Intravitreal Implants
 From Handwiki
From Handwiki 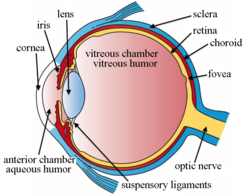
Intravitreal implants are micro device-like inserts injected into the posterior segment of the eye to treat retinal diseases releasing therapeutic drugs at a set rate over a desired period of time.[1][2] The posterior segment of the eye consists of the sclera, choroid, fovea, vitreous humor, optic nerve, and retina.[3][4]
Applications
Non-biodegradable implants
Inserts made with non-biodegradable materials such as polymers require a surgical removal of the implant after the end of the treatment period.[2] Examples of these materials consist of polymers such as ethylene-vinyl acetate (EVA), polyvinyl alcohol (PVA), polyurethane (PU) and poly siloxane (PS).[4] An advantage to these non-biodegradable implants is that they do not cause any immune response towards the retina and the release of the drug substance can be controlled by "layering polymers of different permeability."[2]
- Fluocinolone acetonide (Iluvien)[5]
Biodegradable implants
Biodegradable implants are made of materials, typically, either water-soluble or metabolizable to degrade into un-harmful byproducts which can be safely excreted by the human body.[2][4] It is important to note the release of the therapeutic drug is determined by the degradation of the implant and the diffusion rate of the drug substance.[2] Indicating that the higher the molecular weight of the polymer and drug substance used, the slower the release of the drug into the vitreous humor.[2]
- Dexamethasone (Ozurdex)[5]
- Bimatoprost (Durysta)[5]
References
- ↑ "Nanomedicine and drug delivery to the retina: current status and implications for gene therapy". Naunyn-Schmiedeberg's Archives of Pharmacology 395 (12): 1477–1507. December 2022. doi:10.1007/s00210-022-02287-3. PMID 36107200.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Intravitreal Systems For Targeted Drug Delivery To The Posterior Eye Segment: A Systematic Review". Russian Open Medical Journal 11 (2): e0213. 2022-06-25. doi:10.15275/rusomj.2022.0213. https://romj.org/2022-0213.
- ↑ (in en) Smart Biomaterial Devices (0 ed.). CRC Press. 2016-12-19. doi:10.1201/9781315371559. ISBN 978-1-4987-0701-5. https://www.taylorfrancis.com/books/9781498707015.
- ↑ 4.0 4.1 4.2 "Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials". Pharmaceutics 13 (5): 701. May 2021. doi:10.3390/pharmaceutics13050701. PMID 34065798.
- ↑ 5.0 5.1 5.2 "The Use of Sustained Release Intravitreal Steroid Implants in Non-Infectious Uveitis Affecting the Posterior Segment of the Eye". Ophthalmology and Therapy 11 (2): 479–487. April 2022. doi:10.1007/s40123-022-00456-4. PMID 35092605.
 |
Categories: [Implants (medicine)] [Ophthalmology]
↧ Download as ZWI file | Last modified: 07/15/2024 15:13:58 | 3 views
☰ Source: https://handwiki.org/wiki/Medicine:Intravitreal_implants | License: CC BY-SA 3.0

 KSF
KSF