Psychedelic Drug
 From Handwiki
From Handwiki

Psychedelics are a subclass of hallucinogenic drugs whose primary effect is to trigger non-ordinary states of consciousness (known as psychedelic experiences or "trips").[2][3][4] This causes specific psychological, visual, and auditory changes, and often a substantially altered state of consciousness.[5][6][3][7] Psychedelic states are often compared to meditative, psychodynamic or transcendental types of alterations of mind.[4][5][8] The "classical" psychedelics, the psychedelics with the largest scientific and cultural influence, are mescaline, LSD, psilocybin, and DMT.[5][9][10][4]
Most psychedelic drugs fall into one of the three families of chemical compounds: tryptamines, phenethylamines, or lysergamides and many tend to act via serotonin 2A receptor agonism. When compounds bind to serotonin 5-HT2A receptors,[11] they modulate the activity of key circuits in the brain involved with sensory perception and cognition, however, the exact nature of how psychedelics induce changes in perception and cognition via the 5-HT2A receptor is still unknown, although reduction in default mode network activity and increased functional connectivity between regions in the brain as a result may be one of the most relevant pharmacological mechanisms underpinning the psychedelic experience,[12][13] particularly ego death.[14] The psychedelic experience is often compared to non-ordinary forms of consciousness such as those experienced in meditation,[15][3] mystical experiences,[8][7] and near-death experiences,[7] which also appear to be partially underpinned by altered default mode network activity.[16][17] The phenomenon of ego death is often described as a key feature of the psychedelic experience.[15][3][7]
Many psychedelic drugs are illegal worldwide under the UN conventions, with occasional exceptions for religious use or research contexts. Despite these controls, recreational use of psychedelics is common.[18][19] Legal barriers have made the scientific study of psychedelics more difficult. Research has been conducted, however, and studies show that psychedelics are physiologically safe and rarely lead to addiction.[20][21] Studies conducted using psilocybin in a psychotherapeutic setting reveal that psychedelic drugs may assist with treating depression, alcohol addiction, and nicotine addiction.[22][23] Although further research is needed, existing results suggest that psychedelics could be effective treatments for certain forms of psychopathology.[24][25][26][19] Proponents believe that the increase in consumption of psychedelics in defiance of the law is likely to result in more widespread legalization and decriminalization of the substances (as was the case with cannabis).[27] In 2022, 28% of Americans have tried psychedelics.[28]
Etymology and nomenclature

The term psychedelic was coined by the psychiatrist Humphrey Osmond during written correspondence with author Aldous Huxley and presented to the New York Academy of Sciences by Osmond in 1957.[29] It is irregularly[30] derived from the Greek words ψυχή psychḗ 'soul, mind' and δηλείν dēleín 'to manifest', with the intended meaning "mind manifesting," the implication being that psychedelics can reveal unused potentials of the human mind.[31] The term was loathed by American ethnobotanist Richard Schultes but championed by American psychologist Timothy Leary.[32]
Aldous Huxley had suggested his own coinage phanerothyme (Greek phaneroein- "visible" and Greek thymos "soul", thus "visible soul") to Osmond in 1956.[33] Recently, the term entheogenic has come into use to denote the use of psychedelic drugs, as well as various other types of psychoactive substances, in a religious, spiritual, and mystical context.[34]
In 2004, David E. Nichols wrote the following about the nomenclature used for psychedelic drugs:
Many different names have been proposed over the years for this drug class. The famous German toxicologist Louis Lewin used the name phantastica earlier in this century, and as we shall see later, such a descriptor is not so farfetched. The most popular names—hallucinogen, psychotomimetic, and psychedelic ("mind manifesting")—have often been used interchangeably. Hallucinogen is now, however, the most common designation in the scientific literature, although it is an inaccurate descriptor of the actual effects of these drugs. In the lay press, the term psychedelic is still the most popular and has held sway for nearly four decades. Most recently, there has been a movement in nonscientific circles to recognize the ability of these substances to provoke mystical experiences and evoke feelings of spiritual significance. Thus, the term entheogen, derived from the Greek word entheos, which means "god within", was introduced by Ruck et al. and has seen increasing use. This term suggests that these substances reveal or allow a connection to the "divine within". Although it seems unlikely that this name will ever be accepted in formal scientific circles, its use has dramatically increased in the popular media and on internet sites. Indeed, in much of the counterculture that uses these substances, entheogen has replaced psychedelic as the name of choice and we may expect to see this trend continue.[34]
Robin Carhart-Harris and Guy Goodwin write that the term psychedelic is preferable to hallucinogen for describing classical psychedelics because of the term hallucinogen's "arguably misleading emphasis on these compounds' hallucinogenic properties."[35]
Examples

- LSD (Lysergic acid diethylamide) is a derivative of lysergic acid, which is obtained from the hydrolysis of ergotamine. Ergotamine is an alkaloid found in the fungus claviceps purpurea, which primarily infects rye. LSD is both the prototypical psychedelic and the prototypical lysergamide. As a lysergamide, LSD contains both a tryptamine and phenethylamine group within its structure. As a result of containing a phenethylamine group LSD agonises dopamine receptors as well as serotonin receptors,[36] making it more energetic in effect in contrast to the more sedating effects of psilocin, which isn't a dopamine agonist.[37]
- Psilocin (4-HO-DMT) is the dephosphorylated active metabolite of the indole alkaloid psilocybin and a substituted tryptamine, which is produced in over 200 species of fungi. Of the Classical psychedelics psilocybin has attracted the greatest academic interest regarding its ability to manifest mystical experiences,[38] although all psychedelics are capable of doing so to variable degrees. O-Acetylpsilocin (4-AcO-DMT) is an acetylated analog of psilocin. Additionally, replacement of a methyl group at the dimethylated nitrogen with an isopropyl or ethyl group yields 4-HO-MIPT and 4-HO-MET, respectively.[39]
- Mescaline (3,4,5-trimethoxyphenethylamine) is a phenthylamine alkaloid found in various species of cacti, the most well known being Peyote (Lophophora williamsii) and San Pedro (Echinopsis pachanoi). Mescaline has effects comparable to those of LSD and psilocybin, albeit with a greater emphasis on colors and patterns.[40]
- DMT (N,N-dimethyltryptamine) is an indole alkaloid found in various species of plants. Traditionally it is consumed by tribes in South America in the form of ayahuasca. A brew is used that consists of DMT-containing plants as well as plants containing MAOIs, specifically harmaline, which allows DMT to be consumed orally without being rendered inactive by monoamine oxidase enzymes in the digestive system.[41] In the Western world DMT is more commonly consumed via the vaporisation of freebase DMT. Whereas Ayahuasca typically lasts for several hours, inhalation has an onset measured in seconds and has effects measured in minutes, being significantly more intense.[42] Particularly in vaporised form, DMT has the ability to cause users to enter a hallucinatory realm fully detached from reality, being typically characterised by hyperbolic geometry, and described as defying visual or verbal description.[43] Users have also reported encountering and communicating with entitites within this hallucinatory state.[44] DMT is the archetypal substituted tryptamine, being the structural scaffold of psilocybin and - to a lesser extent - the lysergamides.
- 2C-B (2,5-dimethoxy-4-bromophenethylamine) is a substituted phenthylamine first synthesised in 1974 by Alexander Shulgin.[45] 2C-B is both a psychedelic and a mild entactogen, with its psychedelic effects increasing and its entactogenic effects decreasing with dosage. 2C-B is the most well known compound in the 2C family, their general structure being discovered as a result of modifying the structure of mescaline.[45]
Uses
Traditional
Psychedelics have a long history of use in traditional medicine and traditional religion, for their perceived ability to promote physical and mental healing. In this context, they are often known as entheogens. Native American practitioners using mescaline-containing cacti (most notably peyote, San Pedro, and Peruvian torch) have reported success in treating alcoholism, and Mazatec practitioners routinely use psilocybin mushrooms for divination and healing.[citation needed] The psychoactive brew ayahuasca is used in Peru and other parts of South America for spiritual and physical healing as well as in religious festivals.[46]
Psychedelic therapy
Psychedelic therapy (or psychedelic-assisted therapy) is the proposed use of psychedelic drugs to treat mental disorders.[25][47] As of 2021, psychedelic drugs are controlled substances in most countries and psychedelic therapy is not legally available outside clinical trials, with some exceptions.[47][48]
The procedure for psychedelic therapy differs from that of therapies using conventional psychiatric medications. While conventional medications are usually taken without supervision at least once daily, in contemporary psychedelic therapy the drug is administered in a single session (or sometimes up to three sessions) in a therapeutic context.[49] The therapeutic team prepares the patient for the experience beforehand and helps them integrate insights from the drug experience afterwards.[50][51] After ingesting the drug, the patient normally wears eyeshades and listens to music to facilitate focus on the psychedelic experience, with the therapeutic team interrupting only to provide reassurance if adverse effects such as anxiety or disorientation arise.[50][51]
As of 2022, the body of high-quality evidence on psychedelic therapy remains relatively small and more, larger studies are needed to reliably show the effectiveness and safety of psychedelic therapy's various forms and applications.[24][25] On the basis of favorable early results, ongoing research is examining proposed psychedelic therapies for conditions including major depressive disorder,[24][52] and anxiety and depression linked to terminal illness.[24][53] The United States Food and Drug Administration has granted "breakthrough therapy" status, which expedites the assessment of promising drug therapies for potential approval,[note 1] to psilocybin therapy for treatment-resistant depression and major depressive disorder.[47]
Recreational
Recreational use of psychedelics is common.[18][19]
Microdosing
Psychedelic microdosing is the practice of using sub-threshold doses (microdoses) of psychedelics in an attempt to improve creativity, boost physical energy level, emotional balance, increase performance on problems-solving tasks and to treat anxiety, depression and addiction.[55][56] The practice of microdosing has become more widespread in the 21st century with more people claiming long-term benefits from the practice.[57][58]
Pharmacology
Psychedelics are 5-HT2A receptor agonists (serotonin 2A receptor agonists).[2][59][60][61]
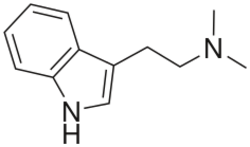
Tryptamines
Tryptamine, along with other trace amines, is found in the central nervous system of mammals. It is hypothesized to play a role as a neuromodulator on classical monoamine neurotransmitters, such as dopamine, serotonin, and norepinephrine (epinephrine). Tryptamine acts as a non-selective serotonin receptor agonist to activate serotonin receptors, and a serotonin–norepinephrine–dopamine releasing agent (SNDRA) to release more monoamine neurotransmitter, with a preference for evoking serotonin and dopamine release over norepinephrine (epinephrine) release.[62][63][64] Psychedelic tryptamines found in nature include psilocin, DMT, 5-MeO-DMT, and tryptamines that have been synthesized in the laboratory include 4-HO-MET,[65] 4-HO-MiPT,[39] and 5-MeO-DALT.[66]
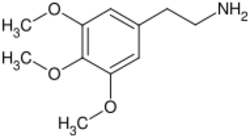
Phenethylamines
Phenethylamine is also a trace amine but to a lesser extent acts as a neurotransmitter in the human central nervous system (CNS). Phenethylamine instead regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1), which plays a significant role in regulating neurotransmission in dopamine, norepinephrine, and serotonin neurons in the CNS and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons.[67][68] When VMAT2 is inhibited monoamine neurotransmitters such as dopamine cannot be released into the synapse via typical release mechanisms.[69] Mescaline is a naturally occurring psychedelic protoalkaloid of the substituted phenethylamine class.
Lysergamides
.svg.png)
Amides of lysergic acid are collectively known as lysergamides, and include a number of compounds with potent agonist and/or antagonist activity at various serotonin and dopamine receptors. Lysergamides contain both Tryptamine and Phenethylamine structure although it is class as a complex Tryptamine. LSD (Lysergic Acid Diethylamide) is one of many lysergamides. A wide range of lysergamides have emerged in recent years, inspired by existing scientific literature. Others, have appeared from chemical research.[70] 1P-LSD is a derivative and functional analogue of LSD and a homologue of ALD-52. It modifies the LSD molecule by adding a propionyl group to the nitrogen molecule of LSD's indole.[71]
Psychedelic experiences
Although several attempts have been made, starting in the 19th and 20th centuries, to define common phenomenological structures of the effects produced by classic psychedelics, a universally accepted taxonomy does not yet exist.[72][73] At lower doses, features of psychedelic experiences include sensory alterations, such as the warping of surfaces, shape suggestibility, and color variations. Users often report intense colors that they have not previously experienced, and repetitive geometric shapes are common. Higher doses often cause intense and fundamental alterations of sensory perception, such as synesthesia or the experience of additional spatial or temporal dimensions.[74]
Classic psychedelics are considered to be those found in nature like psilocybin, DMT, mescaline, and LSD which is derived from naturally occurring ergotamine, and non-classic psychedelics are considered to be newer analogs and derivatives of pharmacophore lysergamides, tryptamine, and phenethylamine structures like 2C-B. Many of these psychedelics cause remarkably similar effects, despite their different chemical structure. However, many users report that the three major families have subjectively different qualities in the "feel" of the experience, which are difficult to describe. Some compounds, such as 2C-B, have extremely tight "dose curves", meaning the difference in dose between a non-event and an overwhelming disconnection from reality can be very slight. There can also be very substantial differences between the drugs; for instance, 5-MeO-DMT rarely produces the visual effects typical of other psychedelics.[75] Tryptamines are well documented to cause classic psychedelic states, such as increased empathy, visual distorsions (drifting, morphing, breathing, melting of various surfaces and objects), auditory hallucinations, ego dissolution or ego death with high enough dose, mystical and spiritual experiences, closed eye hallucinations and complete detachment from reality with a high enough dose.[76]
Potential adverse effects
Despite the contrary perception of much of the public, psychedelic drugs are not addictive and are physiologically safe.[20][21][22] As of 2016, there have been no known deaths due to overdose of LSD, psilocybin, or mescaline.[22]
Risks do exist during an unsupervised psychedelic experience, however; Ira Byock wrote in 2018 in the Journal of Palliative Medicine that psilocybin is safe when administered to a properly screened patient and supervised by a qualified professional with appropriate set and setting. However, he called for an "abundance of caution" because in the absence of these conditions a range of negative reactions is possible, including "fear, a prolonged sense of dread, or full panic." He notes that driving or even walking in public can be dangerous during a psychedelic experience because of impaired hand-eye coordination and fine motor control.[77] In some cases, individuals taking psychedelics have performed dangerous or fatal acts because they believed they possessed superhuman powers.[22]
The usage of most psychedelics entails some risk of eliciting flashbacks of the drug experience even after the effects have worn off, albeit rarely.[78] HPPD or other after-effects may also occur. Psilocybin-induced states of mind share features with states experienced in psychosis, and while a causal relationship between psilocybin and the onset of psychosis has not been established as of 2011, researchers have called for investigation of the relationship.[78] Many of the persistent negative perceptions of psychological risks are unsupported by the currently available scientific evidence, with the majority of reported adverse effects not being observed in a regulated and/or medical context.[79] A population study on associations between psychedelic use and mental illness published in 2013 found no evidence that psychedelic use was associated with increased prevalence of any mental illness.[80]
Potential therapeutic effects

Psychedelic substances which may have therapeutic uses include psilocybin, LSD, and mescaline.[26] During the 1950s and 1960s, lack of informed consent in some scientific trials on psychedelics led to significant, long-lasting harm to some participants.[26] Since then, research regarding the effectiveness of psychedelic therapy has been conducted under strict ethical guidelines, with fully informed consent and a pre-screening to avoid people with psychosis taking part.[26] Although the history behind these substances has hindered research into their potential medicinal value, scientists are now able to conduct studies and renew research that was halted in the 1970s. Some research has shown that these substances have helped people with such mental disorders as obsessive-compulsive disorder (OCD), post-traumatic stress disorder (PTSD), alcoholism, depression, and cluster headaches.[19]
It has long been known that psychedelics promote neurite growth and neuroplasticity and are potent psychoplastogens.[81][82][83] There is evidence that psychedelics induce molecular and cellular adaptations related to neuroplasticity and that these could potentially underlie therapeutic benefits.[84][85] Psychedelics have also been shown to have potent anti-inflammatory activity and therapeutic effects in animal models of inflammatory diseases including asthma,[86] and cardiovascular disease and diabetes.[87]
Shamanic, spiritual and religious use

A number of frequently mentioned or traditional psychedelics such as Ayahuasca (which contains DMT), San Pedro (which contains mescaline), Psilocybe mushrooms (which contain psilocin/psilocybin) and Tabernanthe iboga (which contains the unique psychedelic ibogaine) all have a long and extensive history of spiritual, shamanic and traditional usage by indigenous peoples in various world regions, particularly in Latin America but in the case of iboga, Gabon, Africa.[88]
Different countries have come to be associated with particular psychedelic entheogens such as ayahuasca's spiritual importance in regions of Peru near the Amazon Basin[89] and the entheogenic use of psilocybe mushrooms by the native Mazatec people of Oaxaca, Mexico.[90]
Surrounding culture

Psychedelic culture includes manifestations such as psychedelic music,[91] psychedelic art,[92] psychedelic literature,[93] psychedelic film,[94] and psychedelic festivals.[95] Examples of psychedelic music would be rock bands like the Grateful Dead and Jefferson Airplane. Many psychedelic bands and elements of the psychedelic subculture originated in San Francisco during the mid to late 1960s[96]
Legal status
Many psychedelics are classified under Schedule I of the United Nations Convention on Psychotropic Substances of 1971 as drugs with the greatest potential to cause harm and no acceptable medical uses.[97] In addition, many countries have analogue laws; for example, in the United States , the Federal Analogue Act of 1986 automatically forbids any drugs sharing similar chemical structures or chemical formulas to illicit or prohibited substances if sold for human consumption.[98]
See also
- Aztec use of entheogens
- Bwiti
- Cognitive liberty
- Concord Prison Experiment
- Designer Drugs
- Dissociative drug
- Drug harmfulness
- Entheogenic drugs and the archaeological record
- Entheogenics and the Maya
- Hallucinogenic fish
- Hallucinogenic plants in Chinese herbals
- Hamilton's Pharmacopeia
- History of lysergic acid diethylamide
- Ibogaine
- List of psychedelic plants
- Marsh Chapel Experiment
- Morning glory
- Mystical psychosis
- PiHKAL
- Serotonergic psychedelic
- Tabernanthe iboga
- TiHKAL
- Research chemical
- List of psychedelic drugs
- List of designer drugs
Categories
Notes
- ↑ The Food and Drug Administration describes the designation of breakthrough therapy as "a process designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s)."[54]
References
- ↑ "Peyote San Pedro Cactus – Shamanic Sacraments". D.M.Taylor. http://mescaline.com/exp.
- ↑ 2.0 2.1 Aghajanian, G (August 1999). "Serotonin and Hallucinogens". Neuropsychopharmacology 21 (2): 16S–23S. doi:10.1016/S0893-133X(98)00135-3. PMID 10432484.
- ↑ 3.0 3.1 3.2 3.3 Millière, Raphaël; Carhart-Harris, Robin L.; Roseman, Leor; Trautwein, Fynn-Mathis; Berkovich-Ohana, Aviva (2018). "Psychedelics, Meditation, and Self-Consciousness". Frontiers in Psychology 9: 1475. doi:10.3389/fpsyg.2018.01475. PMID 30245648.
- ↑ 4.0 4.1 4.2 Pollan, Michael (2018). How to Change Your Mind: What the New Science of Psychedelics Teaches Us About Consciousness, Dying, Addiction, Depression, and Transcendence
- ↑ 5.0 5.1 5.2 Leary, Timothy; Metzner, Ralph (1964). The Psychedelic Experience: A Manual Based on The Tibetan Book of the Dead
- ↑ McKenna, Terence (1989). True Hallucinations: Being an Account of the Author's Extraordinary Adventures in the Devil's Paradise
- ↑ 7.0 7.1 7.2 7.3 Timmermann, Christopher; Roseman, Leor; Williams, Luke; Erritzoe, David; Martial, Charlotte; Cassol, Héléna; Laureys, Steven; Nutt, David et al. (2018). "DMT Models the Near-Death Experience". Frontiers in Psychology 9: 1424. doi:10.3389/fpsyg.2018.01424. PMID 30174629.
- ↑ 8.0 8.1 R. R. Griffiths; W. A. Richards; U. McCann; R. Jesse (7 July 2006). "Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance". Psychopharmacology 187 (3): 268–283. doi:10.1007/s00213-006-0457-5. PMID 16826400.
- ↑ McKenna, Terence (1992). Food of the Gods: The Search for the Original Tree of Knowledge A Radical History of Plants, Drugs, and Human Evolution
- ↑ W. Davis (1996), One River: Explorations and Discoveries in the Amazon Rain Forest. New York, Simon and Schuster, Inc. p. 120.
- ↑ Basic neurochemistry : molecular, cellular and medical aspects. Siegel, George J. (7th ed.). Amsterdam. 11 November 2005. ISBN 978-0-08-047207-2. OCLC 123438340. https://www.worldcat.org/oclc/123438340.
- ↑ Smigielski, Lukasz; Scheidegger, Milan; Kometer, Michael; Vollenweider, Franz X. (August 2019). "Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects" (in en). NeuroImage 196: 207–215. doi:10.1016/j.neuroimage.2019.04.009. PMID 30965131. https://linkinghub.elsevier.com/retrieve/pii/S1053811919302952.
- ↑ Palhano-Fontes, Fernanda; Andrade, Katia C.; Tofoli, Luis F.; Santos, Antonio C.; Crippa, Jose Alexandre S.; Hallak, Jaime E. C.; Ribeiro, Sidarta; Araujo, Draulio B. de (2015-02-18). "The Psychedelic State Induced by Ayahuasca Modulates the Activity and Connectivity of the Default Mode Network" (in en). PLOS ONE 10 (2): e0118143. doi:10.1371/journal.pone.0118143. ISSN 1932-6203. PMID 25693169. Bibcode: 2015PLoSO..1018143P.
- ↑ Tagliazucchi, Enzo; Roseman, Leor; Kaelen, Mendel; Orban, Csaba; Muthukumaraswamy, Suresh; Murphy, Kevin; Laufs, Helmut; Leech, Robert et al. (2016). "Increased Global Functional Connectivity Correlates with LSD-Induced Ego Dissolution" (in en). Current Biology 26 (8): 1043–1050. doi:10.1016/j.cub.2016.02.010. PMID 27085214. https://linkinghub.elsevier.com/retrieve/pii/S0960982216300628.
- ↑ 15.0 15.1 Letheby, Chris; Gerrans, Philip (2017). "Self unbound: ego dissolution in psychedelic experience". Neuroscience of Consciousness 3 (1): nix016. doi:10.1093/nc/nix016. PMID 30042848. "The connection with findings about PCC deactivation in ‘effortless awareness’ meditation is obvious, and bolstered by the finding that acute ayahuasca intoxication increases mindfulness-related capacities.".
- ↑ Brewer, Judson A.; Worhunsky, Patrick D.; Gray, Jeremy R.; Tang, Yi-Yuan; Weber, Jochen; Kober, Hedy (2011-12-13). "Meditation experience is associated with differences in default mode network activity and connectivity". Proceedings of the National Academy of Sciences 108 (50): 20254–20259. doi:10.1073/pnas.1112029108. PMID 22114193. Bibcode: 2011PNAS..10820254B.
- ↑ Rim, James I.; Ojeda, Jesse Caleb; Svob, Connie; Kayser, Jürgen; Drews, Elisa; Kim, Youkyung; Tenke, Craig E.; Skipper, Jamie et al. (2019). "Current Understanding of Religion, Spirituality, and Their Neurobiological Correlates" (in en). Harvard Review of Psychiatry 27 (5): 303–316. doi:10.1097/HRP.0000000000000232. ISSN 1465-7309. PMID 31490186.
- ↑ 18.0 18.1 Krebs, Teri S; Johansen, Pål-Ørjan (28 March 2013). "Over 30 million psychedelic users in the United States". F1000Research 2: 98. doi:10.12688/f1000research.2-98.v1. PMID 24627778.
- ↑ 19.0 19.1 19.2 19.3 Garcia-Romeu, Albert; Kersgaard, Brennan; Addy, Peter H. (August 2016). "Clinical applications of hallucinogens: A review.". Experimental and Clinical Psychopharmacology 24 (4): 229–268. doi:10.1037/pha0000084. PMID 27454674.
- ↑ 20.0 20.1 Le Dain, Gerald (1971). The Non-medical Use of Drugs: Interim Report of the Canadian Government's Commission of Inquiry. p. 106. "Physical dependence does not develop to LSD"
- ↑ 21.0 21.1 Lüscher, Christian; Ungless, Mark A. (14 November 2006). "The Mechanistic Classification of Addictive Drugs". PLOS Medicine 3 (11): e437. doi:10.1371/journal.pmed.0030437. PMID 17105338.
- ↑ 22.0 22.1 22.2 22.3 Nichols, D. E. (3 February 2016). "Psychedelics". Pharmacological Reviews 68 (2): 264–355. doi:10.1124/pr.115.011478. PMID 26841800.
- ↑ Rich Haridy (24 October 2018). "Psychedelic psilocybin therapy for depression granted Breakthrough Therapy status by FDA". https://newatlas.com/psilocybin-magic-mushrooms-depression-fda-breakthrough-therapy/56928/.
- ↑ 24.0 24.1 24.2 24.3 Bender, David; Hellerstein, David J. (2022). "Assessing the risk–benefit profile of classical psychedelics: a clinical review of second-wave psychedelic research". Psychopharmacology 239 (6): 1907–1932. doi:10.1007/s00213-021-06049-6. PMID 35022823.
- ↑ 25.0 25.1 25.2 Reiff, Collin M.; Richman, Elon E.; Nemeroff, Charles B.; Carpenter, Linda L.; Widge, Alik S.; Rodriguez, Carloyn I.; Kalin, Ned H.; McDonald, William M. et al. (2020). "Psychedelics and Psychedelic-Assisted Psychotherapy". The American Journal of Psychiatry 177 (5): 391–410. doi:10.1176/appi.ajp.2019.19010035. PMID 32098487.
- ↑ 26.0 26.1 26.2 26.3 Tupper, Kenneth W.; Wood, Evan; Yensen, Richard; Johnson, Matthew W. (2015-10-06). "Psychedelic medicine: a re-emerging therapeutic paradigm". CMAJ: Canadian Medical Association Journal 187 (14): 1054–1059. doi:10.1503/cmaj.141124. ISSN 0820-3946. PMID 26350908.
- ↑ "Scofflaws Lead the Way To Legalizing Psychedelic Drugs". August 3, 2022. https://reason.com/2022/08/03/scofflaws-lead-the-way-to-legalizing-psychedelic-drugs/.
- ↑ "One in four Americans say they’ve tried at least one psychedelic drug". July 28, 2022. https://today.yougov.com/topics/lifestyle/articles-reports/2022/07/28/one-in-four-americans-have-tried-psychedelic-drugs.
- ↑ Tanne, Janice Hopkins (2004). "Humphrey Osmond". BMJ 328 (7441): 713. doi:10.1136/bmj.328.7441.713.
- ↑ Oxford English Dictionary, 3rd edition, September 2007, s.v., Etymology
- ↑ A. Weil, W. Rosen. (1993), From Chocolate To Morphine: Everything You Need To Know About Mind-Altering Drugs. New York, Houghton Mifflin Company. p. 93
- ↑ W. Davis (1996), "One River: Explorations and Discoveries in the Amazon Rain Forest". New York, Simon and Schuster, Inc. p. 120.
- ↑ iia700700.us.archive.org
- ↑ 34.0 34.1 "Hallucinogens". Pharmacology & Therapeutics 101 (2): 131–81. February 2004. doi:10.1016/j.pharmthera.2003.11.002. PMID 14761703.
- ↑ Carhart-Harris, Robin; Guy, Goodwin (2017). "The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future". Neuropsychopharmacology 42 (11): 2105–2113. doi:10.1038/npp.2017.84. PMID 28443617.
- ↑ Creese, Ian; Burt, David R.; Snyder, Solomon H. (1975-12-01). "The dopamine receptor: Differential binding of d-LSD and related agents to agonist and antagonist states" (in en). Life Sciences 17 (11): 1715–1719. doi:10.1016/0024-3205(75)90118-6. ISSN 0024-3205. PMID 1207384. https://www.sciencedirect.com/science/article/abs/pii/0024320575901186.
- ↑ Passie, Torsten; Seifert, Juergen; Schneider, Udo; Emrich, Hinderk M. (October 2002). "The pharmacology of psilocybin" (in en). Addiction Biology 7 (4): 357–364. doi:10.1080/1355621021000005937. PMID 14578010. http://doi.wiley.com/10.1080/1355621021000005937.
- ↑ Griffiths, R. R.; Richards, W. A.; McCann, U.; Jesse, R. (August 2006). "Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance" (in en). Psychopharmacology 187 (3): 268–283. doi:10.1007/s00213-006-0457-5. ISSN 0033-3158. PMID 16826400. http://link.springer.com/10.1007/s00213-006-0457-5.
- ↑ 39.0 39.1 "4-HO-MiPT" (in en-US). 2020-01-06. https://psychedelicreview.com/compound/4-ho-mipt/.
- ↑ Freye, E. (2009). Pharmacology and abuse of cocaine, amphetamines, ecstasy and related designer drugs : a comprehensive review on their mode of action, treatment of abuse and intoxication. Dordrecht: Springer. ISBN 978-90-481-2448-0. OCLC 489218895. https://www.worldcat.org/oclc/489218895.
- ↑ Riba, Jordi; Valle, Marta; Urbano, Gloria; Yritia, Mercedes; Morte, Adelaida; Barbanoj, Manel J. (July 2003). "Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion, and Pharmacokinetics" (in en). Journal of Pharmacology and Experimental Therapeutics 306 (1): 73–83. doi:10.1124/jpet.103.049882. ISSN 0022-3565. PMID 12660312. http://jpet.aspetjournals.org/lookup/doi/10.1124/jpet.103.049882.
- ↑ Haroz, Rachel; Greenberg, Michael I. (November 2005). "Emerging drugs of abuse". The Medical Clinics of North America 89 (6): 1259–1276. doi:10.1016/j.mcna.2005.06.008. ISSN 0025-7125. PMID 16227062. https://pubmed.ncbi.nlm.nih.gov/16227062.
- ↑ Strassman, Ruck J.; Qualls, Clifford R.; Uhlenhuth, Eberhard H.; Kellner, Robert (1994-02-01). "Dose-Response Study of N,N-Dimethyltryptamine in Humans: II. Subjective Effects and Preliminary Results of a New Rating Scale". Archives of General Psychiatry 51 (2): 98–108. doi:10.1001/archpsyc.1994.03950020022002. ISSN 0003-990X. PMID 8297217. https://doi.org/10.1001/archpsyc.1994.03950020022002.
- ↑ Davis, Alan K; Clifton, John M; Weaver, Eric G; Hurwitz, Ethan S; Johnson, Matthew W; Griffiths, Roland R (September 2020). "Survey of entity encounter experiences occasioned by inhaled N,N -dimethyltryptamine: Phenomenology, interpretation, and enduring effects" (in en). Journal of Psychopharmacology 34 (9): 1008–1020. doi:10.1177/0269881120916143. ISSN 0269-8811. PMID 32345112.
- ↑ 45.0 45.1 Shulgin, Alexander T. (1991). Pihkal : a chemical love story. Ann Shulgin. Berkeley, CA: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628. https://www.worldcat.org/oclc/25627628.
- ↑ Ismael Eduardo Apud Peláez. (2020). Ayahuasca: Between Cognition and Culture. Publicacions Universitat Rovira i Virgili. ISBN 978-84-8424-834-7. OCLC 1229544084. https://www.worldcat.org/oclc/1229544084.
- ↑ 47.0 47.1 47.2 Marks, Mason; Cohen, I. Glenn (2021). "Psychedelic therapy: a roadmap for wider acceptance and utilization". Nature Medicine 27 (10): 1669–1671. doi:10.1038/s41591-021-01530-3. PMID 34608331.
- ↑ Pilecki, Brian; Luoma, Jason B.; Bathje, Geoff J.; Rhea, Joseph; Narloch, Vilmarie Fraguada (2021). "Ethical and legal issues in psychedelic harm reduction and integration therapy". Harm Reduction Journal 18 (1): 40. doi:10.1186/s12954-021-00489-1. PMID 33827588.
- ↑ Nutt, David; Erritzoe, David; Carhart-Harris, Robin (2020). "Psychedelic Psychiatry's Brave New World". Cell 181 (1): 24–28. doi:10.1016/j.cell.2020.03.020. PMID 32243793.
- ↑ 50.0 50.1 Johnson, M. W.; Richards, W. A.; Griffiths, R. R. (2008). "Human hallucinogen research: guidelines for safety". Journal of Psychopharmacology 22 (6): 603–620. doi:10.1177/0269881108093587. PMID 18593734.
- ↑ 51.0 51.1 Garcia-Romeu, Albert; Richards, William A. (2018). "Current perspectives on psychedelic therapy: use of serotonergic hallucinogens in clinical interventions". International Review of Psychiatry 30 (4): 291–316. doi:10.1080/09540261.2018.1486289. ISSN 0954-0261. PMID 30422079.
- ↑ Romeo, Bruno; Karila, Laurent; Martelli, Catherine; Benyamina, Amine (2020). "Efficacy of psychedelic treatments on depressive symptoms: A meta-analysis". Journal of Psychopharmacology 34 (10): 1079–1085. doi:10.1177/0269881120919957. PMID 32448048.
- ↑ Schimmel, Nina; Breeksema, Joost J.; Smith-Apeldoorn, Sanne Y.; Veraart, Jolien; van den Brink, Wim; Schoevers, Robert A. (2022). "Psychedelics for the treatment of depression, anxiety, and existential distress in patients with a terminal illness: a systematic review". Psychopharmacology 239 (15–33): 15–33. doi:10.1007/s00213-021-06027-y. PMID 34812901. https://research.rug.nl/en/publications/d86d811c-a066-4d34-bc83-df904e91a765.
- ↑ "Breakthrough Therapy". United States Food and Drug Administration. 1 April 2018. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/breakthrough-therapy.
- ↑ Fadiman, James (2016-01-01). "Microdose research: without approvals, control groups, double blinds, staff or funding". Psychedelic Press XV. https://www.researchgate.net/publication/308138461.
- ↑ Brodwin, Erin (30 January 2017). "The truth about 'microdosing,' which involves taking tiny amounts of psychedelics like LSD". Business Insider. http://www.businessinsider.com/microdosing-lsd-effects-risks-2017-1.
- ↑ Dahl, Henrik (7 July 2015). "A Brief History of LSD in the Twenty-First Century". Psychedelic Press UK. http://psypressuk.com/2015/07/07/a-brief-history-of-lsd-in-the-twenty-first-century/.
- ↑ "Narrative identity, rationality, and microdosing classic psychedelics". The International Journal on Drug Policy 70: 33–39. August 2019. doi:10.1016/j.drugpo.2019.04.013. PMID 31071597.
- ↑ "Crystal Structure of LSD and 5-HT2AR Part 2: Binding Details and Future Psychedelic Research Paths" (in en-US). 2020-10-05. https://psychedelicreview.com/crystal-structure-of-lsd-and-5-ht2ar-part-2-binding-details-and-future-psychedelic-research-paths/.
- ↑ Nichols, David E. (2018), Halberstadt, Adam L.; Vollenweider, Franz X.; Nichols, David E., eds., "Chemistry and Structure–Activity Relationships of Psychedelics" (in en), Behavioral Neurobiology of Psychedelic Drugs, Current Topics in Behavioral Neurosciences (Berlin, Heidelberg: Springer) 36: pp. 1–43, doi:10.1007/7854_2017_475, ISBN 978-3-662-55880-5, PMID 28401524, https://doi.org/10.1007/7854_2017_475, retrieved 5 March 2022
- ↑ Nichols, David E. (2016-04-01). "Psychedelics" (in en). Pharmacological Reviews 68 (2): 264–355. doi:10.1124/pr.115.011478. ISSN 0031-6997. PMID 26841800. PMC 4813425. https://pharmrev.aspetjournals.org/content/68/2/264.
- ↑ Wölfel, Reinhard; Graefe, Karl-Heinz (February 1992). "Evidence for various tryptamines and related compounds acting as substrates of the platelet 5-hydroxytryptamine transporter" (in en). Naunyn-Schmiedeberg's Archives of Pharmacology 345 (2): 129–136. doi:10.1007/BF00165727. ISSN 0028-1298. PMID 1570019.
- ↑ Shimazu, Seiichiro; Miklya, Ildikó (May 2004). "Pharmacological studies with endogenous enhancer substances: β-phenylethylamine, tryptamine, and their synthetic derivatives" (in en). Progress in Neuro-Psychopharmacology and Biological Psychiatry 28 (3): 421–427. doi:10.1016/j.pnpbp.2003.11.016. PMID 15093948.
- ↑ Blough, Bruce E.; Landavazo, Antonio; Partilla, John S.; Baumann, Michael H.; Decker, Ann M.; Page, Kevin M.; Rothman, Richard B. (2014-06-12). "Hybrid Dopamine Uptake Blocker–Serotonin Releaser Ligands: A New Twist on Transporter-Focused Therapeutics" (in en). ACS Medicinal Chemistry Letters 5 (6): 623–627. doi:10.1021/ml500113s. ISSN 1948-5875. PMID 24944732.
- ↑ "4-HO-MET" (in en-US). https://thedrugclassroom.com/video/4-ho-met/.
- ↑ Shulgin, Alexander T. (Alexander Theodore) (1997). Tihkal : the continuation. Shulgin, Ann. (1st ed.). Berkeley, CA: Transform Press. ISBN 0-9630096-9-9. OCLC 38503252. https://www.worldcat.org/oclc/38503252.
- ↑ Miller, Gregory M. (2010-12-16). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. ISSN 0022-3042. PMID 21073468.
- ↑ Grandy, Gina (2020-04-23). "Guest editorial" (in en). Gender in Management 35 (3): 257–260. doi:10.1108/GM-05-2020-238. ISSN 1754-2413.
- ↑ Little, Karley Y.; Krolewski, David M.; Zhang, Lian; Cassin, Bader J. (January 2003). "Loss of Striatal Vesicular Monoamine Transporter Protein (VMAT2) in Human Cocaine Users" (in en). American Journal of Psychiatry 160 (1): 47–55. doi:10.1176/appi.ajp.160.1.47. ISSN 0002-953X. PMID 12505801.
- ↑ Brandt, Simon D.; Kavanagh, Pierce V.; Westphal, Folker; Stratford, Alexander; Odland, Anna U.; Klein, Adam K.; Dowling, Geraldine; Dempster, Nicola M. et al. (2020-04-20). "Return of the lysergamides. Part VI: Analytical and behavioural characterization of 1‐cyclopropanoyl‐ d ‐lysergic acid diethylamide (1CP‐LSD)" (in en). Drug Testing and Analysis 12 (6): 812–826. doi:10.1002/dta.2789. ISSN 1942-7603. PMID 32180350. PMC 9191646. https://researchonline.ljmu.ac.uk/id/eprint/12478/1/DTA-20-0053.R1_accepted_uncorrected.pdf.
- ↑ Grumann, Christina; Henkel, Kerstin; Brandt, Simon D.; Stratford, Alexander; Passie, Torsten; Auwärter, Volker (2020). "Pharmacokinetics and subjective effects of 1P-LSD in humans after oral and intravenous administration" (in en). Drug Testing and Analysis 12 (8): 1144–1153. doi:10.1002/dta.2821. ISSN 1942-7611. PMID 32415750.
- ↑ Preller, Katrin H.; Vollenweider, Franz X. (2016). "Phenomenology, Structure, and Dynamic of Psychedelic States". Behavioral Neurobiology of Psychedelic Drugs. 36. Berlin, Heidelberg: Springer Berlin Heidelberg. 221–256. doi:10.1007/7854_2016_459. ISBN 978-3-662-55878-2.
- ↑ Swanson, Link R. (2018-03-02). "Unifying Theories of Psychedelic Drug Effects". Frontiers in Pharmacology 9: 172. doi:10.3389/fphar.2018.00172. ISSN 1663-9812. PMID 29568270.
- ↑ Luke, David (28 November 2013). "Rock Art or Rorschach: Is there More to Entoptics than Meets the Eye?". Time and Mind 3 (1): 9–28. doi:10.2752/175169710x12549020810371.
- ↑ Nichols, David E. (April 2016). "Psychedelics". Pharmacological Reviews 68 (2): 264–355. doi:10.1124/pr.115.011478. PMID 26841800.
- ↑ Berry, Mark D. (July 2004). "Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators". Journal of Neurochemistry 90 (2): 257–271. doi:10.1111/j.1471-4159.2004.02501.x. ISSN 0022-3042. PMID 15228583.
- ↑ Byock, Ira (2018). "Taking Psychedelics Seriously". Journal of Palliative Medicine 21 (4): 417–421. doi:10.1089/jpm.2017.0684. PMID 29356590.
- ↑ 78.0 78.1 van Amsterdam, Jan; Opperhuizen, Antoon; van den Brink, Wim (2011). "Harm potential of magic mushroom use: A review". Regulatory Toxicology and Pharmacology 59 (3): 423–429. doi:10.1016/j.yrtph.2011.01.006. PMID 21256914.
- ↑ Schlag, Anne K; Aday, Jacob; Salam, Iram; Neill, Jo C; Nutt, David J (2022-02-02). "Adverse effects of psychedelics: From anecdotes and misinformation to systematic science". Journal of Psychopharmacology 36 (3): 258–272. doi:10.1177/02698811211069100. ISSN 0269-8811. PMID 35107059.
- ↑ Krebs, Teri S.; Johansen, Pål-Ørjan; Lu, Lin (19 August 2013). "Psychedelics and Mental Health: A Population Study". PLOS ONE 8 (8): e63972. doi:10.1371/journal.pone.0063972. PMID 23976938. Bibcode: 2013PLoSO...863972K.
- ↑ Jones, K.A.; Srivastave, D.P.; Allen, J.A.; Roth, B.L.; Penzes, P. (2009). "Psychedelics Promote Structural and Functional Neural Plasticity". Proc Natl Acad Sci U S A 106 (46): 19575–19580. doi:10.1073/pnas.0905884106. PMID 19889983.
- ↑ Yoshida, H.; Kanamaru, C.; Ohtani, A.; Senzaki, K.; Shiga, T. (2011). "Subtype specific roles of serotonin receptors in the spine formation of cortical neurons in vitro". Neurosci Res. 71 (3): 311–314. doi:10.1016/j.neures.2011.07.1824. PMID 21802453.
- ↑ Ly, Calvin; Greb, Alexandra C.; Cameron, Lindsay P.; Wong, Jonathan M.; Barragan, Eden V.; Wilson, Paige C.; Burbach, Kyle F.; Soltanzadeh Zarandi, Sina et al. (June 2018). "Psychedelics Promote Structural and Functional Neural Plasticity". Cell Reports 23 (11): 3170–3182. doi:10.1016/j.celrep.2018.05.022. PMID 29898390.
- ↑ de Vos, Cato M. H.; Mason, Natasha L.; Kuypers, Kim P. C. (2021). "Psychedelics and Neuroplasticity: A Systematic Review Unraveling the Biological Underpinnings of Psychedelics". Frontiers in Psychiatry 12: 1575. doi:10.3389/fpsyt.2021.724606. ISSN 1664-0640. PMID 34566723.
- ↑ Calder, Abigail E.; Hasler, Gregor (2022-09-19). "Towards an understanding of psychedelic-induced neuroplasticity" (in en). Neuropsychopharmacology: 1–9. doi:10.1038/s41386-022-01389-z. ISSN 1740-634X. https://www.nature.com/articles/s41386-022-01389-z.
- ↑ Nau, F.; Miller, J.; Saravia, J.; Ahlert, T.; Yu, B.; Happel, K.I; Cormier, S.A; Nichols, C.D. (2015). "Serotonin 5-HT₂ receptor activation prevents allergic asthma in a mouse model". American Journal of Physiology. Lung Cellular and Molecular Physiology 308 (2): 191–198. doi:10.1152/ajplung.00138.2013. PMID 25416380.
- ↑ Flanagan, T.W.; Sebastian, M.N.; Battaglia, D.M.; Foster, T.P.; Maillet, E.L.; Nichols, C.D. (2019). "Activation of 5-HT2 Receptors Reduces Inflammation in Vascular Tissue and Cholesterol Levels in High-Fat Diet-Fed Apolipoprotein E Knockout Mice". Sci. Rep. 9 (1): 13444–198. doi:10.1038/s41598-019-49987-0. PMID 31530895. Bibcode: 2019NatSR...913444F.
- ↑ Carlini, E. A.; Maia, Lucas O. (2020). "Plant and Fungal Hallucinogens as Toxic and Therapeutic Agents" (in en). Plant Toxins. Toxinology (Springer Netherlands): 1–44. doi:10.1007/978-94-007-6728-7_6-2. ISBN 978-94-007-6728-7. https://link.springer.com/referenceworkentry/10.1007/978-94-007-6728-7_6-2. Retrieved 23 February 2022.
- ↑ "Overviews Shamanism – On the Origin of Ayahuasca". Ayahuasca.com. 2008. http://www.ayahuasca.com/ayahuasca-overviews/on-the-origins-of-ayahuasca/.
- ↑ "History of Psychedelics: How the Mazatec Tribe Brought Entheogens to the World". 28 October 2015. https://psychedelictimes.com/history-of-psychedelics-how-the-mazatec-tribe-brought-entheogens-to-the-world/.
- ↑ Hicks, Michael (15 January 2000). Sixties Rock: Garage, Psychedelic, and Other Satisfactions. Chicago, IL: University of Illinois Press. pp. 63–64. ISBN 0-252-06915-3.
- ↑ Krippner, Stanley (2017). "Ecstatic Landscapes: The Manifestation of Psychedelic Art". Journal of Humanistic Psychology 57 (4): 415–435. doi:10.1177/0022167816671579.
- ↑ Dickins, Robert (2013). "Preparing the Gaia connection: An ecological exposition of psychedelic literature 1954-1963". European Journal of Ecopsychology 4: 9–18. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.854.6673&rep=rep1&type=pdf. Retrieved 7 January 2021.
- ↑ Gallagher, Mark (2004). "Tripped Out: The Psychedelic Film and Masculinity". Quarterly Review of Film and Video 21 (3): 161–171. doi:10.1080/10509200490437817.
- ↑ St John, Graham. "Neotrance and the Psychedelic Festival." Dancecult: Journal of Electronic Dance Music Culture, 1(1) (2009).
- ↑ Hirschfelder, Adam (Jan 14, 2016). "The Trips Festival explained". https://experiments.californiahistoricalsociety.org/what-was-the-trips-festival/.
- ↑ Rucker, James J. H. (2015). "Psychedelic drugs should be legally reclassified so that researchers can investigate their therapeutic potential". British Medical Journal 350: h2902. doi:10.1136/bmj.h2902. PMID 26014506.
- ↑ "U.S.C. Title 21 - FOOD AND DRUGS". https://www.govinfo.gov/content/pkg/USCODE-2011-title21/html/USCODE-2011-title21-chap13.htm.
Further reading
- Echard, William (2017). Psychedelic Popular Music: A History through Musical Topic Theory. Bloomington, IN: Indiana University Press. doi:10.2307/j.ctt1zxxzgx. ISBN 978-0253026453.
- Halberstadt, Adam L., ed (2018). Behavioral Neurobiology of Psychedelic Drugs. 36. Berlin, Heidelberg: Springer. ISBN 978-3-662-55878-2.
- Jay, Mike (2019). Mescaline: A Global History of the First Psychedelic. New Haven, CT: Yale University Press. doi:10.2307/j.ctvgc61q9. ISBN 9780300257502.
- Letheby, Chris (2021). Philosophy of Psychedelics. Oxford: Oxford University Press. doi:10.1093/med/9780198843122.001.0001. ISBN 978-0-19-884312-2.
- Richards, William A. (2016). Sacred Knowledge: Psychedelics and Religious Experiences. New York: Columbia University Press. ISBN 978-0-231-54091-9.
- Siff, Stephen (2015). Acid Hype: American News Media and the Psychedelic Experience. Champaign, Illinois: University of Illinois Press. ISBN 978-0-252-09723-2.
- Winstock, Ar; Timmerman, C; Davies, E; Maier, Lj; Zhuparris, A; Ferris, Ja; Barratt, Mj; Kuypers, Kpc (2021). Global Drug Survey (GDS) 2020 Psychedelics Key Findings Report.
External links
- Psychedelic Timeline by Tom Frame. Psychedelic Times.
PsychonautWiki.org a community-driven online encyclopedia.
| Wikiquote has quotations related to: Psychedelics |
 |
Categories: [Hallucinations]
↧ Download as ZWI file | Last modified: 04/15/2024 04:53:15 | 18 views
☰ Source: https://handwiki.org/wiki/Unsolved:Psychedelic_drug | License: CC BY-SA 3.0

 KSF
KSF