasburger’s Lehrbuch der Botanik, by permission of Gustav Fischer. | Fig. 13.—Ascobolus furfuraceus. Diagrammatic section of the fructification. (After Janczewski.) |
| Fig. 12.—Peziza aurantiaca. (After Krombholz, nat. size.) |
m, Mycelium. c, Archicarp. l, Pollinodium. s, Ascogenous filaments. a, Asri. r, p, The sterile tissue from which the paraphyses h spring. |
Discomycetes.—Used in its widest sense this includes the Hysteriaceae, Phacidiaceae, Helvellaceae, &c. The group is characterized in general by the possession of an ascocarp which, though usually a completely closed structure during the earlier stages of development, at maturity opens out to form a bowl or saucer-shaped organ, thus completely exposing the layer of asci which forms the hymenium. Such an ascocarp goes by the name of apothecium. Owing to the shape of the fruit-body many of these forms are known as cup-fungi, the cup or apothecium often attaining a large size, sometimes several inches across (fig. 12). Functional male and female organs have been shown to exist in Pyronema and Boudiera; in Lachnea stercorea both ascogonia and antheridia are present, but the antheridium is non-functional, the ascogonial (female) nuclei fusing in pairs; this is also the case in Humaria granulata and Ascobolus furfuraceus, where the antheridium is entirely absent. In H. rutilans, however, both sexual organs are absent and the ascogenous hyphae arise apogamously from the ordinary hyphae of the mycelim. In all these cases the ascogonium and antheridium contain numerous nuclei; they are to be looked upon as gametangia in which there is no differentiation of gametes, and since they act as single gametes they are termed coenogametes. In some forms as in Ascobolus the ascogonium is multicellular, the various cells communicating by pores in the transverse walls (fig. 13).
 |
| From Strasburger’s Lehrbuch der Botanik, by permission of Gustav Fischer. |
| Fig. 14.—Perithecium of Podospora fimiseda in longitudinal section. After v. Tavel. |
|
s, Asci. a, Paraphyses. e, Periphyses. m, Mycelial hyphae. |
In the Helvellaceae there is no apothecium but a large irregular fruit body which at maturity bears the asci on its surface. The development is only slightly known, but there is some evidence for believing that the fruit-body is closed in its very early stages.
The genus Peziza (in its widest sense) may be taken as the type of the group. Most of them grow on living plants or on dead vegetable remains, very often on fallen wood; a number, however, are found growing on earth which is rich in humus. The genus Sclerotinia may be mentioned here; a number of forms have been investigated by Woronin. The conidia are fragrant and are carried by bees to the stigma of the bilberry; here they germinate with the pollen and the hyphae pass with the pollen tubes down the style; the former infect the ovules and produce sclerotia, therein reducing the fruits to a mummified condition. From the sclerotia later the apothecium develops. One species, S. heteroica, is heteroecious; the ascospores infecting the leaves of Vaccinium uliginosum, while the conidia which then arise infect only Ledum palustre. This is the only case of heteroecism known in the vegetable kingdom outside the Uredineae.
Pyrenomycetes.—This is an extraordinarily large and varied group of forms which mostly live parasitically or saprophytically on vegetable tissue, but a few are parasitic on insect-larvae. The group is characterized by a special type of ascocarp, the perithecium. This is typically of a flask-shaped form opening with a small pore at the top. The asci live at the bottom often mixed with paraphyses, while the upper “neck” of the flask is lined with special hyphae, the periphyses, which aid in the ejection of the spores (fig. 14). The simpler forms bear the perithecia directly on the mycelium, but the more highly developed forms often bear them on a special mycelial development—the stroma, which is often of large size and special shape and colour, and of dense consistence. The cytological details of development of the perithecia are not well known; most of them appear to develop their ascogenous hyphae in an apogamous way without any connexion with an ascogonium. Besides the special ascocarps, accessory reproductive organs are known in the majority of cases in the form of conidia.
Tuberineae.—These are a small group of fungi including the well-known truffles. They are found living saprophytically (in part parasitically) underground in forests. The asci are developed in the large dense fruit bodies (cleistothecia) and the spores escape by the decay of the wall. The fruit-body is of complicated structure, but its early stages of development are not known. Many of the fruit-bodies have a pleasant flavour and are eaten under the name of truffles (Tuber brumale and other species). The exact life-history of the truffle is not known.
 |
| From Strasburger’s Lehrbuch der Botanik, by permission of Gustav Fischer. |
| Fig. 15.—Armillaria mellea. (After Ruhland.) |
|
A, Young basidium with the two primary nuclei. B, After fusion of the two nuclei. Hypholoma appendiculatum. C, A basidium before the four nuclei derived from the secondary nucleus of the basidium have passed into the four basidiospores. D, Passage of a nucleus through the sterigma into the basidiospore. |
Laboulbeniineae are a group of about 150 species of fungi found on insects, especially beetles, and principally known from the researches of Thaxter in America. The plant is a small, dark brown, erect structure (receptacle) of a few cells, and 1-10 mm. high, attached to the insect by the lowermost end (foot), and easily mistaken for a hair or similar appendage of the insect. The receptacle ends above in appendages, each consisting of one or a few cells, some of which are the male organs, others the female organs, and others again may be barren hairs. The male organ (antheridium) consists of a few cells, the terminal one of which either abstricts from its end, or emits from its interior the non-motile spermatia, reminding us of those of the Florideae. The female organ is essentially a flask-shaped structure; the neck of the flask growing out as the trichogyne, and the belly composed of an axial carpogenic cell surrounded by investing cells, and with one cell (trichophoric) between it and the trichogyne. These three elements—trichogyne, trichophoric cell, and carpogenic cell—are regarded as the procarp. The spermatia have been shown by Thaxter to fuse with the trichogyne, after which the axial cell below (carpogenic cell) undergoes divisions, and ultimately forms asci containing ascospores, while cells investing this form a perithecium, the whole structure reminding us essentially of the fructification of a Pyrenomycete. Many modifications in details occur, and the plants may be dioecious. No injury is done to the infested insects. It has lately been shown that there is a fusion of nuclei in connexion with ascus formation, so that there can be no doubt of the position of this extraordinary group of plants among the Ascomycetes. The various cells of these organisms are connected by large pits which are traversed by thick protoplasmic threads connecting one cell with the next. In this point and in their method of fertilization the Laboulbeniineae suggest a possible relationship of Ascomycetes and the Red Algae.
Basidiales.—This very large group of plants is characterized by the possession of a special type of conidiophore—the basidium, which gives its name to the group. The basidium is a unicellular or multicellular structure from which four basidiospores arise as outgrowths; it starts as a binucleate structure, but soon, like the ascus, becomes uninucleate by the fusion of the two nuclei. Then two successive nuclear divisions occur resulting in the formation of four nuclei which later migrate respectively into the four basidiospores (fig. 15). The Basidiales are further characterized by the complete loss of normal sexuality, but at some time or other in the life-history there takes place an association of two nuclei in a cell; the two nuclei are derived from separate cells or possibly in some cases are sister nuclei of the same cell. The two nuclei when once associated are termed “conjugate” nuclei, and they always divide at the same time, a half of each passing into each cell. This conjugate condition is finally brought to a close by the nuclear fusion in the basidium. Between the nuclear association and the nuclear fusion in the basidium many thousands of cell generations may be intercalated. This nuclear association of equivalent nuclei apparently represents a reduced sexual process (like the fusion of female nuclei in Humaria granulata and of vegetative nuclei in H. rutilans, among the Ascomycetes) in which, however, the actual fusion (normally, in a sexual process, occurring immediately after association) is delayed until the formation of the basidium. During the tetrad division in the basidium nuclear reduction occurs. There is thus in all the Basidiales an alternation of generations, obscured, however, by the apogamous transition from the gametophyte to sporophyte. The sporophyte may be considered to begin at the stage of nuclear association and end with the nuclear reduction in the basidium.
 | |
| Fig. 16.—Puccinia graminis. | |
|
A, Mass of teleutospores (t) on a leaf of couch-grass. e, Epidermis ruptured. b, Sub-epidermal fibres. (After De Bary.) |
B, Part of vertical section through leaf of Berberis vulgaris, with a, aecidium fruits, p, peridium, and sp, spermogonia. (After Sachs.) C, Mass of uredospores (ur), with one teleutospore (t). sh, Sub-hymenial hyphae. (After De Bary.) |
Uredineae.—This is a large group of about 2000 forms. They are all intercellular parasites living mostly on the leaves of higher plants. Owing to the presence of oily globules of an orange-yellow or rusty-red colour in their hyphae and spores they are termed Rust-Fungi. They are distinguished from the other fungi and the rest of the Basidiales by the great variety of the spores and the great elaboration of the life-history to be found in many cases. Five different kinds of spores may be present—teleutospores, sporidia (= basidiospores), aecidiospores, spermatia and uredospores (fig. 16). The teleutospore, with the sporidia which arise from it, is always present, and the division into genera is based chiefly on its characters. The teleutospore puts forth on germination a four-celled structure, the promycelium or basidium, and this bears later four sporidia or basidiospores, one on each cell. When the sporidia infect a plant the mycelium so produced gives origin to aecidiospores and spermatia; the aecidiospores on infection produce a mycelium which bears uredospores and later teleutospores. This is the life-history of the most complicated forms, of the so-called eu forms. In the opsis forms the uredospores are absent, the mycelium from the aecidiospores producing directly the teleutospores. In brachy and hemi the aecidiospores are absent, the mycelium from the sporidia giving origin directly to the uredospores; the former possess spermatia, in the latter they are absent. In lepto and micro forms both aecidiospores and uredospores are absent, the sporidia producing a mycelium which gives rise directly to teleutospores; in the lepto forms the teleutospores can germinate directly, in the micro forms only after a period of rest. We have thus a series showing a progressive reduction in the complexity of the life-history, the lepto and micro forms having a life-history like that of the Basidiomycetes. The eu and opsis forms may exhibit the remarkable phenomenon of heteroecism, i.e. the dependence of the fungus on two distinct host-plants for the completion of the life-history. Heteroecism is very common in this group and is now known in over one hundred and fifty species. In all cases of heteroecism the sporidia infect one host leading to the production of aecidiospores and spermatia (if present), while the aecidiospores are only able to infect another host on which the uredospores (if present) and the teleutospores are developed. A few examples are appended:
| Species. | Teleutospores on | Aecidiospores on |
| Coleosporium Senecionis | Pinus | Senecio |
| Melampsora Rostrupi | Populus | Mecurialis |
| Pucciniastrum Goeppertiana | Vaccinium | Abies |
| Gymnosporangium Sabinae | Juniperus | Pyrus |
| Uromyces Pisi | Pisum, &c. | Euphorbia |
| Puccinia graminis | Triticum, &c. | Berberis |
| P. dispersa | Secale, &c. | Anchusa |
| P. coronata | Agrostis | Rhamnus |
| P. Ari-Phalaridis | Phalaris | Arum |
| P. Caricis | Carex | Urtica |
| Cronartium Ribicola | Ribes | Pinus |
| Chrysomyxa Rhododendri | Rhododendron | Picea |
Some of the Uredineae also exhibit the peculiarity of the development of biologic forms within a single morphological species, sometimes termed specialization of parasitism; this will be dealt with later under the section Physiology.
 |
| From Strasburger’s Lehrbuch der Botanik, by permission of Gustav Fischer. |
| Fig. 17.—Phragmidium Violaceum. (After Blackman.) |
|
A, Portion of a young aecidium. st, Sterile cell. a, Fertile cells; at a2 the passage of a nucleus from the adjoining cell is seen. B, Formation of the first spore-mother-cell (sm), from the basal cell (a) of one of the rows of spores. C, A further stage in which from sm1 the first aecidiospore (a) and the intercalary cell (z) have arisen. sm2, The second spore-mother-cell. D, Ripe aecidiospore |
Cytology of Uredineae.—The study of the nuclear behaviour of the cells of the Uredineae has thrown great light on the question of sexuality. This group like the rest of the Basidiales exhibits an association of nuclei at some point in its life-history, but unlike the case of the Basidiomycetes the point of association in the Uredineae is very well defined in all those forms which possess aecidiospores. We find thus that in the eu and opsis forms the association of nuclei takes place at the base of the aecidium which produces the aecidiospores. There we find an association of nuclei either by the fusion of two similar cells as described by Christmann or by the migration of the nucleus of a vegetative cell into a special cell of the aecidium. After this association the nuclei continue in the conjugate condition so that the aecidiospores, the uredospore-bearing mycelium, the uredospores and the young teleutospores all contain two paired nuclei in their cells (fig. 17). Before the teleutospore reaches maturity the nuclei fuse, and the uninucleate condition then continues again until aecidium formation. In the hemi, brachy, micro and lepto forms, which possess no aecidium, we find that the association takes place at various points in the ordinary mycelium but always before the formation of the uredospores in the hemi and brachy forms, and before the formation of teleutospores in micro and lepto form. Whether the association of nuclei in the ordinary mycelium takes place by the migration of a nucleus from one cell to another or whether two daughter nuclei become conjugate in one cell, is not yet clear. The most reasonable interpretation of the spermatia is that they are abortive male cells. They have never been found to cause infection, and they have not the characters of conidia; the large size of their nuclei, the reduction of their cytoplasm and the absence of reserve material and their thin cell wall all point to their being male gametes. Although in the forms without aecidia the two generations are not sharply marked off from one another, we may look up the generation with single nuclei in the cells as the gametophyte and that with conjugate nuclei as the sporophyte. The subjoined diagram will indicate the relationship of the forms.
Basidiomycetes.—This group is characterized by its greatly reduced life-history as compared with that of the eu forms among the Uredineae. All the forms have the same life-history as the lepto forms of that group, so that there is no longer any trace of sexual organs. There is also a further reduction in that the basidium is not derived from a teleutospore but is borne directly on the mycelium. Formerly, before the relationship of promycelium and basidium were understood, the Uredineae were considered as quite independent of the Basidiomycetes. Later, however, these Uredineae were placed as a mere subdivision of the Basidiomycetes. Although the Uredineae clearly lead on to the Basidiomycetes, yet owing to their retaining in many cases definite traces of sexual organs they are clearly a more primitive group. Their marked parasitic habit also separates them off, so that they are best included with the Basidiomycetes in a larger cohort which may be called Basidiales. Most of Basidiomycetes are characterized by the large sporophore on which the basidia with its basidiospores are borne.
 |
| From Annals of Botany, by permission of the Clarendon Press. |
| Fig. 18. |
It must be clearly borne in mind that though the Basidiomycetes show no traces of differentiated sexual organs yet, like the micro and lepto forms of the Uredineae, they still show (in the association of nuclei and later fusion of nuclei in the basidium), a reduced fertilization which denotes their derivation, through the Uredineae, from more typically sexual forms. No one has yet made out in any form the exact way in which the association of nuclei takes place in the group. The mycelium is always found to contain conjugate nuclei before the formation of basidia, but the point at which the conjugate condition arises seems very variable. Miss Nichols finds that it occurs very soon after the germination of the spore in Coprinus, but no fusion of cells or migration of nuclei was to be observed.
 | |
| Fig. 19.—Amanita muscaria. | |
|
A, The young plant. B, The mature plant. C, Longitudinal section of mature plant. p, The pileus. |
g, The gills. a, The annulus, or remnant of velum partiale, v, Remains of volva or velum universale. s, The stalk. |
Protobasidiomycetes.—This, by far the smaller division of Basidiomycetes, includes those forms which have a septate basidium. There are three families—Auriculariaceae, Pilacreaceae and Tremellinaceae. The first named contains a small number of forms with the basidium divided like the promycelium of the Uredineae. They are characterized by their gelatinous consistence and large size of their sporophore. Hirneola (Auricularia) Auricula-Judae is the well-known Jew’s Ear, so named from the resemblance of the sporophore to a human ear.
The Pilacreaceae are a family found by Brefeld to contain the genus Pilacre. P. Petersii has a transversely divided basidium as in Auriculariaceae, but the basidia are surrounded with a peridium-like sheath. The Tremellinaceae are characterized by the possession of basidia which are divided by two vertical walls at right angles to one another. From each of the four segments in the case of Tremella a long outgrowth arises which reaches to the surface of the hymenium and bears the basidiospores. In Dacryomyces only two outgrowths and two spores are produced.
Autobasidiomycetes.—In this by far the larger division of the Basidiomycetes the basidia are undivided and the four basidiospores are borne on short sterigmata nearly always at the apex of the basidium. The group may be divided into two main divisions, Hymenomycetes and Gasteromycetes.
 |
| Fig. 20.—Agaricus mucidus. Portion of hymenium. s, Sporidia; st, sterigmata; g, sterile cells; c, cystidium, with operculum o. |
Hymenomycetes are a very large group containing over 11,000 species, most of which live in soil rich in humus or on fallen wood or stems, a few only being parasites. In the simplest forms (e.g. Exobasidium) the basidia are borne directly on the ordinary mycelium, but in the majority of cases the basidia are found developed in layers (hymenium) on special sporophores of characteristic form in the various groups. In these sporophores (such as the well-known toadstools and mushrooms where the ordinary vegetative mycelium is underground) we have structures specially developed for bearing the basidiospores and protecting them from rain, &c., and for the distribution of the spores—see earlier part of article on distribution of spores (figs. 19 and 20). The underground mycelium in many cases spreads wider and wider each year, often in a circular manner, and the sporophores springing from it appear in the form of a ring—the so-called fairy rings. Armillaria melleus and Polyporus annosus are examples of parasitic forms which attack and destroy living trees, while Merulius lacrymans is the well-known “dry rot” fungus.
Gasteromycetes are characterized by having closed sporophores or fruit-bodies which only open after the spores are ripe and then often merely by a small pore. The fruit-bodies are of very various shapes, showing a differentiation into an outer peridium and an inner spore-bearing mass, the gleba. The gleba is usually differentiated into a number of chambers which are lined directly by the hymenium (basidial layer), or else the chambers contain an interwoven mass of hyphae, the branches of which bear the basidia. By the breaking down of the inner tissues the spores often come to lie as a loose powdery mass in the interior of the hollow fruit-body, mixed sometimes with a capillitium. The best-known genera are Bovista, Lycoperdon (puff-ball) Scleroderma, Geaster (earth-star, q.v.). In the last-named genus the peridium is double and the outer layer becomes ruptured and spreads out in the form of star-shaped pieces; the inner layer, however, merely opens at the apex by a small pore.
The most complex members of the Gasteromycetes belong to the Phalloideae, which is sometimes placed as a distinct division of the Autobasidiomycetes. Phallus impudicus, the stink-horn, is occasionally found growing in woods in Britain. The fruit-body before it ruptures may reach the size of a hen’s egg and is white in colour; from this there grows out a hollow cylindrical structure which can be distinguished at the distance of several yards by its disgusting odour. It is highly poisonous.
Physiology.—The physiology of the fungi comes under the head of that of plants generally, and the works of Pfeffer, Sachs, Vines, Darwin and Klebs may be consulted for details. But we may refer generally here to certain phenomena peculiar to these plants, the life-actions of which are restricted and specialized by their peculiar dependence on organic supplies of carbon and nitrogen, so that most fungi resemble the colourless cells of higher plants in their nutrition. Like these they require water, small but indispensable quantities of salts of potassium, magnesium, sulphur and phosphorus, and supplies of carbonaceous and nitrogenous materials in different stages of complexity in the different cases. Like these, also, they respire oxygen, and are independent of light; and their various powers of growth, secretion, and general metabolism, irritability, and response to external factors show similar specific variations in both cases. It is quite a mistake to suppose that, apart from the chlorophyll function, the physiology of the fungus-cell is fundamentally different from that of ordinary plant-cells. Nevertheless, certain biological phenomena in fungi are especially pronounced, and of these the following require particular notice.
Parasitism. —Some fungi, though able to live as saprophytes, occasionally enter the body of living plants, and are thus termed facultative parasites. The occasion may be a wound (e.g. Nectria, Dasyscypha, &c.), or the enfeeblement of the tissues of the host, or invigoration of the fungus, the mycelium of which then becomes strong enough to overcome the host’s resistance (Botrytis). Many fungi, however, cannot complete their life-history apart from the host-plant. Such obligate parasites may be epiphytic (Erysipheae), the mycelium remaining on the outside and at most merely sending haustoria into the epidermal cells, or endophytic (Uredineae, Ustilagineae, &c.), when the mycelium is entirely inside the organs of the host. An epiphytic fungus is not necessarily a parasite, however, as many saprophytes (moulds, &c.) germinate and develop a loose mycelium on living leaves, but only enter and destroy the tissues after the leaf has fallen; in some cases, however, these saprophytic epiphytes can do harm by intercepting light and air from the leaf (Fumago, &c.), and such cases make it difficult to draw the line between saprophytism and parasitism. Endophytic parasites may be intracellular, when the fungus or its mycelium plunges into the cells and destroys their contents directly (Olpidium, Lagenidium, Sclerotinia, &c.), but they are far more frequently intercellular, at any rate while young, the mycelium growing in the lacunae between the cells (Peronospora, Uredineae) into which it may send short (Cystopus), or long and branched (Peronospora Calotheca) haustoria, or it extends in the middle lamella (Ustilago), or even in the solid substance of the cell-wall (Botrytis). No sharp lines can be drawn, however, since many mycelia are intercellular at first and subsequently become intracellular (Ustilagineae), and the various stages doubtless depend on the degrees of resistance which the host tissues are able to offer. Similar gradations are observed in the direct effect of the parasite on the host, which may be local (Hemileia) when the mycelium never extends far from the point of infection, or general (Phytophthora) when it runs throughout the plant. Destructive parasites rapidly ruin the whole plant-body (Pythium), whereas restrained parasites only tax the host slightly, and ill effects may not be visible for a long time, or only when the fungus is epidemic (Rhytisma). A parasite may be restricted during a long incubation-period, however, and rampant and destructive later (Ustilago). The latter fact, as well as the extraordinary fastidiousness, so to speak, of parasites in their choice of hosts or of organs for attack, point to reactions on the part of the host-plant, as well as capacities on that of the parasite, which may be partly explained in the light of what we now know regarding enzymes and chemotropism. Some parasites attack many hosts and almost any tissue or organ (Botrytis cinerea), others are restricted to one family (Cystopus Candidus) or genus (Phytophthora infestans) or even species (Pucciniastrum Padi), and it is customary to speak of root-parasites, leaf-parasites, &c., in expression of the fact that a given parasite occurs only on such organs—e.g. Dematophora necatrix on roots, Calyptospora Goeppertiana on stems, Ustilago Scabiosae in anthers, Claviceps purpurea in ovaries, &c. Associated with these relations are the specializations which parasites show in regard to the age of the host. Many parasites can enter a seedling, but are unable to attack the same host when older—e.g. Pythium, Phytophthora omnivora.
Chemotropism.—Taken in conjunction with Pfeffer’s beautiful discovery that certain chemicals exert a distinct attractive influence on fungus hyphae (chemotropism), and the results of Miyoshi’s experimental application of it, the phenomena of enzyme-secretion throw considerable light on the processes of infection and parasitism of fungi. Pfeffer showed that certain substances in definite concentrations cause the tips of hyphae to turn towards them; other substances, though not innutritious, repel them, as also do nutritious bodies if too highly concentrated. Marshall Ward showed that the hyphae of Botrytis pierce the cell-walls of a lily by secreting a cytase and dissolving a hole through the membrane. Miyoshi then demonstrated that if Botrytis is sown in a lamella of gelatine, and this lamella is superposed on another similar one to which a chemotropic substance is added, the tips of the hyphae at once turn from the former and enter the latter. If a thin cellulose membrane is interposed between the lamellae, the hyphae nevertheless turn chemotropically from the one lamella to the other and pierce the cellulose membrane in the process. The hyphae will also dissolve their way through a lamella of collodion, paraffin, parchment paper, elder-pith, or even cork or the wing of a fly, to do which it must excrete very different enzymes. If the membrane is of some impermeable substance, like gold leaf, the hyphae cannot dissolve its way through, but the tip finds the most minute pore and traverses the barrier by means of it, as it does a stoma on a leaf We may hence conclude that a parasitic hyphae pierces some plants or their stomata and refuses to enter others, because in the former case there are chemotropically attractive substances present which are absent from the latter, or are there replaced by repellent poisonous or protective substances such as enzymes or antitoxins.
Specialization of Parasitism.—The careful investigations of recent years have shown that in several groups of fungi we cannot be content to distinguish as units morphologically different species, but we are compelled to go deeper and analyse further the species. It has been shown especially in the Uredineae and Erysiphaceae that many forms which can hardly be distinguished morphologically, or which cannot be differentiated at all by structural characters, are not really homogeneous but consist of a number of forms which are sharply distinguishable by their infecting power. Eriksson found, for example, that the well-known species Puccinia graminis could be split up into a number of forms which though morphologically similar were physiologically distinct. He found that the species really consisted of six distinct races, each having a more or less narrow range of grasses on which it can live. The six races he named P. graminis Secalis, Tritici, Avenae, Airae, Agrostis, Poae. The first named will grow on rye and barley but not on wheat or oat. The form Tritici is the least sharply marked and will grow on wheat, barley, rye and oat but not on the other grasses. The form Avenae will grow on oat and many grasses but not on the other three cereals mentioned. The last three forms grow only on the genera Aira, Agrostis and Poa respectively. All these forms have of course their aecidium-stage on the barberry. The terms biologic forms, biological species, physiological species, physiological races, specialized forms have all been applied to these; perhaps the term biologic forms is the most satisfactory. A similar specialization has been observed by Marshall Ward in the Puccinia parasitic on species of Bromus, and by Neger, Marchal and especially Salmon in the Erysiphaceae. In the last-named family the single morphological species Erysiphe graminis is found growing on the cereals, barley, oat, wheat, rye and a number of wild grasses (such as Poa, Bromus, Dactylis). On each of these host-plants the fungus has become specialized so that the form on barley cannot infect the other three cereals or the wild grasses and so on. Just as the uredospores and aecidiospores both show these specialized characters in the case of Puccinia graminis so we find that both the conidia and ascospores of E. graminis show this phenomenon. Salmon has further shown in investigating the relation of E. graminis to various species of the genus, Bromus, that certain species may act as “bridging species,” enabling the transfer of a biologic form to a host-plant which it cannot normally infect. Thus the biologic form on B. racemosus cannot infect B. commutatus. If, however, conidia from B. racemosus are sown on B. hordaceus, the conidia which develop on that plant are now able to infect B. commutatus; thus B. hordaceus acts as a bridging species. Salmon also found that injury of a leaf by mechanical means, by heat, by anaesthetics, &c., would affect the immunity of the plant and allow infection by conidia which was not able to enter a normal leaf. The effect of the abnormal conditions is probably to stop the production of, or weaken or destroy the protective enzymes or antitoxins, the presence of which normally confers immunity on the leaf.
Symbiosis.—The remarkable case of life in common first observed in lichens, where a fungus and an alga unite to form a compound organism—the lichen—totally different from either, has now been proved to be universal in these plants, and lichens are in all cases merely algae enmeshed in the interwoven hyphae of fungi (see LICHENS). This dualism, where the one constituent (alga) furnishes carbohydrates, and the other (fungus) ensures a supply of mineral matters, shade and moisture, has been termed symbiosis. Since then numerous other cases of symbiosis have been demonstrated. Many trees are found to have their smaller roots invaded by fungi and deformed by their action, but so far from these being injurious, experiments go to show that this mycorhiza (fungus-root) is necessary for the well-being of the tree. This is also the case with numerous other plants of moors and woodlands—e.g. Ericaceae, Pyrolaceae, Gentianaceae, Orchidaceae, ferns, &c. Recent experiments have shown that the difficulties of getting orchid seeds to germinate are due to the absence of the necessary fungus, which must be in readiness to infect the young seedling immediately after it emerges from the seed. The well-known failures with rhododendrons, heaths, &c., in ordinary garden soils are also explained by the need of the fungus-infected peat for their roots. The rôle of the fungus appears to be to supply materials from the leaf-mould around, in forms which ordinary root-hairs are incapable of providing for the plant; in return the latter supports the fungus at slight expense from its abundant stores of reserve materials. Numerous other cases of symbiosis have been discovered among the fungi of fermentation, of which those between Aspergillus and yeast in saké manufacture, and between yeasts and bacteria in kephir and in the ginger-beer plant are best worked out. For cases of symbiosis see Bacteriology.
Authorities.—General: Engler and Prantl, Die natürlichen Pflanzenfamilien, i. Teil (1892 onwards); Zopf, Die Pilze (Breslau, 1890); De Bary, Comparative Morphology of Fungi, &c. (Oxford, 1887); von Tafel, Vergleichende Morphologie der Pilze (Jena, 1892); Brefeld, Unters. aus dem Gesamtgebiete der Mykologie, Heft i. 13 (1872-1905); Lotsy, Vorträge über botanische Stammesgeschichte (Jena, 1907). Distribution, &c.: Cooke, Introduction to the Study of Fungi (London, 1895); Felix in Zeitschr. d. deutsch. geologisch. Gesellsch. (1894-1896); Staub, Sitzungsber. d. bot. Sec. d. Kgl. ungarischen naturwiss. Gesellsch. zu Budapest (1897). Anatomy, &c.: Bommer, “Sclerotes et cordons mycéliens,” Mém. de l’Acad. Roy. de Belg. (1894); Mangin, “Observ. sur la membrane des mucorinées,” Journ. de Bot. (1899); Zimmermann, Die Morph. und Physiologie des Pflanzenzellkernes (Jena, 1896); Wisselingh, “Microchem. Unters. über die Zellwände d. Fungi,” Pringsh. Jahrb. B. 31, p. 619 (1898); Istvanffvi, “Unters. über die phys. Anat. der Pilze,” Prings. Jahrb. (1896). Spore Distribution: Fulton, “Dispersal of the Spores of Fungi by Insects,” Ann. Bot. (1889); Falck, “Die Sporenverbreitung bei den Basidiomyceten,” Beitr. zur Biol. d. Pflanzen, ix. (1904). Spores and Sporophores: Zopf, Die Pilze; also the works of von Tafel and Brefeld. Classification: van Tieghem, Journ. de bot. p. 77 (1893), and the works of Brefeld, Engler and Prantl, von Tafel, Saccardo and Lotsy already cited, Oomycetes: Wager, “On the Fertilization of Peronospora parasitica,” Ann. Bot. vol. xiv. (1900); Stevens, “The Compound Oosphere of Albugo Bliti,” Bot. Gaz. vol. 28 (1899); “Gametogenesis and Fertilization in Albugo,” ibid. vol. 32 (1901); Miyake, “The Fertilization of Pythium de Baryanum,” Ann. of Bot. vol. xv. (1901); Trow, “On Fertilization in the Saprolegnieae,” Ann. of Bot. vol. xviii. (1904); Thaxter, “New and Peculiar Aquatic Fungi,” Bot. Gaz. vol. 20 (1895); Lagerheim, “Unters. über die Monoblepharideae,” Bih. Svenska Vet. Acad. Handlingar, 25. Afd. iii. (1900); Woronin, “Beitrag zur Kenntnis der Monoblepharideen,” Mém. de l’Acad. Imp. d. Sc. de St-Pétersbourg, 8 sér. vol. 16 (1902). Zygomycetes: Harper, “Cell-division in Sporangia and Asci,” Ann. Bot. vol. xiii. (1899); Klebs, Die Bedingungen der Fortpflanzung, &c. (Jena, 1896), and “Zur Physiologie der Fortpflanzung” Prings. Jahr. (1898 and 1899), “Über Sporodinia grandis,” Bot. Zeit. (1902); Falck, “Die Bedingungen der Zygotenbildung bei Sporodinia grandis,” Cohn’s Beitr. z. Biol. d. Pflanzen, Bd. 8 (1902); Gruber “Verhalten der Zellkerne in den Zygosporen von Sporodinia grandis,” Ber. d. deutschen bot. Ges. Bd. 19 (1901); Blakeslee, “Sexual Reproduction in the Mucorineae,” Proc. Am. Acad. (1904); “Zygospore germination in the Mucorineae,” Annales mycologici (1906). Ustilagineae: Plowright, British Uredineae and Ustilagineae (London, 1889); Massee, British Fungi (Phycomycetes and Ustilagineae) (London, 1891); Brefeld, Unters. aus dem Gesamtgeb. der Mykol. Hefte xi. and xii.; and Falck, “Die Bluteninfektion bei den Brandpilzen,” ibid. Heft xiii. 1905; Dangeard, “La Reproduction sexuelle des Ustilaginées,” C.R., Oct. 9, 1893; Maire, “Recherches cytologiques et taxonomiques sur les Basidiomyceten,” Annexé au Bull. de la Soc. Mycol. de France (1902). Saccharomycetaceae: Jorgensen, The Micro-organisms of Fermentation (1899); Barker, Ann. of Bot. vol. xiv. (1901); “On Spore-formation among the Saccharomycetes,” Journ. of the Fed. Institute of Brewing, vol. 8 (1902); Guillermond, Recherches cytologiques sur lés levures (Paris, 1902); Hansen, Centralbl. f. Bakt. u. Parasitenp. Abt. ii. Bd. 12 (1904). Exoascaceae: Giesenhagen, “Taphrina, Exoascus, Magnusiella” (complete literature given), Bot. Zeit. Bd. 7 (1901). Erysiphaceae: Harper, “Die Entwicklung des Perithecium bei Sphaerotheca castagnei,” Ber. d. deut bot Ges. (1896); “Sexual Reproduction and the Organization of the Nucleus in certain Mildews,” Publ. Carnegie Institution (Washington, 1906); Blackman & Fraser, “Fertilization in Sphaerotheca,” Ann. of Bot. (1905). Perisporiaceae: Brefeld, Untersuchungen aus dem Gesamtgeb. der Mykol. Heft 10 (1891); Fraser and Chamber, Annales mycologici (1907). Discomycetes: Harper, “Über das Verhalten der Kerne bei Ascomyceten,” Jahr. f. wiss. Bot. Bd. 29 (1890); “Sexual Reproduction in Pyronema confluens,” Ann. of Bot. 14 (1900); Claussen, “Zur Entw. der Ascomyceten,” Boudiera, Bot. Zeit. Bd. 63 (1905); Dangeard, “Sur le Pyronema confluens,” Le Botaniste, 9 série (1903) (and numerous papers in same journal earlier and later); Ramlow, “Zur Entwick. von Thelebolus stercoren,” Bot. Zeit. (1906); Woronin, “Über die Sclerotienkrankheit der Vaccineen Beeren,” Mem. de l’Acad. Imp. des Sciences de St-Pétersbourg, 7 série, 36 (1888); Dittrich, “Zur Entwickelungsgeschichte der Helvellineen,” Cohn’s Beitr. z. Biol. d. Pflanzen (1892). Pyrenomycetes: Fisch, “Beitr. z. Entwickelungsgeschichte einiger Ascomyceten,” Bot. Zeit. (1882); Frank, “Über einige neue u. weniger bekannte Pflanzkrankh.,” Landw. Jahrb. Bd. 12 (1883); Ward, “Onygena equina, a horn-destroying fungus,” Phil. Trans., vol. 191 (1899); Dawson, “On the Biology of Poroniapunctata,” Ann. of Bot. 14 (1900). Tuberineae: Buchholtz, “Zur Morphologie u. Systematik der Fungi hypogaei,” Ann. Mycol. Bd. 1 (1903); Fischer in Engler and Prantl, Die natürlichen Pflanzenfamilien (1896). Laboulbeniineae: Thaxter, “Monograph of the Laboulbeniaceae,” Mem. Amer. Acad. of Arts and Sciences, vol. 12 (1895). Uredineae: Eriksson and Henning, Die Getreideroste (Stockholm, 1896); Eriksson, Botan. Gaz. vol. 25 (1896); “On the Vegetative Life of some Uredineae,” Ann. of Bot. (1905); Klebahn, Die wirtwechselnden Rostpilze (Berlin, 1904); Sapin-Trouffy, “Recherches histologiques sur la famille des Urédinées,” Le Botaniste (1896-1897); Blackman, “On the Fertilization, Alternation of Generations and General Cytology of the Uredineae,” Ann. of Bot. vol. 18 (1904); Blackman and Fraser, “Further Studies on the Sexuality of Uredineae,” Ann. of Bot. vol. 20 (1906); Christman, “Sexual Reproduction of Rusts,” Ann. of Bot. vol. 20 (1906); Ward, “The Brooms and their Rust Fungus,” Ann. of Bot. vol. 15 (1901). Basidiomycetes: Dangeard, “La Reprod. sexuelle des Basidiomycètes,” Le Botaniste (1894 and 1900); Maire, “Recherches cytologiques et taxonomiques sur les Basidiomycètes,” Annexe du Bull. de la Soc. Mycol. de France (1902); Möller, “Protobasidiomyceten,” Schimper’s Mitt. aus den Tropen, Heft 8 (Jena, 1895); Nichols, “The Nature and Origin of the Binucleated Cells in certain Basidiomycetes,” Trans. Wisconsin Acad. of Sciences, vol. 15 (1905); Wager, “The Sexuality of the Fungi,” Ann. of Bot. 13 (1899); Woronin, “Exobasidium Vaccinii,” Verh. Naturf. Ges. zu Freiburg, Bd. 4 (1867). Fermentation: Buchner, “Gährung ohne Hefezellen,” Bot. Zeit. Bd. 18 (1898); Albert, Cent. f. Bakt. Bd. 17 (1901); Green, The Soluble Ferments and Fermentation (Cambridge, 1899). Parasitism: “On some Relations between Host and Parasite,” Proc. Roy. Soc. vol. 47 (1890); “A Lily Disease,” Ann. of Botany, vol. 2 (1888); Eriksson & Hennings, Die Getreideroste (vide supra); Ward, “On the Question of Predisposition and Immunity in Plants,” Proc. Cambridge Phil. Soc. vol. 11 (1902); also Annals of Bot. vol. 16 (1902) and vol. 19 (1905); Neger, “Beitr. z. Biol. d. Erysipheen” Flora, Bde. 88 and 90 (1901-1902); Salmon, “Cultural Experiments with ‘Biologic Forms’ of the Erysiphaceae,” Phil. Trans. (1904); “On Erysiphe graminis and its adaptative parasitism within the genus, Bromus,” Ann. Mycol. vol. 11 (1904), also Ann. of Bot. vol. 19 (1905). Symbiosis: Ward, “The Ginger-Beer Plant,” Phil. Trans. Roy. Soc. (1892); “Symbiosis,” Ann. of Bot. 13 (1899); Shalk, “Der Sinn der Mykorrhizenbildung,” Jahrb. f. wiss. Bot. Bd. 34 (1900); Bernard, “On some Different Cases of Germination,” Gardener’s Chronicle (1900); Pierce, Publ. Univ. California (1900).
↧ Download as ZWI file | Last modified: 11/17/2022 15:24:13 | 51 views
☰ Source: https://oldpedia.org/article/britannica11/Fungi | License: Public domain in the USA. Project Gutenberg License
 ZWI signed:
ZWI signed: KSF
KSF