Glycinol (Pterocarpan)
 From Handwiki
From Handwiki 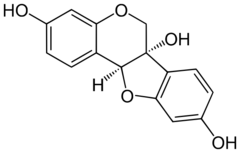
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(6aS,11aS)-6H-[1]Benzofuro[3,2-c][1]benzopyran-3,6a,9(11aH)-triol | |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider |
|
| KEGG |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C15H12O5 |
| Molar mass | 272.25 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
- SizeSet
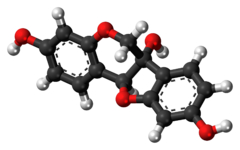
Glycinol is a pterocarpan, a type of natural phenol. It is a phytoalexin found in the soybean (Glycine max). It is formed by the cyclisation of daidzein.[citation needed]
More recent literature supports that glycinol has potent phytoestrogenic activity.[1][2]
The so-called osteogenesis that is causes is postulated to be a preventative factor for osteoporosis.[citation needed]
It can be synthethised chemically and possesses two chiral centers.[3]
Glycinol is the direct precursor of glyceollins through the action of a prenyltransferase.[citation needed]
Experiments show that the 6a oxygen of glycinol is derived from molecular oxygen.[4]
References
- ↑ Boué, Stephen M.; Tilghman, Syreeta L.; Elliott, Steven; Zimmerman, M. Carla; Williams, K. Y.; Payton-Stewart, Florastina; Miraflor, Allen P.; Howell, Melanie H. et al. (2009). "Identification of the Potent Phytoestrogen Glycinol in Elicited Soybean (Glycine max)". Endocrinology 150 (5): 2446–2453. doi:10.1210/en.2008-1235. ISSN 0013-7227. PMID 19116342.
- ↑ Strong, Amy L; Jones, Robert B; Glowacki, Julie; Boue, Stephen M; Burow, Matthew E; Bunnell, Bruce A (2017). "Glycinol enhances osteogenic differentiation and attenuates the effects of age on mesenchymal stem cells". Regenerative Medicine 12 (5): 513–524. doi:10.2217/rme-2016-0148. ISSN 1746-0751. PMID 28718749.
- ↑ Luniwal Amarjit; Khupse Rahul S; Reese Michael; Lei Fang; Erhardt Paul W (2009). "Total Syntheses of Racemic and Natural Glycinol". Journal of Natural Products 72 (11): 2072–2075. doi:10.1021/np900509f. PMID 19943626. http://cat.inist.fr/?aModele=afficheN&cpsidt=22189893.
- ↑ Matthews, David E.; Plattner, Ronald D.; Vanetten, Hans D. (1989). "The 6a oxygen of the pterocarpan glycinol is derived from molecular oxygen". Phytochemistry 28 (1): 113–115. doi:10.1016/0031-9422(89)85020-4. Bibcode: 1989PChem..28..113M. https://zenodo.org/record/1258250.
 |
Categories: [Pterocarpans] [Phytoalexins] [Phenols]
↧ Download as ZWI file | Last modified: 04/02/2024 18:21:49 | 7 views
☰ Source: https://handwiki.org/wiki/Chemistry:Glycinol_(pterocarpan) | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF