Niobium Disulfide
 From Handwiki
From Handwiki  NbS2 exfoliated layer
| |
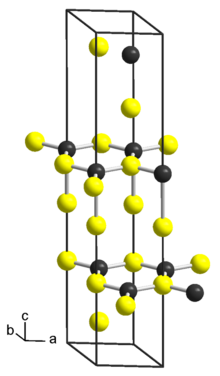 NbS2 structure
| |
| Names | |
|---|---|
| IUPAC name
niobium(IV) sulfde, niobium disulfde
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
PubChem CID
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
NbS2 |
| Molar mass | 157.038 g/mol[1] |
| Appearance | Black crystals[1] |
| Density | 4.4 g/cm3[1] |
Magnetic susceptibility (χ)
|
+120·10−6 cm3/mol[2] |
| Structure[3] | |
Crystal structure
|
Trigonal, hR9, No. 160 |
Space group
|
R3m |
Lattice constant
|
a = 0.333 nm, b = 0.333 nm, c = 1.78 nm α = 90°, β = 90°, γ = 120°
|
Formula units (Z)
|
3 |
| Related compounds | |
Other anions
|
Niobium diselenide, Niobium ditelluride |
Other cations
|
Vanadium disulfide, Tantalum disulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Niobium disulfide is the chemical compound with the formula NbS2. It is a black layered solid that can be exfoliated into ultrathin grayish sheets similar to other transition metal dichalcogenides. These layers exhibit superconductivity, where the transition temperature increases from ca. 2 to 6 K with the layer thickness increasing from 6 to 12 nm, and then saturates with thickness.[4]
References
- ↑ 1.0 1.1 1.2 Lide, D. R., ed (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. p. 4.76. ISBN 0-8493-0486-5.
- ↑ Lee, P.A. (6 December 2012). Optical and Electrical Properties. Springer Science & Business Media. p. 446. ISBN 978-94-010-1478-6. https://books.google.com/books?id=eTvwCAAAQBAJ&pg=PA446.
- ↑ Rajora, O. S.; Curzon, A. E. (1987). "The preparation and X‐ray diffraction study of the layer materials NbSxSe2−x for 0 ≦ x ≦ 2". Physica Status Solidi A 99: 65–72. doi:10.1002/pssa.2210990108.
- ↑ Yan, Rusen; Khalsa, Guru; Schaefer, Brian T.; Jarjour, Alexander; Rouvimov, Sergei; Nowack, Katja C.; Xing, Huili G.; Jena, Debdeep (2019). "Evolution of superconductivity in ultrathin NbS2". Applied Physics Express 12 (2): 023008. doi:10.7567/1882-0786/aaff89.
 |
Categories: [Niobium(IV) compounds] [Disulfides] [Transition metal dichalcogenides] [Monolayers]
↧ Download as ZWI file | Last modified: 12/06/2024 20:17:43 | 9 views
☰ Source: https://handwiki.org/wiki/Chemistry:Niobium_disulfide | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF