Glutamine
 From Nwe
From Nwe 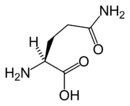  Chemical structure of L-glutamine |
|
Glutamine |
|
| Systematic (IUPAC) name | |
| (2S)-2-amino-4-carbamoyl-butanoic acid | |
| Identifiers | |
| CAS number | 56-85-9 |
| PubChem | 738 |
| Chemical data | |
| Formula | C5H10N2O3 |
| Mol. weight | 146.15 |
| SMILES | N[C@@H](CCC(N)=O)C(O)=O |
| Complete data | |
Glutamine is an α-amino acid that is found in many proteins and is a neutralized version of the acidic amino acid glutamic acid, having an amide side chain that is formed by replacing a side-chain hydroxyl of glutamic acid with an amine functional group. Glutamine is the most abundant amino acid in human beings (Longe 2005a). In addition to being a constituent of proteins, it is important in many metabolic processes, in the elimination of toxic ammonia from the body, and in immunity, as well as a nutritional supplement in treating a variety of diseases.
The L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids common in animal proteins and required for normal functioning in humans. However, it is not considered to be an "essential" amino acid since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions. It is essential in the diet of premature infants, however, who cannot produce glutamine fast enough.
Glutamine plays an important role relative to stress. Stress, such as induced by burns, trauma, excessive exercise, and various diseases, such as cancer, often results in glutamine deficiency (Longe 2005a, 2005b). Although glutamine normally is plentiful in the diet and can be synthesized by the body, human creativity has developed means to synthesize this amino acid, which provides so many valuable functions, and develop supplements to be able to aid the body from recovery from stress.
Glutamine's three letter code is Gln, its one letter code is Q, and its systematic name is 2-Amino-4-carbamoylbutanoic acid(IUPAC-IUB 1983). A three-letter designation for either glutamine (Gln) or glutamic acid (Glu) is Glx and a one-letter abbreviation for either is Z—these are often used in cases in which peptide sequencing reactions may convert glutamine to glutamate (or vice versa), leaving the original identity of the amino acid in doubt. Glutamine is genetically coded for by the RNA codons CAA and CAG.
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In glutamine, only the L-stereoisomer is involved in protein synthesis in mammals.
Glutamine's chemical formula is HOOC-CH(NH2)-(CH2)2-CO-H2N, which is very similar to glutamic acid's formula, HOOC-CH(NH2)-(CH2)2-COOH, but with the -OH replaced by H2N; in other words, a side-chain hydroxyl of glutamic acid replaced with an amine functional group, yielding an amide side chain. Glutamine's general formula is C5H10N2O3.
Biological importance
Like other amino acids, glutamine is biochemically important as a constituent of proteins. It is also important for such metabolic processes as regulation of cell growth and function, gluconeogenesis (generation of glucose from non-sugar carbon substrates like pyruvate, lactate, glycerol, and such glucogenic amino acids as alanine and glutamine); maintenance of acid-base equilibrium in the body; improved kidney cell function; and as a major fuel for intestinal mucosal cells (Longe 2005b). Along with arginine, glutamine is referred to as an immunonutrient because of its role in the functioning of the immune system and as a major fuel for lymphocytes (type of white blood cell) (Longe 2005a, Longe 2005b). It appears to be the rate-limiting factor for the production of glutathione (GSH), a chemical that protects cells against the damage of oxidation (Longe 2005b).
Glutamine is also crucial in nitrogen metabolism. Ammonia (formed by nitrogen fixation) is assimilated into organic compounds by converting glutamic acid to glutamine. The enzyme that accomplishes this is called glutamine synthetase. Glutamine can, hence, be used as a nitrogen donor in the biosynthesis of many compounds, including other amino acids, purines, and pyrimidines.
It also is important as an intermediate in the removal of ammonia from the body. Ammonia is a metabolic product of amino acid deamination (removal of an amine group) and is toxic to the body. In humans, ammonia is quickly converted to urea, which is much less toxic. Essentially, glutuamic acid coupled with ammonia yields glutamine, which is transported to the liver. Glutamine can then yield its ammonia for the formation of urea for excretion. Glutamine is the most plentiful amino acid in the bloodstream (Longe 2005a).
Nutrition and treatment of disease
In addition to be synthesized in the body, glutamine is generally common in the diet and thus healthy people obtain all they need. Glutamine is found in foods high in proteins, such as fish, red meat, beans, dairy products, legumes, peanuts, eggs, and in raw cabbage and beets, although cooking can destroy glutamine in vegetables (Longe 2005a).
However, states of stress, such as induced by cancer and other diseases, as well as burns, trauma, and excessive exercise, often results in glutamine deficiency (Longe 2005a, 2005b). Thus, glutamine, which is important to many functions, including that of the immune system, is sometimes added medically to the body by physicians or through dietary supplements (Longe 2005b).
It is also known that glutamine has various effects in reducing healing time after operations. Hospital waiting times after abdominal surgery are reduced by providing parenteral nutrition regimens containing amounts of glutamine to patients. Clinical trials have revealed that patients on supplementation regimens containing glutamine have improved nitrogen balances, generation of cysteinyl-leukotrienes from polymorphonuclear neutrophil granulocytes, and improved lymphocyte recovery and intestinal permeability (in postoperative patients)—in comparison to those who had no glutamine within their dietary regime; all without any side-effects (Morlion 1998).
There have been several recent studies into the effects of glutamine and what properties it possesses, and, there is now a significant body of evidence that links glutamine-enriched diets with intestinal effects; aiding maintenance of gut barrier function, intestinal cell proliferation and differentiation, as well as generally reducing septic morbidity, and the symptoms of Irritable Bowel Syndrome. The reason for such "cleansing" properties is thought to stem from the fact that the intestinal extraction rate of glutamine is higher than that for other amino acids, and is therefore thought to be the most viable option when attempting to alleviate conditions relating to the gastrointestinal tract (Boza 2001).
These conditions were discovered after comparing plasma concentration within the gut between glutamine-enriched and non glutamine-enriched diets. However, even though glutamine is thought to have "cleansing" properties and effects, it is unknown to what extent glutamine has clinical benefits, due to the varied concentrations of glutamine in varieties of food (Boza 2001).
Glutamine is a supplement that is used in weightlifting, bodybuilding, endurance and other sports, as well as by those who suffer from muscular cramps or pain—particularly elderly people. The main use of glutamine within the diet of either group is as a means of replenishing the body's supply of amino acids that have been used during exercise or everyday activities.
Studies which are looking into problems with excessive consumption of glutamine thus far have proved inconclusive. Normal supplementation is healthy mainly because glutamine is helpful after prolonged periods of exercise (for example, a workout or exercise in which amino acids are required for use) and replenishes amino acid supply. This is the main reason glutamine is recommended during fasting or for people who suffer from physical trauma, immune deficiencies, or cancer. A secondary benefit to bettering body immunity is fortification of the intestinal tract, responsible for roughly 70 percent of the body's immunity.
References
ISBN links support NWE through referral fees
- Boza, J.J., M. Dangin, D. Moennoz, F. Montigon, J. Vuichoud, A. Jarret, E. Pouteau, G. Gremaud, S. Oguey-Araymon, D. Courtois, A. Woupeyi, P. A. Finot, and O. Ballevre. 2001. Free and protein-bound glutamine have identical splanchnic extraction in healthy human volunteers Am J Physiol Gastrointest Liver Physiol. 281(1): G267-74. PMID 11408280 Retrieved December 9, 2007.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology IUPAC-IUB. Retrieved December 9, 2007.
- Jiang, Z. M., J. D. Cao, X. G. Zhu, W. X. Zhao, J. C. Yu, E. L. Ma, X. R. Wang, M. W. Zhu, H. Shu, and Y. W. Liu. 1999. The impact of alanyl-glutamine on clinical safety, nitrogen balance, intestinal permeability, and clinical outcome in postoperative patients: A randomised, double-blind, controlled study of 120 patients. JPEN J Parenter Enteral Nutr. 23(5 Suppl):S62-6. PMID 10483898 Retrieved December 9, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536
- Longe, J. L. 2005a. The Gale Encyclopedia of Alternative Medicine. Detroit: Thomson Gale. ISBN 0787674249
- Longe, J. L. 2005b. The Gale Encyclopedia of Cancer: A Guide to Cancer and its Treatments. Detroit: Thomson Gale. ISBN 1414403623
- McAnena, O. J., F. A. Moore, E. E. Moore, T. N. Jones, and P. Parsons. 1991. Selective uptake of glutamine in the gastrointestinal tract: confirmation in a human study. Br J Surg. 78(4): 480-2. PMID 1903318 Retrieved December 9, 2007.
- Morlion, B. J., P. Stehle, P. Wachtler, H. P. Siedhoff, M. Koller, W. Konig, P. Furst, and C. Puchstein. 1998. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery. Ann Surg. 227(2): 302-308. PMID 9488531 Retrieved December 9, 2007.
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
↧ Download as ZWI file | Last modified: 02/04/2023 08:53:15 | 16 views
☰ Source: https://www.newworldencyclopedia.org/entry/Glutamine | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: KSF
KSF