Platinum Hexafluoride
 From Handwiki
From Handwiki 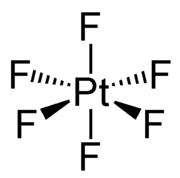
| |
| |
| Names | |
|---|---|
| IUPAC name
Platinum(VI) fluoride
| |
| Other names
Platinum hexafluoride
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
PtF6 |
| Molar mass | 309.07 g/mol |
| Appearance | dark-red crystals |
| Density | 3.83 g/cm3 |
| Melting point | 61.3 °C (142.3 °F; 334.4 K) |
| Boiling point | 69.14 °C (156.45 °F; 342.29 K) |
Solubility in water
|
Reacts with water |
| Structure | |
Crystal structure
|
Orthorhombic, oP28 |
Space group
|
Pnma, No. 62 |
Coordination geometry
|
octahedral (Oh) |
Dipole moment
|
0 |
| Hazards | |
| Main hazards | Strong oxidizer |
| NFPA 704 (fire diamond) | 
0
4
3 OX |
| Related compounds | |
Related compounds
|
Platinum(IV) fluoride Platinum(V) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
- SizeSet
Platinum hexafluoride is the chemical compound with the formula PtF6, and is one of seventeen known binary hexafluorides. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation state. With only four d-electrons, it is paramagnetic with a triplet ground state. PtF6 is a strong fluorinating agent and one of the strongest oxidants, capable of oxidising xenon and O2. PtF6 is octahedral in both the solid state and in the gaseous state. The Pt-F bond lengths are 185 picometers.[1]
Synthesis
PtF6 was first prepared by reaction of fluorine with platinum metal.[2] This route remains the method of choice.[1]
- Pt + 3 F2 → PtF6
PtF6 can also be prepared by disproportionation of the pentafluoride (PtF5), with the tetrafluoride (PtF4) as a byproduct. The required PtF5 can be obtained by fluorinating PtCl2:
- 2 PtCl2 + 5 F2 → 2 PtF5 + 2 Cl2
- 2 PtF5 → PtF6 + PtF4
Hexafluoroplatinates
Platinum hexafluoride can gain an electron to form the hexafluoroplatinate anion, PtF−6. It is formed by reacting platinum hexafluoride with relatively uncationisable elements and compounds, for example with xenon to form "XePtF6" (actually a mixture of XeFPtF5, XeFPt2F11, and Xe2F3PtF6), known as xenon hexafluoroplatinate. The discovery of this reaction in 1962 proved that noble gases form chemical compounds. Previous to the experiment with xenon, PtF6 had been shown to react with oxygen to form [O2]+[PtF6]−, dioxygenyl hexafluoroplatinate.
See also
- Hexafluoride
- Chloroplatinic acid
References
- ↑ 1.0 1.1 Drews, T.; Supel, J.; Hagenbach, A.; Seppelt, K. "Solid State Molecular Structures of Transition Metal Hexafluorides" Inorganic Chemistry 2006, volume 45, pp 3782-3788.doi:10.1021/ic052029f
- ↑ Weinstock, B.; Claassen, H. H.; Malm, J. G. (1957). "Platinum Hexafluoride". Journal of the American Chemical Society 79 (21): 5832. doi:10.1021/ja01578a073.
General reading
- Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN:0-12-352651-5.
 |
Categories: [Platinum compounds] [Hexafluorides] [Platinum group halides] [Fluorinating agents] [Octahedral compounds] [Gases with color]
↧ Download as ZWI file | Last modified: 05/28/2024 16:09:40 | 9 views
☰ Source: https://handwiki.org/wiki/Chemistry:Platinum_hexafluoride | License: CC BY-SA 3.0

 KSF
KSF