Annulation
 From Handwiki
From Handwiki
In organic chemistry, annulation (from la anellus 'little ring'; occasionally annelation) is a chemical reaction in which a new ring is constructed on a molecule.[1]
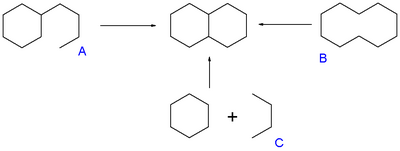
Examples are the Robinson annulation, Danheiser annulation and certain cycloadditions. Annular molecules are constructed from side-on condensed cyclic segments, for example helicenes and acenes. In transannulation a bicyclic molecule is created by intramolecular carbon-carbon bond formation in a large monocyclic ring. An example is the samarium(II) iodide induced ketone - alkene cyclization of 5-methylenecyclooctanone which proceeds through a ketyl intermediate:[2]
Benzannulation
The term benzannulated compounds refers to derivatives of cyclic compounds (usually aromatic) which are fused to a benzene ring. Examples are listed in the table below:
| Benzannulated derivative | Source of cyclic compound |
|---|---|
| Benzopyrene | Pyrene |
| Quinoline | Pyridine |
| Isoquinoline | |
| Chromene | Pyran |
| Isochromene | |
| Indole | Pyrrole |
| Isoindole | |
| Benzofuran | Furan |
| Isobenzofuran | |
| Benzimidazole | Imidazole |
In contemporary chemical literature, the term benzannulation also means "construction of benzene rings from acyclic precursors".[3]thumb|upright=1.4|Protonation of [[Verkade base induces a transannular bonding, giving an atrane.[4]]]
Transannular interaction
A transannular interaction in chemistry is any chemical interaction (favorable or nonfavorable) between different non-bonding molecular groups in a large ring or macrocycle.[5] See for example atranes.
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "annulation". doi:10.1351/goldbook.A00367IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "annelation". doi:10.1351/goldbook.A00365.html
- ↑ Construction of Bicyclic Ring Systems via a Transannular SmI2-Mediated Ketone-Olefin Cyclization StrategyGary A. Molander, Barbara Czakó, and Michael Rheam J. Org. Chem.; 2007; 72(5) pp 1755 - 1764; (Article) doi:10.1021/jo062292d
- ↑ Swami, Bhawna; Yadav, Deepak; Menon, Rajeev S. (January 2022). "Benzannulation Reactions: A Case for Perspective Change From Arene Decoration to Arene Construction" (in en). The Chemical Record 22 (1): e202100249. doi:10.1002/tcr.202100249. ISSN 1527-8999. PMID 34796605. https://onlinelibrary.wiley.com/doi/10.1002/tcr.202100249.
- ↑ Verkade, John G.; Urgaonkar, Sameer; Verkade, John G.; Urgaonkar, Sameer (2012). "Proazaphosphatrane". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00702.pub2. ISBN 978-0471936237.
- ↑ Experimental evidence in support of transannular interactions in diketones Kata Mlinaric-Majerski, Marijana Vinkovic, Danko Škare, Alan P. Marchand Arkivoc DS-339E 2002 Online Article
 |
Categories: [Organic reactions]
↧ Download as ZWI file | Last modified: 07/30/2024 10:15:24 | 7 views
☰ Source: https://handwiki.org/wiki/Chemistry:Annulation | License: CC BY-SA 3.0


 KSF
KSF