Pathophysiology Of Hypertension
 From Mdwiki
From Mdwiki
Pathophysiology is a branch of medicine which explains the function of the body as it relates to diseases and conditions. The pathophysiology of hypertension is an area which attempts to explain mechanistically the causes of hypertension, which is a chronic disease characterized by elevation of blood pressure. Hypertension can be classified by cause as either essential (also known as primary or idiopathic) or secondary. About 90–95% of hypertension is essential hypertension.[1][2][3][4]
Some authorities define essential hypertension as that which has no known explanation, while others define its cause as being due to overconsumption of sodium and underconsumption of potassium. Secondary hypertension indicates that the hypertension is a result of a specific underlying condition with a well-known mechanism, such as chronic kidney disease, narrowing of the aorta or kidney arteries, or endocrine disorders such as excess aldosterone, cortisol, or catecholamines. Persistent hypertension is a major risk factor for hypertensive heart disease, coronary artery disease, stroke, aortic aneurysm, peripheral artery disease, and chronic kidney disease.[5]
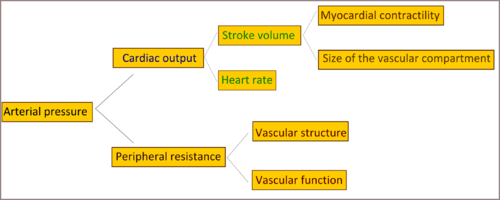
Cardiac output and peripheral resistance are the two determinants of arterial pressure.[6] Cardiac output is determined by stroke volume and heart rate; stroke volume is related to myocardial contractility and to the size of the vascular compartment. Peripheral resistance is determined by functional and anatomic changes in small arteries and arterioles.
Genetics[edit | edit source]
Single gene mutations can cause Mendelian forms of high blood pressure;[7] ten genes have been identified which cause these monogenic forms of hypertension.[7][8]
These mutations affect blood pressure by altering kidney salt handling.[9][10] There is greater similarity in blood pressure within families than between families, which indicates a form of inheritance,[11] and this is not due to shared environmental factors.[12] With the aid of genetic analysis techniques, a statistically significant linkage of blood pressure to several chromosomal regions, including regions linked to familial combined hyperlipidemia, was found.[13][14][15][16][17] These findings suggest that there are many genetic loci, in the general population, each with small effects on blood pressure. Overall, however, identifiable single-gene causes of hypertension are uncommon, consistent with a multifactorial cause of essential hypertension.[2][10][18][19]
Autonomic nervous system[edit | edit source]
The autonomic nervous system plays a central role in maintaining cardiovascular homeostasis via pressure, volume, and chemoreceptor signals. It does this by regulating the peripheral vasculature, and kidney function, which in turn affect cardiac output, vascular resistance, and fluid retention. Excess activity of the sympathetic nervous system increases blood pressure and contributes to hypertension.[20][21][22][23][24]
The mechanisms of increased sympathetic nervous system activity in hypertension involve alterations in baroreflex and chemoreflex pathways at both peripheral and central levels. Arterial baroreceptors are reset to a higher pressure in hypertensive patients, and this peripheral resetting reverts to normal when arterial pressure is normalized.[25][26][27] Furthermore, there is central resetting of the aortic baroreflex in hypertensive patients, resulting in suppression of sympathetic inhibition after activation of aortic baroreceptor nerves. This baroreflex resetting seems to be mediated, at least partly, by a central action of angiotensin II.[28][29][30] Additional small-molecule mediators that suppress baroreceptor activity and contribute to exaggerated sympathetic drive in hypertension include reactive oxygen species and endothelin.[31][32] Some studies shown that hypertensive patients manifest greater vasoconstrictor responses to infused norepinephrine than normotensive controls.[33] And that hypertensive patients do not show the normal response to increased circulating norepinephrine levels which generally induces downregulation of noradrenergic receptor, and it is believed that this abnormal response is genetically inherited.[34]
Exposure to stress increases sympathetic outflow, and repeated stress-induced vasoconstriction may result in vascular hypertrophy, leading to progressive increases in peripheral resistance and blood pressure.[2] This could partly explain the greater incidence of hypertension in lower socioeconomic groups, since they must endure greater levels of stress associated with daily living. Persons with a family history of hypertension manifest augmented vasoconstrictor and sympathetic responses to laboratory stressors, such as cold pressor testing and mental stress, that may predispose them to hypertension. This is particularly true of young African Americans. Exaggerated stress responses may contribute to the increased incidence of hypertension in this group.[35]
Resistant hypertension can be treated by electrically stimulating the baroreflex with a pacemaker-like device.[36]
Renin–angiotensin–aldosterone system[edit | edit source]
Another system maintaining the extracellular fluid volume, peripheral resistance, and that if disturbed may lead to hypertension, is the renin–angiotensin–aldosterone system. Renin is a circulating enzyme that participates in maintaining extracellular volume and arterial vasoconstriction, therefore contributing to regulation of blood pressure. It performs this function by breaking down (hydrolysing) angiotensinogen, secreted from the liver, into the peptide angiotensin I. Angiotensin I is further cleaved by an enzyme that is located primarily but not exclusively in the pulmonary circulation bound to endothelium; that enzyme is angiotensin converting enzyme (ACE). This cleavage produces angiotensin II, the most vasoactive peptide.[37][38] Angiotensin II is a potent constrictor of all blood vessels. It acts on the musculature of arteries, raising peripheral resistance and thereby elevating blood pressure. Angiotensin II also causes the adrenal glands to release aldosterone, which stimulates the epithelial cells of the kidneys to increase re-absorption of salt and water, leading to raised blood volume and raised blood pressure. So elevated renin levels in the blood (normally 1.98-2.46 ng/ml in the upright position)[39] leads to hypertension.[2][40]
Recent studies claim that obesity is a risk factor for hypertension because of activation of the renin–angiotensin system (RAS) in adipose tissue,[41][42] and also linked renin–angiotensin system with insulin resistance, and claims that anyone can cause the other.[43] Local production of angiotensin II in various tissues, including the blood vessels, heart, adrenals, and brain, is controlled by ACE and other enzymes, including the serine protease chymase. The activity of local renin–angiotensin systems and alternative pathways of angiotensin II formation may make an important contribution to remodeling of resistance vessels and the development of target organ damage (i.e. left ventricular hypertrophy, congestive heart failure, atherosclerosis, stroke, end-stage kidney disease, myocardial infarction, and arterial aneurysm) in hypertensive persons.[40]
Endothelial dysfunction[edit | edit source]
The endothelium of blood vessels produces an extensive range of substances that influence blood flow and, in turn, is affected by changes in the blood and the pressure of blood flow. For example, local nitric oxide and endothelin, which are secreted by the endothelium, are the major regulators of vascular tone and blood pressure. In patients with essential hypertension, the balance between the vasodilators and the vasoconstrictors is upset, which leads to changes in the endothelium and sets up a "vicious cycle" that contributes to the maintenance of high blood pressure. In patients with hypertension, endothelial activation and damage also lead to changes in vascular tone, vascular reactivity, and coagulation and fibrinolytic pathways. Alterations in endothelial function are a reliable indicator of target organ damage and atherosclerotic disease, as well as prognosis.[44]
Evidence suggests that oxidant stress alters many functions of the endothelium, including modulation of vasomotor tone. Inactivation of nitric oxide (NO) by superoxide and other reactive oxygen species (ROS) seems to occur in conditions such as hypertension.[45][46][47] Normally nitric oxide is an important regulator and mediator of numerous processes in the nervous, immune and cardiovascular systems, including smooth muscle relaxation thus resulting in vasodilation of the artery and increasing blood flow, suppressor of migration and proliferation of vascular smooth-muscle cells.[2] It has been suggested that angiotensin II enhances formation of the oxidant superoxide at concentrations that affect blood pressure minimally.[48]
Endothelin is a potent vasoactive peptide produced by endothelial cells that has both vasoconstrictor and vasodilator properties. Circulating endothelin levels are increased in some hypertensive patients,[49][50] particularly African Americans and persons with hypertension.[49][51][52][53]
Sodium/potassium ratio hypothesis of essential hypertension[edit | edit source]
A 2007 review article states that while excessive sodium consumption has long been recognized as contributing to the risk of hypertension, "potassium, the main intracellular cation, has usually been viewed as a minor factor in the pathogenesis of hypertension. However, abundant evidence indicates that a potassium deficit has a critical role in hypertension and its cardiovascular sequelae." The authors state that modern, western, high sodium, low potassium diets result in corresponding changes in intracellular concentration of these, the two most important cations in animal cells. This imbalance leads to contraction of vascular smooth muscle, restricting blood flow and so driving up blood pressure. The authors cite studies which showing that potassium supplementation is effective in reducing hypertension.[54]
Epidemiological support for this hypothesis can be found in a 2014 meta-analysis which states that "the sodium-to-potassium ratio appears to be more strongly associated with blood pressure outcomes than either sodium or potassium alone in hypertensive adult populations.".[55]
References[edit | edit source]
- ↑ Carretero OA, Oparil S (January 2000). "Essential hypertension. Part I: definition and etiology". Circulation. 101 (3): 329–35. doi:10.1161/01.CIR.101.3.329. PMID 10645931. Archived from the original on 2012-07-07. Retrieved 2009-06-05.
- ↑ 2.0 2.1 2.2 2.3 2.4 Oparil S, Zaman MA, Calhoun DA (November 2003). "Pathogenesis of hypertension". Ann. Intern. Med. 139 (9): 761–76. doi:10.7326/0003-4819-139-9-200311040-00011. PMID 14597461. S2CID 32785528.
- ↑ Hall, John E.; Guyton, Arthur C. (2006). Textbook of medical physiology. St. Louis, Mo: Elsevier Saunders. p. 228. ISBN 978-0-7216-0240-0.
- ↑ "Hypertension: eMedicine Nephrology". Archived from the original on 2019-08-01. Retrieved 2009-06-05.
- ↑ Pierdomenico SD, Di Nicola M, Esposito AL, et al. (June 2009). "Prognostic Value of Different Indices of Blood Pressure Variability in Hypertensive Patients". American Journal of Hypertension. 22 (8): 842–47. doi:10.1038/ajh.2009.103. PMID 19498342.
- ↑ Klabunde, Richard E. (2007). "Cardiovascular Physiology Concepts – Mean Arterial Pressure". Archived from the original on October 2, 2009. Retrieved 2008-09-29.
- ↑ 7.0 7.1 Lifton RP, Gharavi AG, Geller DS (February 2001). "Molecular mechanisms of human hypertension". Cell. 104 (4): 545–56. doi:10.1016/S0092-8674(01)00241-0. PMID 11239411. S2CID 9401969.
- ↑ Wilson FH, Disse-Nicodème S, Choate KA, et al. (August 2001). "Human hypertension caused by mutations in WNK kinases". Science. 293 (5532): 1107–12. doi:10.1126/science.1062844. PMID 11498583. S2CID 22700809. Archived from the original on 2022-05-18. Retrieved 2021-09-13.
- ↑ Guyton AC (June 1991). "Blood pressure control--special role of the kidneys and body fluids". Science. 252 (5014): 1813–16. Bibcode:1991Sci...252.1813G. doi:10.1126/science.2063193. PMID 2063193.
- ↑ 10.0 10.1 Corvol P, Persu A, Gimenez-Roqueplo AP, Jeunemaitre X (June 1999). "Seven lessons from two candidate genes in human essential hypertension: angiotensinogen and epithelial sodium channel". Hypertension. 33 (6): 1324–31. doi:10.1161/01.hyp.33.6.1324. PMID 10373210.
- ↑ Feinleib M, Garrison RJ, Fabsitz R, et al. (October 1977). "The NHLBI twin study of cardiovascular disease risk factors: methodology and summary of results". American Journal of Epidemiology. 106 (4): 284–85. doi:10.1093/oxfordjournals.aje.a112464. PMID 562066. Archived from the original on 2013-04-15. Retrieved 2009-06-08.
- ↑ Biron P, Mongeau JG, Bertrand D (October 1976). "Familial aggregation of blood pressure in 558 adopted children". Canadian Medical Association Journal. 115 (8): 773–74. PMC 1878814. PMID 974967.
- ↑ Hsueh WC, Mitchell BD, Schneider JL, et al. (June 2000). "QTL influencing blood pressure maps to the region of PPH1 on chromosome 2q31-34 in Old Order Amish". Circulation. 101 (24): 2810–16. doi:10.1161/01.cir.101.24.2810. PMID 10859286. Archived from the original on 2013-02-23. Retrieved 2009-06-08.
- ↑ Levy D, DeStefano AL, Larson MG, et al. (October 2000). "Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study". Hypertension. 36 (4): 477–83. doi:10.1161/01.hyp.36.4.477. PMID 11040222.
- ↑ Kristjansson K, Manolescu A, Kristinsson A, et al. (June 2002). "Linkage of essential hypertension to chromosome 18q". Hypertension. 39 (6): 1044–49. doi:10.1161/01.HYP.0000018580.24644.18. PMID 12052839.
- ↑ Hunt SC, Ellison RC, Atwood LD, Pankow JS, Province MA, Leppert MF (July 2002). "Genome scans for blood pressure and hypertension: the National Heart, Lung, and Blood Institute Family Heart Study". Hypertension. 40 (1): 1–6. doi:10.1161/01.HYP.0000022660.28915.B1. PMID 12105129.
- ↑ Selby JV, Newman B, Quiroga J, Christian JC, Austin MA, Fabsitz RR (April 1991). "Concordance for dyslipidemic hypertension in male twins". JAMA: The Journal of the American Medical Association. 265 (16): 2079–84. doi:10.1001/jama.265.16.2079. PMID 2013927.
- ↑ Niu T, Yang J, Wang B, et al. (February 1999). "Angiotensinogen gene polymorphisms M235T/T174M: no excess transmission to hypertensive Chinese". Hypertension. 33 (2): 698–702. doi:10.1161/01.hyp.33.2.698. PMID 10024331.
- ↑ Luft FC (May 2000). "Molecular genetics of human hypertension". Current Opinion in Nephrology and Hypertension. 9 (3): 259–66. doi:10.1097/00041552-200005000-00009. PMID 10847327.
- ↑ Somers VK, Anderson EA, Mark AL (January 1993). "Sympathetic neural mechanisms in human hypertension". Current Opinion in Nephrology and Hypertension. 2 (1): 96–105. doi:10.1097/00041552-199301000-00015. PMID 7922174.
- ↑ Takahashi H (August 2008). "[Sympathetic hyperactivity in hypertension]". Nippon Rinsho. Japanese Journal of Clinical Medicine (in 日本語). 66 (8): 1495–502. PMID 18700548.
- ↑ Esler M (June 2000). "The sympathetic system and hypertension". American Journal of Hypertension. 13 (6 Pt 2): 99S–105S. doi:10.1016/S0895-7061(00)00225-9. PMID 10921528.
- ↑ Mark AL (December 1996). "The sympathetic nervous system in hypertension: a potential long-term regulator of arterial pressure". Journal of Hypertension Supplement. 14 (5): S159–65. PMID 9120673.
- ↑ Brook RD, Julius S (June 2000). "Autonomic imbalance, hypertension, and cardiovascular risk". American Journal of Hypertension. 13 (6 Pt 2): 112S–122S. doi:10.1016/S0895-7061(00)00228-4. PMID 10921530.
- ↑ Chapleau MW, Hajduczok G, Abboud FM (April 1988). "Mechanisms of resetting of arterial baroreceptors: an overview". The American Journal of the Medical Sciences. 295 (4): 327–34. doi:10.1097/00000441-198804000-00019. PMID 2834951.
- ↑ Guo GB, Thames MD, Abboud FM (August 1983). "Arterial baroreflexes in renal hypertensive rabbits. Selectivity and redundancy of baroreceptor influence on heart rate, vascular resistance, and lumbar sympathetic nerve activity". Circulation Research. 53 (2): 223–34. doi:10.1161/01.res.53.2.223. PMID 6883646.
- ↑ Xie PL, Chapleau MW, McDowell TS, Hajduczok G, Abboud FM (August 1990). "Mechanism of decreased baroreceptor activity in chronic hypertensive rabbits. Role of endogenous prostanoids". The Journal of Clinical Investigation. 86 (2): 625–30. doi:10.1172/JCI114754. PMC 296770. PMID 2117025.
- ↑ Lohmeier TE (June 2001). "The sympathetic nervous system and long-term blood pressure regulation". American Journal of Hypertension. 14 (6 Pt 2): 147S–154S. doi:10.1016/S0895-7061(01)02082-9. PMID 11411750.
- ↑ Guo GB, Abboud FM (May 1984). "Impaired central mediation of the arterial baroreflex in chronic renal hypertension". The American Journal of Physiology. 246 (5 Pt 2): H720–7. doi:10.1152/ajpheart.1984.246.5.H720. PMID 6720985.
- ↑ Abboud FM (February 1974). "Effects of sodium, angiotensin, and steroids on vascular reactivity in man". FASEB J. 33 (2): 143–49. PMID 4359754.
- ↑ Li Z, Mao HZ, Abboud FM, Chapleau MW (October 1996). "Oxygen-derived free radicals contribute to baroreceptor dysfunction in atherosclerotic rabbits". Circulation Research. 79 (4): 802–11. doi:10.1161/01.res.79.4.802. PMID 8831504. Archived from the original on 2013-02-23. Retrieved 2009-06-08.
- ↑ Chapleau MW, Hajduczok G, Abboud FM (July 1992). "Suppression of baroreceptor discharge by endothelin at high carotid sinus pressure". The American Journal of Physiology. 263 (1 Pt 2): R103–8. doi:10.1152/ajpregu.1992.263.1.R103. PMID 1636777.
- ↑ Ziegler MG, Mills P, Dimsdale JE (July 1991). "Hypertensives' pressor response to norepinephrine. Analysis by infusion rate and plasma levels". American Journal of Hypertension. 4 (7 Pt 1): 586–91. doi:10.1093/ajh/4.7.586. PMID 1873013.
- ↑ Bianchetti MG, Beretta-Piccoli C, Weidmann P, Ferrier C (April 1986). "Blood pressure control in normotensive members of hypertensive families". Kidney International. 29 (4): 882–88. doi:10.1038/ki.1986.81. PMID 3520094.
- ↑ Calhoun DA, Mutinga ML, Collins AS, Wyss JM, Oparil S (December 1993). "Normotensive blacks have heightened sympathetic response to cold pressor test". Hypertension. 22 (6): 801–05. doi:10.1161/01.hyp.22.6.801. PMID 8244512.
- ↑ Wallbach, M; Koziolek, MJ (9 November 2017). "Baroreceptors in the carotid and hypertension-systematic review and meta-analysis of the effects of baroreflex activation therapy on blood pressure". Nephrology, Dialysis, Transplantation. 33 (9): 1485–1493. doi:10.1093/ndt/gfx279. PMID 29136223.
- ↑ Fujino T, Nakagawa N, Yuhki K, et al. (September 2004). "Decreased susceptibility to renovascular hypertension in mice lacking the prostaglandin I2 receptor IP". J. Clin. Invest. 114 (6): 805–12. doi:10.1172/JCI21382. PMC 516260. PMID 15372104.
- ↑ Brenner & Rector's The Kidney, 7th ed., Saunders, 2004. pp.2118-2119.Full Text with MDConsult subscription Archived 2016-03-03 at the Wayback Machine
- ↑ Hamilton Regional Laboratory Medicine Program - Laboratory Reference Centre Manual. Renin Direct Archived 2012-02-24 at the Wayback Machine
- ↑ 40.0 40.1 McConnaughey MM, McConnaughey JS, Ingenito AJ (June 1999). "Practical considerations of the pharmacology of angiotensin receptor blockers". Journal of Clinical Pharmacology. 39 (6): 547–59. doi:10.1177/00912709922008155. PMID 10354958. S2CID 34396502. Archived from the original on 2019-12-17. Retrieved 2009-06-09.[permanent dead link]
- ↑ Segura J, Ruilope LM (October 2007). "Obesity, essential hypertension and renin–angiotensin system". Public Health Nutrition. 10 (10A): 1151–55. doi:10.1017/S136898000700064X. PMID 17903324.
- ↑ Hasegawa H, Komuro I (April 2009). "[The progress of the study of RAAS]". Nippon Rinsho. Japanese Journal of Clinical Medicine (in 日本語). 67 (4): 655–61. PMID 19348224.
- ↑ Saitoh S (April 2009). "[Insulin resistance and renin–angiotensin–aldosterone system]". Nippon Rinsho. Japanese Journal of Clinical Medicine (in 日本語). 67 (4): 729–34. PMID 19348235.
- ↑ O'Brien, Eoin; Beevers, D. G.; Lip, Gregory Y. H. (2007). ABC of hypertension. London: BMJ Books. ISBN 978-1-4051-3061-5.
- ↑ Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M (November 1991). "Does superoxide underlie the pathogenesis of hypertension?". Proceedings of the National Academy of Sciences of the United States of America. 88 (22): 10045–48. Bibcode:1991PNAS...8810045N. doi:10.1073/pnas.88.22.10045. PMC 52864. PMID 1658794.
- ↑ Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG (February 1997). "Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension". Circulation. 95 (3): 588–93. doi:10.1161/01.cir.95.3.588. PMID 9024144. Archived from the original on 2013-02-23. Retrieved 2009-06-09.
- ↑ Cai H, Harrison DG (November 2000). "Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress". Circulation Research. 87 (10): 840–44. doi:10.1161/01.res.87.10.840. PMID 11073878.
- ↑ Fukui T, Ishizaka N, Rajagopalan S, et al. (January 1997). "p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats". Circulation Research. 80 (1): 45–51. doi:10.1161/01.res.80.1.45. PMID 8978321. Archived from the original on 2013-02-23. Retrieved 2009-06-09.
- ↑ 49.0 49.1 Touyz RM, Schiffrin EL (June 2003). "Role of endothelin in human hypertension". Canadian Journal of Physiology and Pharmacology. 81 (6): 533–41. doi:10.1139/y03-009. PMID 12839265. Archived from the original on 2012-12-16. Retrieved 2009-06-09.
- ↑ Shreenivas S, Oparil S (2007). "The role of endothelin-1 in human hypertension". Clinical Hemorheology and Microcirculation. 37 (1–2): 157–78. PMID 17641406. Archived from the original on 2020-03-12. Retrieved 2009-06-09.
- ↑ Ergul S, Parish DC, Puett D, Ergul A (October 1996). "Racial differences in plasma endothelin-1 concentrations in individuals with essential hypertension". Hypertension. 28 (4): 652–5. doi:10.1161/01.hyp.28.4.652. PMID 8843893. Archived from the original on 2013-02-23. Retrieved 2009-06-09.
- ↑ Grubbs AL, Ergul A (2001). "A review of endothelin and hypertension in African-American individuals". Ethnicity & Disease. 11 (4): 741–48. PMID 11763297.
- ↑ Campia U, Cardillo C, Panza JA (June 2004). "Ethnic differences in the vasoconstrictor activity of endogenous endothelin-1 in hypertensive patients". Circulation. 109 (25): 3191–95. doi:10.1161/01.CIR.0000130590.24107.D3. PMID 15148269. Archived from the original on 2013-02-23. Retrieved 2009-06-09.
- ↑ Adrogué, HJ; Madias, NE (10 May 2007). "Sodium and potassium in the pathogenesis of hypertension" (PDF). The New England Journal of Medicine. 356 (19): 1966–78. doi:10.1056/NEJMra064486. PMID 17494929. Archived (PDF) from the original on 27 January 2021. Retrieved 13 September 2021.
- ↑ Perez, V; Chang, ET (November 2014). "Sodium-to-potassium ratio and blood pressure, hypertension, and related factors". Advances in Nutrition. 5 (6): 712–41. doi:10.3945/an.114.006783. PMC 4224208. PMID 25398734.
Categories: [Hypertension] [Pathophysiology] [Cardiology] [Cardiovascular diseases] [Medical conditions related to obesity]
↧ Download as ZWI file | Last modified: 07/09/2023 22:44:40 | 3 views
☰ Source: https://mdwiki.org/wiki/Pathophysiology_of_hypertension | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF