Alkyne
 From Nwe
From Nwe Alkynes are hydrocarbons that have at least one triple bond between two carbon atoms, with the formula CnH2n-2. The alkynes are traditionally known as acetylenes or the acetylene series, although the name acetylene is also used to refer specifically to the simplest member of the series, known as ethyne (C2H2) using formal IUPAC nomenclature.
Chemical properties
Unlike alkanes and to a lesser extent, alkenes, alkynes are unstable and very reactive. 1-Alkynes are fairly acidic and have pKa values (25) between that of ammonia (35) or ethanol with 16. This acidity is due to the ability for the negative charge in the acetylide conjugate base to be stabilized as a result of the high s character of the sp orbital in which the electron pair resides. Electrons in a s orbital benefit from closer proximity to the positively charged atom nucleus and are therefore lower in energy.
A terminal alkyne with a strong base such as sodium, sodium amide, n-butyllithium or a Grignard reagent gives the anion of the terminal alkyne (a metal acetylide):
- 2 RC≡CH + 2 Na → 2 RC≡CNa + H2
More generally:
- RC≡CH + B → RC≡C− + HB+, where B denotes a strong base.
The acetylide anion is synthetically useful because as a strong nucleophile, it can participate in C−C bond forming reactions.
It is also possible to form copper and silver alkynes, from this group of compounds silver acetylide is an often used example.
Structure
The carbon atoms in an alkyne bond are sp hybridized—they each have two p orbitals and two sp hybrid orbitals. Overlap of an sp orbital from each atom forms one sp-sp sigma bond. Each p orbital on one atom overlaps one on the other atom, forming two pi bonds, giving a total of three bonds. The remaining sp orbital on each atom can form a sigma bond to another atom, for example to hydrogen atoms in the parent compound acetylene. The two sp orbitals on an atom are on opposite sides of the atom—in acetylene, the H-C-C bond angles are 180°. Because a total of two electrons take part in bonding this triple bond it is very strong with a bond strength of 837 kJ/mol. The sigma bond contributes 369 kJ/mol, the first pi bond contributes 268 kJ/mol and the second pi bond is weak with 202 kJ/mol bond strength. The CC bond distance with 121 picometers is also much less than that of the alkene bond which is 134 pm or the alkane bond with 153 pm.
The simplest alkyne is ethyne (acetylene): H-C≡C-H
Terminal and internal alkynes
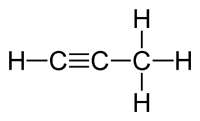
Terminal alkynes have a hydrogen atom bonded to at least one of the sp hybridized carbons (those involved in the triple bond. An example would be methylacetylene (1-propyne using IUPAC nomenclature).
Internal alkynes have something other than hydrogen attached to the sp hybridized carbons, usually another carbon atom, but could be a heteroatom. A good example is 2-pentyne, in which there is a methyl group on one side of the triple bond and an ethyl group on the other side.
Synthesis
Alkynes are generally prepared by dehydrohalogenation of vicinal alkyl dihalides or the reaction of metal acetylides with primary alkyl halides. In the Fritsch-Buttenberg-Wiechell rearrangement an alkyne is prepared starting from a vinyl bromide.
Alkynes can be prepared from aldehydes using the Corey-Fuchs reaction or the Seyferth-Gilbert homologation.
Reactions
Alkynes are involved in many organic reactions.
- electrophilic addition reactions
- addition of hydrogen to give the alkene or the alkane
- addition of halogens to give the vinyl halides or alkyl halides
- addition of hydrogen halides to give the corresponding vinyl halides or alkyl halides
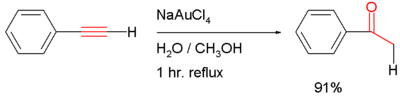
- addition of water to give the carbonyl compound (often through the enol intermediate), for example the hydrolysis of phenylacetylene to acetophenone with sodium tetrachloroaurate in water/methanol (scheme shown below)[1] or (Ph3P)AuCH3 [2]:
- Cycloadditions
- Diels-Alder reaction with 2-pyrone to an aromatic compound after elimination of carbon dioxide
- Azide alkyne Huisgen cycloaddition to triazoles
- Bergman cyclization of enediynes to an aromatic compound
- Alkyne trimerisation to aromatic compounds
- [2+2+1]cycloaddition of an alkyne, alkene and carbon monoxide in the Pauson–Khand reaction
- Metathesis
- scrambling of alkynes in alkyne metathesis to new alkyne compounds
- reaction with alkenes to butadienes in enyne metathesis
- nucleophilic substitution reactions of metal acetylides
- new carbon-carbon bond formation with alkyl halides
- nucleophilic addition reactions of metal acetylides
- reaction with carbonyl compounds to an intermediate alkoxide and then to the hydroxyalkyne after acidic workup.
- hydroboration of alkynes with organoboranes to vinylic boranes
- followed by reduction by oxidation with hydrogen peroxide to the corresponding aldehyde or ketone
- oxidative cleavage with potassium permanganate to the carboxylic acids
- migration of the alkyne along a hydrocarbon chain by treatment with a strong base
- Coupling reaction with other alkynes to di-alkynes in the Cadiot-Chodkiewicz coupling, Glaser coupling and the Eglinton coupling.
See also
Notes
- ↑ Fukuda, Y. and K. Utimoto. "Effective transformation of unactivated alkynes into ketones or acetals with a gold(III) catalyst." J. Org. Chem. 56, 3729–3731, 1991. DOI:10.1021/jo00011a058 Retrieved September 17, 2007.
- ↑ Mizushima, E., D.M. Cui, D.C.D. Nath, T. Hayashi, and M. Tanaka. "Au(I)-Catalyzed hydratation of alkynes: 2,8-nonanedione." Organic Syntheses. Vol. 83, p.55, 2005. Link Retrieved September 17, 2007.
References
ISBN links support NWE through referral fees
- McMurry, John. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole, 2004. ISBN 0534420052
- Morrison, Robert T., and Robert N. Boyd. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1992. ISBN 0-13-643669-2
- Solomons, T.W. Graham, and Fryhle, Craig B. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley, 2004. ISBN 0471417998
| Functional groups |
|---|
| Chemical class: Alcohol • Aldehyde • Alkane • Alkene • Alkyne • Amide • Amine • Azo compound • Benzene derivative • Carboxylic acid • Cyanate • Ester • Ether • Haloalkane • Imine • Isocyanide • Isocyanate • Ketone • Nitrile • Nitro compound • Nitroso compound • Peroxide • Phosphoric acid • Pyridine derivative • Sulfone • Sulfonic acid • Sulfoxide • Thioether • Thiol • Toluene derivative |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
↧ Download as ZWI file | Last modified: 02/03/2023 21:55:48 | 26 views
☰ Source: https://www.newworldencyclopedia.org/entry/Alkyne | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: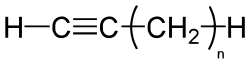
 KSF
KSF