Ferrate
 From Handwiki
From Handwiki Ferrate loosely refers to a material that can be viewed as containing anionic iron complexes. Examples include tetrachloroferrate ([FeCl4]2−), oxyanions (FeO2−4), tetracarbonylferrate ([Fe(CO)4]2−), the organoferrates.[1][page needed] The term ferrate derives from the Latin word for iron, ferrum.
- Ferrates
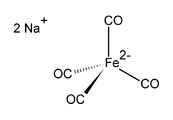
Disodium salt of tetracarbonylferrate.
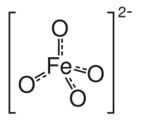
Structure of ferrate(VI), [FeO4]2−.

1-Butyl-3-methylimidazolium salt of [FeCl4]−.
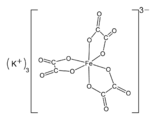
Potassium tris(oxalato)ferrate.
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
 |
Categories: [Iron compounds] [Anions] [Ferrates]
↧ Download as ZWI file | Last modified: 11/07/2024 08:10:24 | 8 views
☰ Source: https://handwiki.org/wiki/Chemistry:Ferrate | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF