Rhenium(Iv) Oxide
 From Handwiki
From Handwiki 
| |
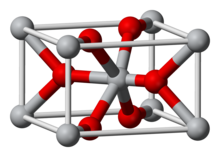 Re O
| |
| Names | |
|---|---|
| IUPAC name
Rhenium(IV) oxide
| |
| Other names
Rhenium dioxide
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
ReO2 |
| Molar mass | 218.206 g/mol |
| Appearance | gray orthorhombic crystals |
| Density | 11.4 g/cm3[1] |
| Melting point | decomposes at 1000 °C[2] |
Solubility in water
|
insoluble |
| Solubility in alkali | insoluble |
Magnetic susceptibility (χ)
|
+44.0·10−6 cm3/mol |
| Structure | |
Crystal structure
|
Orthorohmbic, oP12 |
Space group
|
Pbcn, No. 60 |
| Hazards | |
| Safety data sheet | Aldrich MSDS |
| NFPA 704 (fire diamond) | 
0
1
0 |
| Related compounds | |
Other anions
|
Rhenium(VII) oxide Rhenium(III) oxide Rhenium(III) chloride |
Other cations
|
manganese(IV) oxide Technetium(IV) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rhenium(IV) oxide or rhenium dioxide is the inorganic compound with the formula ReO2. This gray to black crystalline solid is a laboratory reagent that can be used as a catalyst. It adopts the rutile structure.
Synthesis and reactions
It forms via comproportionation:[3]
- 2 Re2O7 + 3 Re → 7 ReO2
Single crystals are obtained by chemical transport, using iodine as the transporting agent.:[4]
- ReO2 + I2 ⇌ ReO2I2
At high temperatures it undergoes disproportionation:
- 7 ReO2 → 2 Re2O7 + 3 Re
It forms rhenates with alkaline hydrogen peroxide and oxidizing acids.[5] In molten sodium hydroxide it forms sodium rhenate:[6]
- 2 NaOH + ReO2 → Na2ReO3 + H2O
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). CRC Press. p. 484. ISBN 0-8493-0594-2. https://books.google.com/books?id=lFjg0L-uOxoC&q="Rhenium(IV)+oxide"&pg=PT869. Retrieved 2008-06-05.
- ↑ Perry, Dale L.; Phillips, Sidney L. (1995). Handbook of Inorganic Compounds. San Diego: CRC Press. p. 328. ISBN 0-8493-8671-3. https://books.google.com/books?id=0fT4wfhF1AsC&q="Rhenium(IV)+oxide"&pg=PA328. Retrieved 2008-06-05.
- ↑ G. Glemser "Rhenium (IV) Oxide" Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1480.
- ↑ Rogers, D. B.; Butler, S. R.; Shannon, R. D. (1972). "Single Crystals of Transition‐Metal Dioxides". Single Crystals of Transition-Metal Dioxides. Inorganic Syntheses. XIII. pp. 135–145. doi:10.1002/9780470132449.ch27. ISBN 9780470131725.
- ↑ "RHENIUM DIOXIDE - Manufacturer". Aaamolybdenum.com. Archived from the original on 2003-02-09. https://web.archive.org/web/20030209232809/http://www.aaamolybdenum.com/RheniumDioxide.html. Retrieved 2012-08-06.
- ↑ G. Glemser "Sodium Rhenate (IV)" Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1483.
 |
Categories: [Transition metal oxides]
↧ Download as ZWI file | Last modified: 02/20/2024 03:16:27 | 10 views
☰ Source: https://handwiki.org/wiki/Chemistry:Rhenium(IV)_oxide | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF