Nuclear Accident
 From Conservapedia
From Conservapedia In the brief history of man's harnessing of nuclear power, three prominent nuclear accidents have occurred. On a time scale, that would indicate that future accidents are certainly possible if not likely. While the nuclear industry and the nuclear portions of the military attempt to maintain their nuclear assets in a safe condition, the possibility does exist that another accident could occur. The possibility that an accident could occur does not necessarily indicate that this should be at the top of one's threats matrix, however, as these accidents are localized to the areas around the affected plant.
Contents
Understanding Nuclear Energy[edit]
Nuclear energy is not visible and there is significant fear surrounding it. Everything we interact with is made of molecules which are made of atoms. Each atom is made up of a nucleus (in the middle) and an electron cloud that orbits it. Inside the nucleus are particles called protons (positively charged) and neutrons (neutral, or no charge). The electron cloud has electrons (negatively charged). In addition to these nuclear particles, there are 3 types of radioactive particles that should be discussed before understanding the processes involved in an accident and the appropriate response one should take. Those particles are the alpha, beta, and gamma particles.
Alpha Radiation[edit]
An alpha particle is equivalent to a Helium atom without any electrons – two protons and two neutrons. It is created when an unstable atom sheds those four particles to become more stable. An analogy would be a canoe filled with four couples paddling in a river. The canoe, with that amount of loading, is unstable. To become more stable, two of the couples jump from the canoe arm-in-arm. Now the canoe is more stable and can continue on its merry way while the two couples stick together to stay safe. In nuclear terms, the alpha particle is relatively large and slow moving. What that means to someone exposed to alpha radiation (alpha particles being emitted) is that they can protect themselves with something as thin as a sheet of paper. In an area with alpha radiation, an N95 mask would suffice, though a more heavy duty respirator would be better protection. Some characteristics of alpha radiation are:
- Most alpha radiation is not able to penetrate human skin.
- Alpha-emitting materials can be harmful to humans if the materials are ‘‘‘inhaled, swallowed, or absorbed’’’ through open wounds.
- Instruments cannot detect alpha radiation through even a thin layer of water, dust, paper, or other material, because alpha radiation is not penetrating.
- Alpha radiation travels only a short distance (a few inches) in air, but is not an external hazard.
- Alpha radiation is not able to penetrate clothing.
Examples of some alpha emitters: radium, radon, uranium, thorium.
Beta Radiation[edit]
Beta radiation is a smaller lighter particle than alpha, but heavier than gammas. They are a short-range negatively charged particle and are actually an ejected electron. Some characteristics of beta radiation are:
- Beta radiation may travel several feet in air and is moderately penetrating.
- Beta radiation can penetrate human skin to the "germinal layer," where new skin cells are produced. If ‘’’high’’’ levels of beta-emitting contaminants are allowed to remain on the skin for a prolonged period of time, they may cause skin injury.
- Beta-emitting contaminants may be harmful if deposited internally.
- Some beta emitters, however, produce very low-energy, poorly penetrating radiation that may be difficult or impossible to detect. Examples of these difficult-to-detect beta emitters are hydrogen-3 (tritium), carbon-14, and sulfur-35. The first isotope listed is one of the most common purposefully encountered in the firearms world. Handgun and rifle “Night sights” are coated with a tritium-laced phosphor. The beta particles (electrons) make the phosphor glow so it can be seen in the dark. Tritium replaced radium in the 1960s, since radium is an alpha emitter and was ingested by the women who painted watch dials when they licked their brushes.
- Clothing provides some protection against beta radiation.
Examples of some pure beta emitters: strontium-90, carbon-14, tritium, and sulfur-35.
Gamma Radiation[edit]
Gamma radiation is basically a photon (demonstrating the properties of both a wave and a particle at the same time) that can occur at various energy levels depending on what emits it. Similar to gamma radiation are X-rays. Some characteristics of these radiations are:
- Gamma radiation or x-rays are able to travel many feet in air and many inches in human tissue. They readily penetrate most materials and are sometimes called "penetrating" radiation.
- X-rays are like gamma rays, but more energetic and will penetrate more deeply through matter.
- Gamma radiation and x-rays are electromagnetic radiation like visible light, radiowaves, and ultraviolet light. These electromagnetic radiations differ ‘’only in the amount of energy they have’’. Gamma rays and x-rays are the most energetic of these.
- Dense materials like steel or lead are needed for shielding from gamma radiation. Clothing provides little shielding from penetrating radiation, but will prevent contamination of the skin by gamma-emitting radioactive materials.
- Because it penetrates easily, Gamma radiation is easily detected by survey meters with a sodium iodide detector probe.
Examples of some gamma emitters: iodine-131, cesium-137, cobalt-60, radium-226, and technetium-99m. Your body concentrates iodine in the thyroid and does not care what isotope it is absorbing. This is why potassium iodide, or KI, tablets may be used nearby a nuclear incident. The body absorbs stable iodide isotopes from the tablets so that it will not absorb iodine-131.
Nuclear Power Production[edit]
Nuclear power from fission is generated by smashing neutrons from some source into atoms such as uranium that will absorb those neutrons, become unstable, and then split (i.e., fission). When that split happens, two new elements are made from the single uranium atom – each one smaller than the uranium atom. In addition to the two new atoms, a large amount of energy is released (harnessed by water to create steam) and the splitting uranium emits more neutrons that can go about smashing other uranium atoms causing a chain reaction. If enough splitting, or fission, events occur then the reaction becomes self-sustaining and is called “critical”. When a nuclear reactor is being started up or increased in power, it is slightly supercritical. When it is cooling down or decreasing in power it is slightly subcritical. These terms are often used in fear-mongering to confuse the uneducated. They are simply terms used to describe whether the reaction is speeding up, slowing down, or on the nuclear version of cruise control.
Historical Nuclear Accidents[edit]
- Three Mile Island, 1979 – The core of Three Mile Island Unit 2 melted down after an open valve allowed primary cooling water to leave the reactor coolant system in sufficient quantity that the reactor core was uncovered and could not be cooled sufficiently. There were no casualties, or even injuries, following the accident. There was small amount of radioactivity released, equivalent to a chest x-ray and the maximum dose anyone in the local area received was approximately the amount they would receive naturally in a year. There was a period of mass hysteria over the accident and there was some dispute over the actual release. However, the simple strategy of decreasing time and increasing distance (even moving away from the area) would have fully prevented any effect.
- Chernobyl, 1986
- Daiichi Nuclear Power Plant, 2011, occurred when the plant was hit by a tsunami triggered by the magnitude 9.0 Tōhoku earthquake
See also[edit]
- Nuclear attack
- World War Three Target Structures
- Potassium iodide
- Post Apocalyptic World
- War
- Man-made disasters
- Terrorism
References[edit]
Categories: [Man-Made Disasters] [Threats]
↧ Download as ZWI file | Last modified: 02/19/2023 15:15:15 | 39 views
☰ Source: https://www.conservapedia.com/Nuclear_accident | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: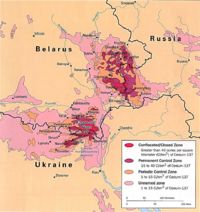
 KSF
KSF