Ethanolamine-O-Sulfate
 From Handwiki
From Handwiki 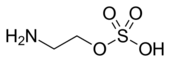
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Aminoethyl hydrogen sulfate | |
| Other names
Aminoethyl sulfate; 2-Aminoethyl hydrogen sulphate; Sulfuric acid mono 2-aminoethyl ester; WAS-34
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| Abbreviations | EOS |
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C2H7NO4S |
| Molar mass | 141.14 g·mol−1 |
| Melting point | 277 °C (531 °F; 550 K) (decomposes) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
GHS hazard statements
|
H302, H315, H319, H335 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
- SizeSet
Ethanolamine-O-sulfate (EOS) is an ester of sulfuric acid and ethanolamine. EOS is a GABA transaminase inhibitor which prevents the metabolism of GABA.[2] It is used as a biochemical tool in studies involving GABA.
EOS is also a diuretic[3] and an anticonvulsant.[4]
References
- ↑ 2-Aminoethyl hydrogen sulfate at Sigma-Aldrich
- ↑ "Ethanolamine-O-sulfate enhances gamma-aminobutyric acid secretion into hypophysial portal blood and lowers serum prolactin concentrations". Neuroendocrinology 37 (5): 397–9. 1983. doi:10.1159/000123580. PMID 6646351.
- ↑ German Patent DE2345291: Diuretic aminoalkyl sulfates; Somani, Pitambar; Martin, Donald Lyons (1974)
- ↑ Anlezark, Gill; Horton, Roger W.; Meldrum, Brian S.; Sawaya, M. Christina B. (1976). "Anticonvulsant action of ethanolamine-O-sulfate and di-n-propylacetate and the metabolism of γ-aminobutyric acid (GABA) in mice with audiogenic seizures". Biochemical Pharmacology 25 (4): 413–417. doi:10.1016/0006-2952(76)90343-9. PMID 779794.
 |
Categories: [Anticonvulsants] [Diuretics] [Sulfate esters]
↧ Download as ZWI file | Last modified: 07/05/2025 14:40:30 | 4 views
☰ Source: https://handwiki.org/wiki/Chemistry:Ethanolamine-O-sulfate | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF