Neurine
 From Handwiki
From Handwiki 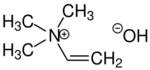
| |
| Names | |
|---|---|
| IUPAC name
Trimethylvinylammonium hydroxide
| |
| Other names
Vitaloid; N,N,N-Trimethylethenaminium hydroxide
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C5H13NO |
| Molar mass | 103.16 |
| Appearance | Syrupy liquid |
Solubility in water
|
Soluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
- SizeSet
Neurine is an alkaloid found in egg yolk, brain, bile and in cadavers. It is formed during putrefaction of biological tissues by the dehydration of choline. It is a poisonous, syrupy liquid with a fishy odor.
Neurine is a quaternary ammonium salt with three methyl groups and one vinyl group attached to the nitrogen atom. Synthetically, neurine can be prepared by the reaction of acetylene with trimethylamine.[1] Neurine is unstable and decomposes readily to form trimethylamine.
References
- ↑ Gardner, C.; Kerrigan, V.; Rose, J. D.; Weedon, B. C. L. (1949-01-01). "169. Acetylene reactions. Part IV. Formation of trimethylvinyl- and tetramethyl-ammonium hydroxide from acetylene and aqueous trimethylamine" (in en). Journal of the Chemical Society (Resumed): 789–792. doi:10.1039/JR9490000789. ISSN 0368-1769. https://pubs.rsc.org/en/content/articlelanding/1949/jr/jr9490000789.
- Merck Index, 11th Edition, 6393.
 |
Categories: [Alkaloids] [Quaternary ammonium compounds] [Vinyl compounds]
↧ Download as ZWI file | Last modified: 08/11/2024 05:29:24 | 22 views
☰ Source: https://handwiki.org/wiki/Chemistry:Neurine | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF